Pharmacokinetics of Isavuconazole During Extracorporeal Membrane Oxygenation Support in Critically Ill Patients: A Case Series
Abstract
1. Introduction
2. Results
2.1. Cohort Description
2.2. Patient Characteristics and Pharmacokinetic Parameters
2.3. ECMO Circuit Effect on Isavuconazole Concentration and Pharmacokinetics
2.4. Clinical Features of the Cohort
3. Discussion
4. Materials and Methods
4.1. Study Design and Population
4.2. Isavuconazole Treatment
4.3. Demographic and Clinical Data
4.4. Blood Sample Collection and Analysis
4.5. Pharmacokinetics Analysis
4.6. Extracorporeal Circuit
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area Under the Curve |
| Cmin | Minimum Plasma Concentration |
| Cmax | Maximum Plasma Concentration |
| T1/2 | Elimination Half-Life |
| kel | Elimination Rate Constant |
| Vd | Volume of Distribution |
| CL | Clearance |
| ECMO | Extracorporeal Membrane Oxygenation |
| ARDS | Acute Respiratory Distress Syndrome |
| TDM | Therapeutic Drug Monitoring |
| PPB | Plasma Protein Binding |
| MOD | Multiple Organ Dysfunction |
| CRRT | Continuous Renal Replacement Therapy |
| CAPA | COVID-19-Associated Pulmonary Aspergillosis |
| V-V ECMO | Veno-Venous ECMO |
| BMI | Body Mass Index |
| AST | Aspartate Aminotransferase |
| ALT | Alanine Aminotransferase |
| ALP | Alkaline Phosphatase |
| GGT | Gamma-Glutamyl Transferase |
| ISA | Isavuconazole |
| HPLC | High-Performance Liquid Chromatography |
| CRRT | Continuous Renal Replacement Therapy |
| PaCO2 | Partial Pressure of Carbon Dioxide |
| SD | Standard Deviation |
| IQR | Interquartile Range |
| SIRS | Systemic Inflammatory Response Syndrome |
| QT | QT interval of the electrocardiogram |
References
- Ellsworth, M.; Ostrosky-Zeichner, L. Isavuconazole: Mechanism of Action, Clinical Efficacy, and Resistance. J. Fungi 2020, 6, 324. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.W.; Moriyama, B.; Petraitiene, R.; Walsh, T.J.; Petraitis, V. Clinical Pharmacokinetics and Pharmacodynamics of Isavuconazole. Clin. Pharmacokinet. 2018, 57, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Van Daele, R.; Debaveye, Y.; Vos, R.; Van Bleyenbergh, P.; Brüggemann, R.J.; Dreesen, E.; Elkayal, O.; Guchelaar, H.J.; Vermeersch, P.; Lagrou, K.; et al. Concomitant Use of Isavuconazole and CYP3A4/5 Inducers: Where Pharmacogenetics Meets Pharmacokinetics. Mycoses 2021, 64, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, S.; Kelly, D.M.; Rameli, P.M.; Kelleher, E.; Martin-Loeches, I. Invasive Pulmonary Aspergillosis in the Intensive Care Unit: Current Challenges and Best Practices. APMIS 2023, 131, 654–667. [Google Scholar] [CrossRef]
- Millar, J.E.; Fanning, J.P.; McDonald, C.I.; McAuley, D.F.; Fraser, J.F. The Inflammatory Response to Extracorporeal Membrane Oxygenation (ECMO): A Review of the Pathophysiology. Crit. Care 2016, 20, 387. [Google Scholar] [CrossRef]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.A.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Flörl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and Managing COVID-19-Associated Pulmonary Aspergillosis: The 2020 ECMM/ISHAM Consensus Criteria for Research and Clinical Guidance. Lancet Infect. Dis. 2021, 21, e149–e162. [Google Scholar] [CrossRef]
- Andes, D.; Kovanda, L.; Desai, A.; Kitt, T.; Zhao, M.; Walsh, T.J. Isavuconazole Concentration in Real-World Practice: Consistency With Results From Clinical Trials. Antimicrob. Agents Chemother. 2018, 62, e00585-18. [Google Scholar] [CrossRef]
- Mikulska, M.; Melchio, M.; Signori, A.; Ullah, N.; Miletich, F.; Sepulcri, C.; Limongelli, A.; Giacobbe, D.R.; Balletto, E.; Russo, C.; et al. Lower Blood Levels of Isavuconazole in Critically Ill Patients Compared with Other Populations: Possible Need for Therapeutic Drug Monitoring. J. Antimicrob. Chemother. 2024, 79, 835–845. [Google Scholar] [CrossRef]
- Lyster, H.; Shekar, K.; Watt, K.; Reed, A.; Roberts, J.A.; Abdul-Aziz, M.-H. Antifungal Dosing in Critically Ill Patients on Extracorporeal Membrane Oxygenation. Clin. Pharmacokinet. 2023, 62, 931–942. [Google Scholar] [CrossRef]
- Pau-Parra, A.; Sosa Garay, M.; Doménech Moral, L.; Díez Poch, M.; Martínez Pla, M.; Gallart, E.; Vima Bofarull, J.; Nuvials, X.; García-García, S.; Doménech Vila, J.M.; et al. Therapeutic Drug Monitoring-Guided High-Dose Isavuconazole Therapy for Invasive Pulmonary Aspergillosis in a Patient on Extracorporeal Membrane Oxygenation Support. J. Chemother. 2025, 1–7. [Google Scholar] [CrossRef]
- Höhl, R.; Bertram, R.; Kinzig, M.; Haarmeyer, G.-S.; Baumgärtel, M.; Geise, A.; Muschner, D.; Prosch, D.; Reger, M.; Naumann, H.-T.; et al. Isavuconazole Therapeutic Drug Monitoring in Critically Ill ICU Patients: A Monocentric Retrospective Analysis. Mycoses 2022, 65, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Perez, L.; Corne, P.; Pasquier, G.; Konecki, C.; Sadek, M.; Le Bihan, C.; Klouche, K.; Mathieu, O.; Reynes, J.; Cazaubon, Y. Population Pharmacokinetics of Isavuconazole in Critical Care Patients with COVID-19-Associated Pulmonary Aspergillosis and Monte Carlo Simulations of High Off-Label Doses. J. Fungi 2023, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Furfaro, E.; Signori, A.; Di Grazia, C.; Dominietto, A.; Raiola, A.M.; Aquino, S.; Ghiggi, C.; Ghiso, A.; Ungaro, R.; Angelucci, E.; et al. Serial Monitoring of Isavuconazole Blood Levels during Prolonged Antifungal Therapy. J. Antimicrob. Chemother. 2019, 74, 2341–2346. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Glampedakis, E.; Boillat-Blanco, N.; Oddo, M.; Pagani, J.-L. Incidence of Invasive Pulmonary Aspergillosis among Critically Ill COVID-19 Patients. Clin. Microbiol. Infect. 2020, 26, 1706–1708. [Google Scholar] [CrossRef]
- Riera, J.; Barbeta, E.; Tormos, A.; Mellado-Artigas, R.; Ceccato, A.; Motos, A.; Fernández-Barat, L.; Ferrer, R.; García-Gasulla, D.; Peñuelas, O.; et al. Effects of Intubation Timing in Patients with COVID-19 throughout the Four Waves of the Pandemic: A Matched Analysis. Eur. Respir. J. 2023, 61, 2201426. [Google Scholar] [CrossRef]
- Patel, J.S.; Kooda, K.; Igneri, L.A. A Narrative Review of the Impact of Extracorporeal Membrane Oxygenation on the Pharmacokinetics and Pharmacodynamics of Critical Care Therapies. Ann. Pharmacother. 2023, 57, 706–726. [Google Scholar] [CrossRef]
- Kovanda, L.L.; Desai, A.V.; Lu, Q.; Townsend, R.W.; Akhtar, S.; Bonate, P.; Hope, W.W. Isavuconazole Population Pharmacokinetic Analysis Using Nonparametric Estimation in Patients with Invasive Fungal Disease (Results from the VITAL Study). Antimicrob. Agents Chemother. 2016, 60, 4568–4576. [Google Scholar] [CrossRef]
- Cresemba® (Isavuconazole). Annex I of Summary of Product Characteristics; European Medicines Agency (EMA): Amsterdam, The Netherlands, 2016; Available online: https://www.ema.europa.eu/es/documents/product-information/cresemba-epar-product-information_es.pdf (accessed on 3 June 2025).
- Shekar, K.; Abdul-Aziz, M.H.; Cheng, V.; Burrows, F.; Buscher, H.; Cho, Y.-J.; Corley, A.; Diehl, A.; Gilder, E.; Jakob, S.M.; et al. Antimicrobial Exposures in Critically Ill Patients Receiving Extracorporeal Membrane Oxygenation. Am. J. Respir. Crit. Care Med. 2023, 207, 704–720. [Google Scholar] [CrossRef]
- Miller, M.; Kludjian, G.; Mohrien, K.; Morita, K. Decreased Isavuconazole Trough Concentrations in the Treatment of Invasive Aspergillosis in an Adult Patient Receiving Extracorporeal Membrane Oxygenation Support. Am. J. Health-Syst. Pharm. 2022, 79, 1245–1249. [Google Scholar] [CrossRef]
- Mendoza-Palomar, N.; Melendo-Pérez, S.; Balcells, J.; Izquierdo-Blasco, J.; Martín-Gómez, M.T.; Velasco-Nuño, M.; Rivière, J.G.; Soler-Palacin, P. Influenza-Associated Disseminated Aspergillosis in a 9-Year-Old Girl Requiring ECMO Support. J. Fungi 2021, 7, 726. [Google Scholar] [CrossRef]
- Zhao, Y.; Seelhammer, T.G.; Barreto, E.F.; Wilson, J.W. Altered Pharmacokinetics and Dosing of Liposomal Amphotericin B and Isavuconazole During Extracorporeal Membrane Oxygenation. Pharmacotherapy 2020, 40, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Kriegl, L.; Hatzl, S.; Zurl, C.; Reisinger, A.C.; Schilcher, G.; Eller, P.; Gringschl, Y.; Muhr, T.; Meinitzer, A.; Prattes, J.; et al. Isavuconazole Plasma Concentrations in Critically Ill Patients during Extracorporeal Membrane Oxygenation. J. Antimicrob. Chemother. 2022, 77, 2500–2505. [Google Scholar] [CrossRef] [PubMed]
- Zurl, C.; Waller, M.; Schwameis, F.; Muhr, T.; Bauer, N.; Zollner-Schwetz, I.; Valentin, T.; Meinitzer, A.; Ullrich, E.; Wunsch, S.; et al. Isavuconazole Treatment in a Mixed Patient Cohort with Invasive Fungal Infections: Outcome, Tolerability and Clinical Implications of Isavuconazole Plasma Concentrations. J. Fungi 2020, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Kovanda, L.L.; Marty, F.M.; Maertens, J.; Desai, A.V.; Lademacher, C.; Engelhardt, M.; Lu, Q.; Hope, W.W. Impact of Mucositis on Absorption and Systemic Drug Exposure of Isavuconazole. Antimicrob. Agents Chemother. 2017, 61, 10-1128. [Google Scholar] [CrossRef]
- Schmitt-Hoffmann, A.; Roos, B.; Spickermann, J.; Heep, M.; Peterfaí, E.; Edwards, D.J.; Stoeckel, K. Effect of Mild and Moderate Liver Disease on the Pharmacokinetics of Isavuconazole after Intravenous and Oral Administration of a Single Dose of the Prodrug BAL8557. Antimicrob. Agents Chemother. 2009, 53, 4885–4890. [Google Scholar] [CrossRef]
- Desai, A.; Schmitt-Hoffmann, A.-H.; Mujais, S.; Townsend, R. Population Pharmacokinetics of Isavuconazole in Subjects with Mild or Moderate Hepatic Impairment. Antimicrob. Agents Chemother. 2016, 60, 3025–3031. [Google Scholar] [CrossRef]
- Bolcato, L.; Thiebaut-Bertrand, A.; Stanke-Labesque, F.; Gautier-Veyret, E. Variability of Isavuconazole Trough Concentrations during Longitudinal Therapeutic Drug Monitoring. J. Clin. Med. 2022, 11, 5756. [Google Scholar] [CrossRef]
- Desai, A.V.; Kovanda, L.L.; Hope, W.W.; Andes, D.; Mouton, J.W.; Kowalski, D.L.; Townsend, R.W.; Mujais, S.; Bonate, P.L. Exposure-Response Relationships for Isavuconazole in Patients with Invasive Aspergillosis and Other Filamentous Fungi. Antimicrob. Agents Chemother. 2017, 61, 10-1128. [Google Scholar] [CrossRef]
- Bertram, R.; Naumann, H.; Bartsch, V.; Hitzl, W.; Kinzig, M.; Haarmeyer, G.; Baumgärtel, M.; Geise, A.; Muschner, D.; Nentwich, J.; et al. Clinical and Demographic Factors Affecting Trough Levels of Isavuconazole in Critically Ill Patients with or without COVID-19. Mycoses 2023, 66, 1071–1078. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, B.; Zheng, Y.; Huang, H.; Wei, Z.; Chen, S.; Huang, W.; Liu, M.; Zhang, Y.; Wu, X. Optimization of Oral Isavuconazole Dose for Population in Special Physiological or Pathological State: A Physiologically Based Pharmacokinetics Model-Informed Precision Dosing. J. Antimicrob. Chemother. 2024, 79, 2379–2389. [Google Scholar] [CrossRef]
- Hatzl, S.; Kriegl, L.; Posch, F.; Schilcher, G.; Eller, P.; Reisinger, A.; Grinschgl, Y.; Muhr, T.; Meinitzer, A.; Hoenigl, M.; et al. Early Attainment of Isavuconazole Target Concentration Using an Increased Loading Dose in Critically Ill Patients with Extracorporeal Membrane Oxygenation. J. Antimicrob. Chemother. 2023, 78, 2902–2908. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.M.E.; Mertens, B.; Spriet, I.; Verweij, P.E.; Schouten, J.; Wauters, J.; Debaveye, Y.; ter Heine, R.; Brüggemann, R.J.M. Population Pharmacokinetics of Total and Unbound Isavuconazole in Critically Ill Patients: Implications for Adaptive Dosing Strategies. Clin. Pharmacokinet. 2023, 62, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Lexicomp. Lexi-Interact Online; Wolters Kluwer Health: Hudson, OH, USA, 1978; Available online: https://www.lexicomp.com (accessed on 2 June 2025).
- Bergmann, F.; Wölfl-Duchek, M.; Jorda, A.; Al Jalali, V.; Leutzendorff, A.; Sanz-Codina, M.; Gompelmann, D.; Trimmel, K.; Weber, M.; Eberl, S.; et al. Pharmacokinetics of Isavuconazole at Different Target Sites in Healthy Volunteers after Single and Multiple Intravenous Infusions. J. Antimicrob. Chemother. 2024, 79, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
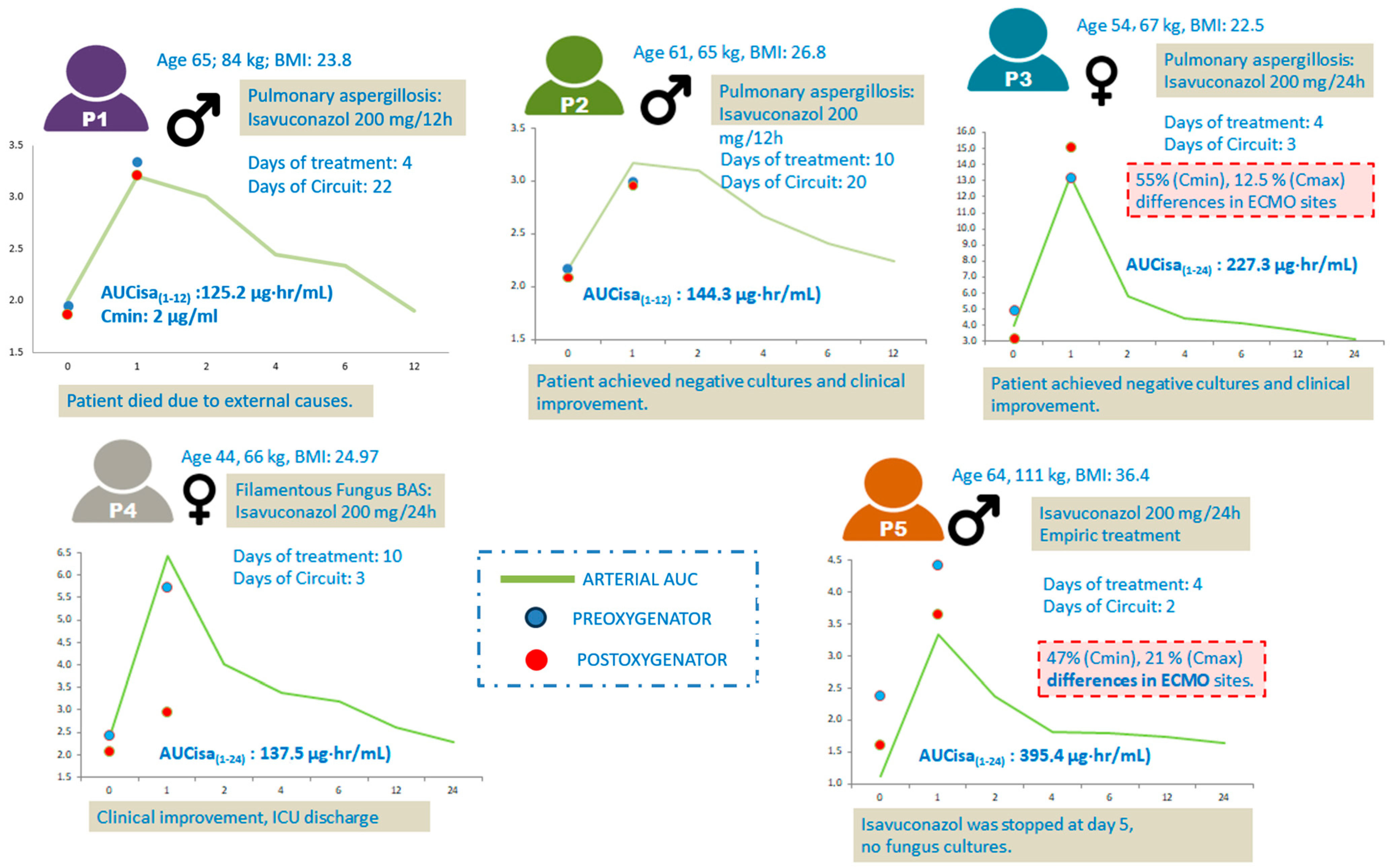
| Patient ID | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Sex | Male | Male | Female | Female | Male |
| Age (years) | 65 | 61 | 54 | 44 | 64 |
| Weight (kg) | 84 | 65 | 67 | 60 | 111 |
| BMI (kg/m2) | 26.8 | 23.9 | 22.6 | 25.0 | 36.4 |
| SOFA score | 12 | 12 | 12 | 11 | 12 |
| Outcomes | Died | Died | |||
| Comorbidities | |||||
| Renal Failure (diálisis) | no | no | no | no | no |
| Isavuconazole indication | CAPA | CAPA | CAPA | CAPA | Empirical treatment for suspected fungal superinfection |
| Laboratory parameters (on AUC day) | |||||
| Serum Creatinine (mg/dL) | 0.92 | 0.70 | 0.74 | 1.21 | 0.46 |
| Bilirubin (mg/dL) | 1.42 | 0.70 | 5.62 | 0.84 | 0.60 |
| ALT (U/L) | 116 | 38 | 41 | 27 | 42 |
| AST (U/L) | 129 | 31 | 141 | 114 | 37 |
| Serum Albumin (g/dL) | 2.4 | 4.1 | 2.4 | 3.6 | 2.8 |
| ECMO characteristics | |||||
| Indication | ARDS | ARDS | ARDS | ARDS | ARDS |
| ECMO modality | V-V ECMO | V-V ECMO | V-V ECMO | V-V ECMO | VV |
| ECMO duration (days) | 24 | 42 | 20 | 15 | 27 |
| Circuit change (day) | 22 | 20 | 3 | 3 | 2 |
| Pharmacokinetics | |||||
| ECMO circuit age at PK sampling (days) | 22 | 20 | 3 | 3 | 2 |
| Isavuconazole IV dosage | 200 mg/BID | 200 mg/BID | 200 mg/QD | 200 mg/QD | 200 mg/QD |
| AUC (µg·h/mL) 0–12 | 125.2 | 144.3 | - | - | - |
| AUC (µg·h/mL) 0–24 | - | - | 227.3 | 137.5 | 395.4 |
| Cmin (mcg/mL) | 2.00 | 2.16 | 3.96 | 2.32 | 1.13 |
| T1/2 (h) | 33.01 | 34.66 | 31.51 | 21.66 | 138.63 |
| Kel | 0.021 | 0.020 | 0.022 | 0.032 | 0.05 |
| Vd (L/kg) | 623 | 630 | 150 | 311 | 598 |
| Cl (L/h) | 1.60 | 1.39 | 0.88 | 1.45 | 0.51 |
| Patient | C0 Pre-ox (mg/L) | C0 Post-ox (mg/L) | C0 Arterial (mg/L) | ΔC0 (%) | C1 Pre-ox (mg/L) | C1 Post-ox (mg/L) | C1 Arterial (mg/L) | ΔC1 (%) |
|---|---|---|---|---|---|---|---|---|
| P1 | 1.95 | 1.86 | 2.00 | −5.0 | 3.34 | 3.21 | 3.20 | −4.05 |
| P2 | 2.17 | 2.09 | 2.16 | −4.0 | 2.99 | 2.96 | 3.17 | −1.01 |
| P3 | 4.94 | 3.18 | 3.96 | −55.0 | 13.2 | 15.1 | 13.3 | 12.58 |
| P4 | 2.43 | 2.34 | 2.32 | −4.0 | 5.74 | 6.11 | 6.42 | 6.06 |
| P5 | 2.38 | 1.62 | 1.13 | −47.0 | 4.43 | 3.65 | 3.34 | −21.37 |
| PK Parameter | Present Cases (ECMO) Median (IQR) 200 mg q/12 h | Present Cases (ECMO) Median (IQR) 200 mg q/24 h | Invasive Fungal Disease [17] (Non-ECMO) Mean ± SD | Prescribing Info: [18] Mean ± SD |
|---|---|---|---|---|
| AUC (μg·h/mL) | 227.3 (182.4–311.4) | 125.2 (81–185.8) | 87.1 ± 41 | 73.2 ± 12.4 |
| Cmin (μg/mL) | 2.0 (1.7–3.1) | 2.4 (1.8–3.2) | – | – |
| t1/2 (h) | 33 (22.7–33.1) | 31.5 (26.6–85.1) | 80.4 ± 33 | – |
| kel (1/h) | 0.021 (0.021–0.023) | 0.022 (0.014–0.027) | – | – |
| Vd (L) | 761 (727–832) | 454 (427–733) | 361.2 ± 166.3 | 304 ± 86.6 |
| CL (L/h) | 1.6 (1.5–3.4) | 0.9 (0.7–1.2) | 2.5 ± 1.6 | 2.8 ± 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doménech-Moral, L.; García-García, S.; Pau-Parra, A.; Sosa, M.; Puertas Sanjuan, A.; Bonilla, C.; Gallart, E.; Castellote, L.; Faixó, P.; Guevara, J.; et al. Pharmacokinetics of Isavuconazole During Extracorporeal Membrane Oxygenation Support in Critically Ill Patients: A Case Series. Antibiotics 2025, 14, 600. https://doi.org/10.3390/antibiotics14060600
Doménech-Moral L, García-García S, Pau-Parra A, Sosa M, Puertas Sanjuan A, Bonilla C, Gallart E, Castellote L, Faixó P, Guevara J, et al. Pharmacokinetics of Isavuconazole During Extracorporeal Membrane Oxygenation Support in Critically Ill Patients: A Case Series. Antibiotics. 2025; 14(6):600. https://doi.org/10.3390/antibiotics14060600
Chicago/Turabian StyleDoménech-Moral, Laura, Sonia García-García, Alba Pau-Parra, Manuel Sosa, Adrian Puertas Sanjuan, Camilo Bonilla, Elisabeth Gallart, Laura Castellote, Patricia Faixó, Jessica Guevara, and et al. 2025. "Pharmacokinetics of Isavuconazole During Extracorporeal Membrane Oxygenation Support in Critically Ill Patients: A Case Series" Antibiotics 14, no. 6: 600. https://doi.org/10.3390/antibiotics14060600
APA StyleDoménech-Moral, L., García-García, S., Pau-Parra, A., Sosa, M., Puertas Sanjuan, A., Bonilla, C., Gallart, E., Castellote, L., Faixó, P., Guevara, J., Vilanova, A., Martínez-Pla, M., Conto, A., Nuvials, X., Lalueza, P., Ferrer, R., Gorgas, M. Q., & Riera, J. (2025). Pharmacokinetics of Isavuconazole During Extracorporeal Membrane Oxygenation Support in Critically Ill Patients: A Case Series. Antibiotics, 14(6), 600. https://doi.org/10.3390/antibiotics14060600






