Efflux-Mediated Resistance in Enterobacteriaceae: Recent Advances and Ongoing Challenges to Inhibit Bacterial Efflux Pumps
Abstract
1. Introduction
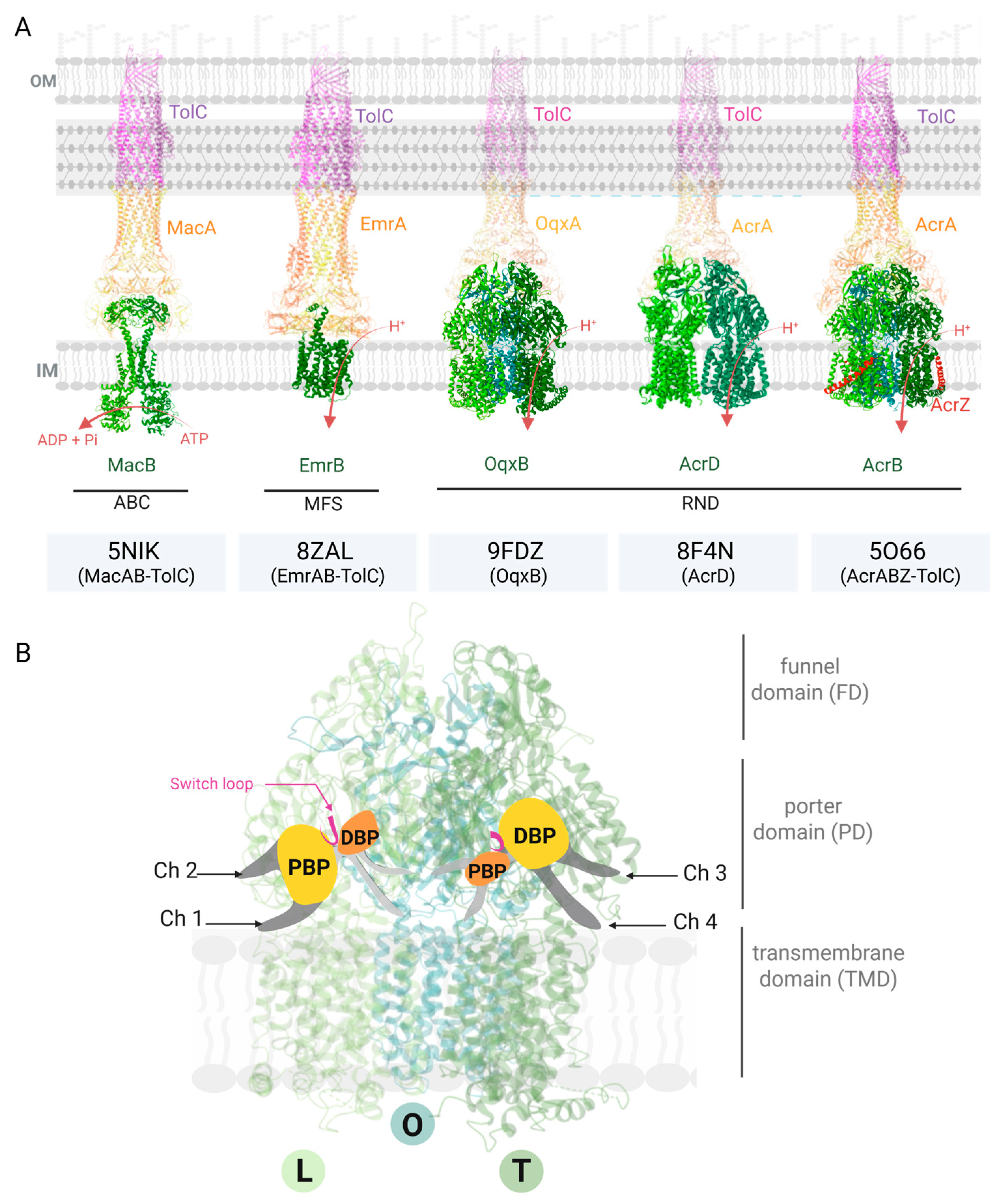
2. Efflux Pump Structure, Function, and Involvement in Drug Resistance
2.1. Structure and Function of Efflux Pumps
2.2. Involvement in Antibiotic Resistance
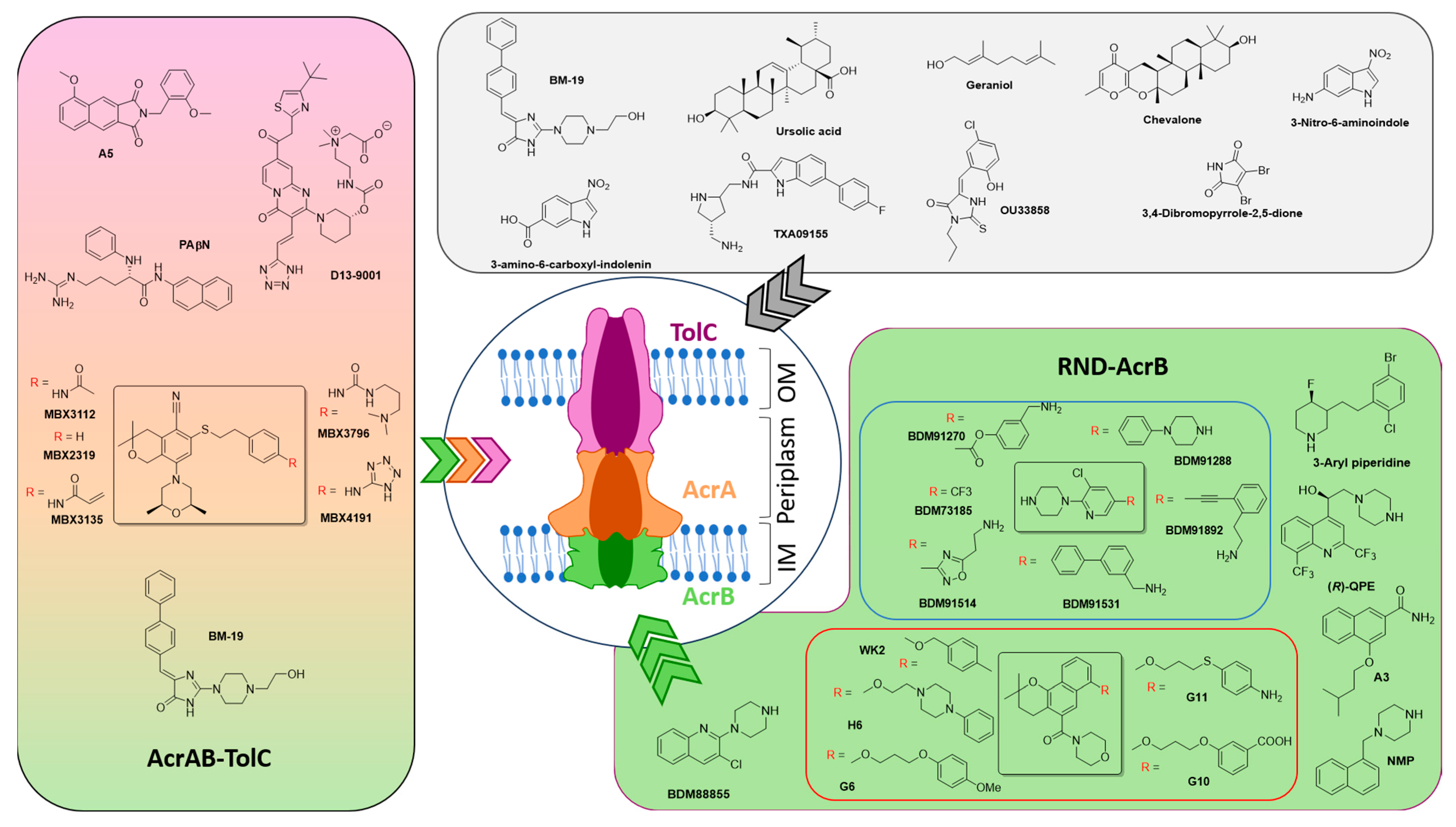
2.3. Possible Targets of EPIs
- -
- By inhibiting the expression of efflux components (such as AcrA, AcrB, and TolC) or interfering with regulatory pathways [42];
- -
- -
- By impairing the energy supply required for active transport (e.g., though PMF dissipation, [36]);
- -
- By physically blocking efflux activity through occlusion of the AcrB pockets or TolC channels (e.g., though steric hindrance in the DBP of AcrB [44]);
- -
- -
- By competing for substrate-binding sites [46];
- -
- By saturating the efflux capacity through excess substrate [4].
3. Possible Adjuvants’ Structure and Origin
4. Perspectives
4.1. Efflux Pump Complexity
4.2. Limitations of Current Methodologies
4.3. Effective Concentration for a Selected Blocker: Assessing Activity with MIC
4.4. Concerns Related to Tissue Penetration and Pharmacokinetics of EPIs
5. Challenges for Clinical Applications of EPIs
- Particular attention should be given to: (i) the interaction between the antibiotic and the efflux pump (recognition, binding, and transport), (ii) the interaction between the adjuvant and the efflux transporter (involving the same mechanisms), and (iii) the concentrations required to restore antimicrobial efficacy against resistant bacterial strains.
- To achieve a meaningful therapeutic effect at a safe dosage (i.e., below toxicity thresholds), the adjuvant must exhibit a higher affinity for the efflux pump components than the antibiotic itself. This relationship can be further explored through modeling and dynamic simulations that incorporate intracellular accumulation data.
- In all cases, it is essential to determine the effective intracellular concentration of the adjuvant necessary to inhibit efflux system activity—analogous to an enzyme inhibitor constant. The molar ratio between the adjuvant and the antibiotic is also critical, considering the potential for off-target effects on cellular organelles such as mitochondria, or on other transport systems involved in vital cellular processes.
- Finally, the chemical stability of the adjuvant at the site of infection must be considered to ensure it maintains its efflux-inhibitory activity at the recommended therapeutic concentration.
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Macesic, N.; Uhlemann, A.-C.; Peleg, A.Y. Multidrug-Resistant Gram-Negative Bacterial Infections. Lancet 2025, 405, 257–272. [Google Scholar] [CrossRef]
- Theuretzbacher, U.; Jumde, R.P.; Hennessy, A.; Cohn, J.; Piddock, L.J.V. Global Health Perspectives on Antibacterial Drug Discovery and the Preclinical Pipeline. Nat. Rev. Microbiol. 2025, 23, 474–490. [Google Scholar] [CrossRef]
- Vergalli, J.; Réfrégiers, M.; Ruggerone, P.; Winterhalter, M.; Pagès, J.-M. Advances in Methods and Concepts Provide New Insight into Antibiotic Fluxes across the Bacterial Membrane. Commun. Biol. 2024, 7, 1508. [Google Scholar] [CrossRef]
- Vergalli, J.; Bodrenko, I.V.; Masi, M.; Moynié, L.; Acosta-Gutiérrez, S.; Naismith, J.H.; Davin-Regli, A.; Ceccarelli, M.; van den Berg, B.; Winterhalter, M.; et al. Porins and Small-Molecule Translocation across the Outer Membrane of Gram-negative bacteria. Nat. Rev. Microbiol. 2020, 18, 164–176. [Google Scholar] [CrossRef]
- Manrique, P.D.; López, C.A.; Gnanakaran, S.; Rybenkov, V.V.; Zgurskaya, H.I. New Understanding of Multidrug Efflux and Permeation in Antibiotic Resistance, Persistence, and Heteroresistance. Ann. N. Y. Acad. Sci. 2023, 1519, 46–62. [Google Scholar] [CrossRef]
- Darby, E.M.; Trampari, E.; Siasat, P.; Gaya, M.S.; Alav, I.; Webber, M.A.; Blair, J.M.A. Molecular Mechanisms of Antibiotic Resistance Revisited. Nat. Rev. Microbiol. 2023, 21, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Piddock, L.J.V.; Alimi, Y.; Anderson, J.; de Felice, D.; Moore, C.E.; Røttingen, J.-A.; Skinner, H.; Beyer, P. Advancing Global Antibiotic Research, Development and Access. Nat. Med. 2024, 30, 2432–2443. [Google Scholar] [CrossRef] [PubMed]
- Henderson, P.J.F.; Maher, C.; Elbourne, L.D.H.; Eijkelkamp, B.A.; Paulsen, I.T.; Hassan, K.A. Physiological Functions of Bacterial “Multidrug” Efflux Pumps. Chem. Rev. 2021, 121, 5417–5478. [Google Scholar] [CrossRef]
- Yamasaki, S.; Zwama, M.; Yoneda, T.; Hayashi-Nishino, M.; Nishino, K. Drug Resistance and Physiological Roles of RND Multidrug Efflux Pumps in Salmonella Enterica, Escherichia Coli and Pseudomonas Aeruginosa. Microbiology 2023, 169, 001322. [Google Scholar] [CrossRef]
- Du, D.; Wang-Kan, X.; Neuberger, A.; van Veen, H.W.; Pos, K.M.; Piddock, L.J.V.; Luisi, B.F. Multidrug Efflux Pumps: Structure, Function and Regulation. Nat. Rev. Microbiol. 2018, 16, 523–539. [Google Scholar] [CrossRef]
- Davin-Regli, A.; Pages, J.-M.; Ferrand, A. Clinical Status of Efflux Resistance Mechanisms in Gram-Negative Bacteria. Antibiotics 2021, 10, 1117. [Google Scholar] [CrossRef]
- Fitzpatrick, A.W.P.; Llabrés, S.; Neuberger, A.; Blaza, J.N.; Bai, X.; Okada, U.; Murakami, S.; van Veen, H.W.; Zachariae, U.; Scheres, S.H.W.; et al. Structure of the MacAB-TolC ABC-Type Tripartite Multidrug Efflux Pump. Nat. Microbiol. 2017, 2, 17070. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Lewis, K.; Matin, A. EmrR Is a Negative Regulator of the Escherichia coli Multidrug Resistance Pump EmrAB. J. Bacteriol. 1995, 177, 2328–2334. [Google Scholar] [CrossRef] [PubMed]
- Yousefian, N.; Ornik-Cha, A.; Poussard, S.; Decossas, M.; Berbon, M.; Daury, L.; Taveau, J.-C.; Dupuy, J.-W.; Đorđević-Marquardt, S.; Lambert, O.; et al. Structural Characterization of the EmrAB-TolC Efflux Complex from E. coli. Biochim. Biophys. Acta-Biomembr. 2021, 1863, 183488. [Google Scholar] [CrossRef]
- Zhang, Z.; Morgan, C.E.; Cui, M.; Yu, E.W. Cryo-EM Structures of AcrD Illuminate a Mechanism for Capturing Aminoglycosides from Its Central Cavity. mBio 2023, 14, e03383-22. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Ning, J.; Sajid, A.; Cheng, G.; Yuan, Z.; Hao, H. The Nature and Epidemiology of OqxAB, a Multidrug Efflux Pump. Antimicrob. Resist. Infect. Control 2019, 8, 44. [Google Scholar] [CrossRef]
- Bharatham, N.; Bhowmik, P.; Aoki, M.; Okada, U.; Sharma, S.; Yamashita, E.; Shanbhag, A.P.; Rajagopal, S.; Thomas, T.; Sarma, M.; et al. Structure and Function Relationship of OqxB Efflux Pump from Klebsiella pneumoniae. Nat. Commun. 2021, 12, 5400. [Google Scholar] [CrossRef] [PubMed]
- Zwama, M.; Yamaguchi, A. Molecular Mechanisms of AcrB-Mediated Multidrug Export. Res. Microbiol. 2018, 169, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.-Q.; Kenzaki, H.; Murakami, S.; Takada, S. Drug Export and Allosteric Coupling in a Multidrug Transporter Revealed by Molecular Simulations. Nat. Commun. 2010, 1, 117. [Google Scholar] [CrossRef] [PubMed]
- Sennhauser, G.; Amstutz, P.; Briand, C.; Storchenegger, O.; Grütter, M.G. Drug Export Pathway of Multidrug Exporter AcrB Revealed by DARPin Inhibitors. PLoS Biol. 2006, 5, e7. [Google Scholar] [CrossRef]
- Kobylka, J.; Kuth, M.S.; Müller, R.T.; Geertsma, E.R.; Pos, K.M. AcrB: A Mean, Keen, Drug Efflux Machine. Ann. N. Y. Acad. Sci. 2020, 1459, 38–68. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Nakashima, R.; Sakurai, K. Structural Basis of RND-Type Multidrug Exporters. Front. Microbiol. 2015, 6, 327. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, R.; Sakurai, K.; Yamasaki, S.; Nishino, K.; Yamaguchi, A. Structures of the Multidrug Exporter AcrB Reveal a Proximal Multisite Drug-Binding Pocket. Nature 2011, 480, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Tam, H.-K.; Foong, W.E.; Oswald, C.; Herrmann, A.; Zeng, H.; Pos, K.M. Allosteric Drug Transport Mechanism of Multidrug Transporter AcrB. Nat. Commun. 2021, 12, 3889. [Google Scholar] [CrossRef]
- Zwama, M.; Yamasaki, S.; Nakashima, R.; Sakurai, K.; Nishino, K.; Yamaguchi, A. Multiple Entry Pathways within the Efflux Transporter AcrB Contribute to Multidrug Recognition. Nat. Commun. 2018, 9, 124. [Google Scholar] [CrossRef]
- Oswald, C.; Tam, H.-K.; Pos, K.M. Transport of Lipophilic Carboxylates is Mediated by Transmembrane Helix 2 in Multidrug Transporter AcrB. Nat. Commun. 2016, 7, 13819. [Google Scholar] [CrossRef]
- Tam, H.-K.; Malviya, V.N.; Foong, W.-E.; Hermann, A.; Malloci, G.; Ruggerone, P.; Vargiu, A.V.; Pos, K.M. Binding and Transport of Carboxylated Drugs by the Multidrug Transporter AcrB. J. Mol. Biol. 2020, 432, 861–877. [Google Scholar] [CrossRef] [PubMed]
- Seeger, M.A.; Schiefner, A.; Eicher, T.; Verrey, F.; Diederichs, K.; Pos, K.M. Structural Asymmetry of AcrB Trimer Suggests a Peristaltic Pump Mechanism. Science 2006, 313, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, J.; Pos, K.M. Molecular Insights into the Determinants of Substrate Specificity and Efflux Inhibition of the RND Efflux Pumps AcrB and AdeB. Microbiology 2024, 170, 001438. [Google Scholar] [CrossRef]
- Nasrollahian, S.; Graham, J.P.; Halaji, M. A Review of the Mechanisms that Confer Antibiotic Resistance in Pathotypes of E. coli. Front. Cell. Infect. Microbiol. 2024, 14, 1387497. [Google Scholar] [CrossRef] [PubMed]
- Dulanto Chiang, A.; Dekker, J.P. Efflux Pump-Mediated Resistance to New Beta Lactam Antibiotics in Multidrug-Resistant Gram-Negative Bacteria. Commun. Med. 2024, 4, 170. [Google Scholar] [CrossRef] [PubMed]
- Maurya, N.; Jangra, M.; Tambat, R.; Nandanwar, H. Alliance of Efflux Pumps with β-Lactamases in Multidrug-Resistant Klebsiella pneumoniae Isolates. Microb. Drug Resist. 2019, 25, 1155–1163. [Google Scholar] [CrossRef]
- Ebbensgaard, A.E.; Løbner-Olesen, A.; Frimodt-Møller, J. The Role of Efflux Pumps in the Transition from Low-Level to Clinical Antibiotic Resistance. Antibiotics 2020, 9, 855. [Google Scholar] [CrossRef]
- Langevin, A.M.; El Meouche, I.; Dunlop, M.J. Mapping the Role of AcrAB-TolC Efflux Pumps in the Evolution of Antibiotic Resistance Reveals Near-MIC Treatments Facilitate Resistance Acquisition. mSphere 2020, 5, e01056-20. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Bhattacharyya, M.; Pfannenstiel, D.M.; Nandi, A.K.; Hwang, Y.; Ho, K.; Harshey, R.M. Efflux-Linked Accelerated Evolution of Antibiotic Resistance at a Population Edge. Mol. Cell 2022, 82, 4368–4385.e6. [Google Scholar] [CrossRef]
- Dong, N.; Zeng, Y.; Wang, Y.; Liu, C.; Lu, J.; Cai, C.; Liu, X.; Chen, Y.; Wu, Y.; Fang, Y.; et al. Distribution and Spread of the Mobilised RND Efflux Pump Gene Cluster tmexCD-toprJ in Clinical Gram-Negative Bacteria: A Molecular Epidemiological Study. Lancet Microbe 2022, 3, e846–e856. [Google Scholar] [CrossRef]
- Liu, C.; Guo, J.; Lu, M.; Shen, N.; Du, P. Dissemination of the Mobilised RND Efflux Pump Gene Cluster tmexCD-toprJ among Klebsiella pneumoniae. Lancet Microbe 2023, 4, e135. [Google Scholar] [CrossRef]
- Gao, H.; Wang, B.; Li, M.; Zhou, P.; Wu, C.; Wan, C.; Shen, L.; Fu, J.; Han, W.; Zhou, Y.; et al. Emergence and Dissemination of Multidrug-Resistant Klebsiella pneumoniae Harboring the Novel tmexCD-toprJ RND Efflux Pump Operon. Front. Cell Infect. Microbiol. 2025, 15, 1579880. [Google Scholar] [CrossRef] [PubMed]
- Plé, C.; Tam, H.-K.; Vieira Da Cruz, A.; Compagne, N.; Jiménez-Castellanos, J.-C.; Müller, R.T.; Pradel, E.; Foong, W.E.; Malloci, G.; Ballée, A.; et al. Pyridylpiperazine-Based Allosteric Inhibitors of RND-Type Multidrug Efflux Pumps. Nat. Commun. 2022, 13, 115. [Google Scholar] [CrossRef]
- Poole, K. Efflux-Mediated Multiresistance in Gram-Negative Bacteria. Clin. Microbiol. Infect. 2004, 10, 12–26. [Google Scholar] [CrossRef]
- Ayhan, D.H.; Tamer, Y.T.; Akbar, M.; Bailey, S.M.; Wong, M.; Daly, S.M.; Greenberg, D.E.; Toprak, E. Sequence-Specific Targeting of Bacterial Resistance Genes Increases Antibiotic Efficacy. PLoS Biol. 2016, 14, e1002552. [Google Scholar] [CrossRef]
- Tikhonova, E.B.; Yamada, Y.; Zgurskaya, H.I. Sequential Mechanism of Assembly of Multidrug Efflux Pump AcrAB-TolC. Chem. Biol. 2011, 18, 454–463. [Google Scholar] [CrossRef]
- Sjuts, H.; Vargiu, A.V.; Kwasny, S.M.; Nguyen, S.T.; Kim, H.-S.; Ding, X.; Ornik, A.R.; Ruggerone, P.; Bowlin, T.L.; Nikaido, H.; et al. Molecular Basis for Inhibition of AcrB Multidrug Efflux Pump by Novel and Powerful Pyranopyridine Derivatives. Proc. Natl. Acad. Sci. USA 2016, 113, 3509–3514. [Google Scholar] [CrossRef]
- Nakashima, R.; Sakurai, K.; Yamasaki, S.; Hayashi, K.; Nagata, C.; Hoshino, K.; Onodera, Y.; Nishino, K.; Yamaguchi, A. Structural Basis for the Inhibition of Bacterial Multidrug Exporters. Nature 2013, 500, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, S.; Altinkanat-Gelmez, G.; Bolelli, K.; Guneser-Merdan, D.; Ufuk Over-Hasdemir, M.; Aki-Yalcin, E.; Yalcin, I. Binding Site Feature Description of 2-Substituted Benzothiazoles as Potential AcrAB-TolC Efflux Pump Inhibitors in E. coli. SAR QSAR Environ. Res. 2015, 26, 853–871. [Google Scholar] [CrossRef]
- Reading, E.; Ahdash, Z.; Fais, C.; Ricci, V.; Wang-Kan, X.; Grimsey, E.; Stone, J.; Malloci, G.; Lau, A.M.; Findlay, H.; et al. Perturbed Structural Dynamics Underlie Inhibition and Altered Efflux of the Multidrug Resistance Pump AcrB. Nat. Commun. 2020, 11, 5565. [Google Scholar] [CrossRef] [PubMed]
- Duffey, M.; Jumde, R.P.; da Costa, R.M.A.; Ropponen, H.-K.; Blasco, B.; Piddock, L.J.V. Extending the Potency and Lifespan of Antibiotics: Inhibitors of Gram-Negative Bacterial Efflux Pumps. ACS Infect. Dis. 2024, 10, 1458–1482. [Google Scholar] [CrossRef]
- Renau, T.E.; Léger, R.; Flamme, E.M.; Sangalang, J.; She, M.W.; Yen, R.; Gannon, C.L.; Griffith, D.; Chamberland, S.; Lomovskaya, O.; et al. Inhibitors of Efflux Pumps in Pseudomonas aeruginosa Potentiate the Activity of the Fluoroquinolone Antibacterial Levofloxacin. J. Med. Chem. 1999, 42, 4928–4931. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Bostian, K.A. Practical Applications and Feasibility of Efflux Pump Inhibitors in the Clinic—A Vision for Applied Use. Biochem. Pharmacol. 2006, 71, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Vargiu, A.V.; Ruggerone, P.; Opperman, T.J.; Nguyen, S.T.; Nikaido, H. Molecular Mechanism of MBX2319 Inhibition of Escherichia coli AcrB Multidrug Efflux Pump and Comparison with Other Inhibitors. Antimicrob. Agents Chemother. 2014, 58, 6224–6234. [Google Scholar] [CrossRef]
- Chen, M.; Shi, X.; Yu, Z.; Fan, G.; Serysheva, I.I.; Baker, M.L.; Luisi, B.F.; Ludtke, S.J.; Wang, Z. In Situ Structure of the AcrAB-TolC Efflux Pump at Subnanometer Resolution. Structure 2022, 30, 107–113.e3. [Google Scholar] [CrossRef] [PubMed]
- Aron, Z.; Opperman, T.J. Optimization of a Novel Series of Pyranopyridine RND Efflux Pump Inhibitors. Curr. Opin. Microbiol. 2016, 33, 1–6. [Google Scholar] [CrossRef]
- Guo, T.; Chen, Y.; Chen, W.; Semple, S.J.; Gu, X.; Polyak, S.W.; Sun, G.; Venter, H.; Ma, S. Design and Synthesis of Benzochromene Derivatives as AcrB Inhibitors for the Reversal of Bacterial Multidrug Resistance. Eur. J. Med. Chem. 2023, 249, 115148. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Weng, J.; Wang, W. Insights into the Inhibitory Mechanism of D13-9001 to the Multidrug Transporter AcrB through Molecular Dynamics Simulations. J. Phys. Chem. B 2016, 120, 2145–2154. [Google Scholar] [CrossRef]
- Bohnert, J.A.; Kern, W.V. Selected Arylpiperazines Are Capable of Reversing Multidrug Resistance in Escherichia coli Overexpressing RND Efflux Pumps. Antimicrob. Agents Chemother. 2005, 49, 849–852. [Google Scholar] [CrossRef]
- Kern, W.V.; Steinke, P.; Schumacher, A.; Schuster, S.; Baum, H.V.; Bohnert, J.A. Effect of 1-(1-Naphthylmethyl)-Piperazine, a Novel Putative Efflux Pump Inhibitor, on Antimicrobial Drug Susceptibility in Clinical Isolates of Escherichia coli. J. Antimicrob. Chemother. 2006, 57, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, A.; Steinke, P.; Bohnert, J.A.; Akova, M.; Jonas, D.; Kern, W.V. Effect of 1-(1-Naphthylmethyl)-Piperazine, a Novel Putative Efflux Pump Inhibitor, on Antimicrobial Drug Susceptibility in Clinical Isolates of Enterobacteriaceae other than Escherichia coli. J. Antimicrob. Chemother. 2006, 57, 344–348. [Google Scholar] [CrossRef]
- Choquet, M.; Lohou, E.; Pair, E.; Sonnet, P.; Mullié, C. Efflux Pump Overexpression Profiling in Acinetobacter baumannii and Study of New 1-(1-Naphthylmethyl)-Piperazine Analogs as Potential Efflux Inhibitors. Antimicrob. Agents Chemother. 2021, 65, e0071021. [Google Scholar] [CrossRef]
- Bohnert, J.A.; Schuster, S.; Kern, W.V.; Karcz, T.; Olejarz, A.; Kaczor, A.; Handzlik, J.; Kieć-Kononowicz, K. Novel Piperazine Arylideneimidazolones Inhibit the AcrAB-TolC Pump in Escherichia coli and Simultaneously Act as Fluorescent Membrane Probes in a Combined Real-Time Influx and Efflux Assay. Antimicrob. Agents Chemother. 2016, 60, 1974–1983. [Google Scholar] [CrossRef]
- Zhang, Y.; Rosado-Lugo, J.D.; Datta, P.; Sun, Y.; Cao, Y.; Banerjee, A.; Yuan, Y.; Parhi, A.K. Evaluation of a Conformationally Constrained Indole Carboxamide as a Potential Efflux Pump Inhibitor in Pseudomonas aeruginosa. Antibiotics 2022, 11, 716. [Google Scholar] [CrossRef]
- Wang, Y.; Alenazy, R.; Gu, X.; Polyak, S.W.; Zhang, P.; Sykes, M.J.; Zhang, N.; Venter, H.; Ma, S. Design and Structural Optimization of Novel 2H-Benzo[h]Chromene Derivatives that Target AcrB and Reverse Bacterial Multidrug Resistance. Eur. J. Med. Chem. 2021, 213, 113049. [Google Scholar] [CrossRef]
- Yamagishi, A.; Nakano, S.; Yamasaki, S.; Nishino, K. An Efflux Inhibitor of the MacAB Pump in Salmonella enterica serovar Typhimurium. Microbiol. Immunol. 2020, 64, 182–188. [Google Scholar] [CrossRef]
- Duffy, E.M.; Buurman, E.T.; Chiang, S.L.; Cohen, N.R.; Uria-Nickelsen, M.; Alm, R.A. The CARB-X Portfolio of Nontraditional Antibacterial Products. ACS Infect. Dis. 2021, 7, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Compagne, N.; Vieira Da Cruz, A.; Müller, R.T.; Hartkoorn, R.C.; Flipo, M.; Pos, K.M. Update on the Discovery of Efflux Pump Inhibitors against Critical Priority Gram-Negative Bacteria. Antibiotics 2023, 12, 180. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.; Vavra, M.; Wirth, D.A.N.; Kern, W.V. Comparative Reassessment of AcrB Efflux Inhibitors Reveals Differential Impact of Specific Pump Mutations on the Activity of Potent Compounds. Microbiol. Spectr. 2024, 12, e03045-23. [Google Scholar] [CrossRef]
- Compagne, N.; Jiménez-Castellanos, J.-C.; Meurillon, V.; Pradel, E.; Vieira Da Cruz, A.; Piveteau, C.; Biela, A.; Eveque, M.; Leroux, F.; Deprez, B.; et al. Optimization of Pyridylpiperazine-Based Inhibitors of the Escherichia coli AcrAB-TolC Efflux Pump. Eur. J. Med. Chem. 2023, 259, 115630. [Google Scholar] [CrossRef]
- Jiménez-Castellanos, J.-C.; Pradel, E.; Compagne, N.; Vieira Da Cruz, A.; Flipo, M.; Hartkoorn, R.C. Characterization of Pyridylpiperazine-Based Efflux Pump Inhibitors for Acinetobacter baumannii. JAC-Antimicrob. Resist. 2023, 5, dlad112. [Google Scholar] [CrossRef] [PubMed]
- Vieira Da Cruz, A.; Jiménez-Castellanos, J.-C.; Börnsen, C.; Van Maele, L.; Compagne, N.; Pradel, E.; Müller, R.T.; Meurillon, V.; Soulard, D.; Piveteau, C.; et al. Pyridylpiperazine Efflux Pump inhibitor Boosts in Vivo Antibiotic Efficacy against K. pneumoniae. EMBO Mol. Med. 2024, 16, 93–111. [Google Scholar] [CrossRef]
- Marshall, R.L.; Lloyd, G.S.; Lawler, A.J.; Element, S.J.; Kaur, J.; Ciusa, M.L.; Ricci, V.; Tschumi, A.; Kühne, H.; Alderwick, L.J.; et al. New Multidrug Efflux Inhibitors for Gram-Negative Bacteria. mBio 2020, 11, e01340-20. [Google Scholar] [CrossRef]
- Le Goff, F.; Hazemann, J.; Christen, L.; Bourquin, G.; Pierlot, G.; Lange, R.; Panchaud, P.; Zumbrunn, C.; Peter, O.; Rueedi, G.; et al. Measurement and Prediction of Small Molecule Retention by Gram-Negative Bacteria Based on a Large-Scale LC/MS Screen. Sci. Rep. 2025, 15, 25431. [Google Scholar] [CrossRef] [PubMed]
- Zgurskaya, H.I.; Malloci, G.; Chandar, B.; Vargiu, A.; Ruggerone, P. Bacterial Efflux Transporters’ Polyspecificity—A Gift and a Curse? Curr. Opin. Microbiol. 2021, 61, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Vergalli, J.; Chauvet, H.; Oliva, F.; Pajović, J.; Malloci, G.; Vargiu, A.V.; Réfrégiers, M.; Ruggerone, P.; Pagès, J.-M. A Framework for Dissecting Affinities of Multidrug Efflux Transporter AcrB to Fluoroquinolones. Commun. Biol. 2022, 5, 1062. [Google Scholar] [CrossRef]
- Ramaswamy, V.K.; Vargiu, A.V.; Malloci, G.; Dreier, J.; Ruggerone, P. Molecular Rationale behind the Differential Substrate Specificity of Bacterial RND Multi-Drug Transporters. Sci. Rep. 2017, 7, 8075. [Google Scholar] [CrossRef]
- Zgurskaya, H.I.; Rybenkov, V.V. Permeability Barriers of Gram-Negative Pathogens. Ann. N. Y. Acad. Sci. 2020, 1459, 5–18. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Cooper, C.J.; Uddin, M.R.; Walker, J.K.; Parks, J.M.; Zgurskaya, H.I. Analysis of Orthogonal Efflux and Permeation Properties of Compounds Leads to the Discovery of New Efflux Pump Inhibitors. ACS Infect. Dis. 2022, 8, 2149–2160. [Google Scholar] [CrossRef]
- Hernando-Amado, S.; Gomis-Font, M.A.; Valverde, J.R.; Oliver, A.; Martínez, J.L. Ceftazidime-Avibactam Use Selects Multidrug-Resistance and Prevents Designing Collateral Sensitivity-Based Therapies against Pseudomonas aeruginosa. Nat. Commun. 2025, 16, 3323. [Google Scholar] [CrossRef] [PubMed]

| Challenge | Description/Comment | Impact on Blocking Bacterial Efflux |
|---|---|---|
| Efflux pump variability (selectivity for the pumps expressed) | Differences in substrate specificity, structure, and expression levels across species and even homologous pumps. Requires quantification of intra-bacterial concentration of antibiotic [4]. A case-by-case approach is required. | Limits generalizability of inhibitors across efflux pump orthologs. |
| Substrate diversity and binding complexity (selectivity for a molecule) | Efflux pumps recognize and expel a broad range of structurally diverse compounds using multiple entry and binding sites. Requires dose-effect of kinetics of EPI action on antibiotic concentration inside bacterial cells [4,71]. | Predicting substrate–EPI interaction is difficult. |
| Experimental inconsistency | Protocols, bacterial strains, and antibiotic/EPI combinations vary between studies. Lack of internal standard/control allowing a real comparison between antibiotics, EPIs, bacterial strains, culture conditions, etc. | Hinders reproducibility and comparison of data. |
| MIC variation and limited readouts | MIC shifts may not reflect the real intracellular drug accumulation or efflux inhibition. Requires accumulation assays, rate-killing assays, and dose effects [4]. | Misestimation of the efficacy of EPI. |
| Dose necessary to control efflux | Difficult to define the concentration needed to impact efflux activity across different pump/antibiotic combinations. Requires bacterial population assay and individual cell assay with a case-by-case approach [4,71]. | Influences the timing and drug efficacy. |
| Diffusion and availability on the internal target | Intracellular accumulation and efflux inhibition may not align with PK-PD. Requires multidisciplinary approaches and the integration of in vitro, in vivo, and in silico results [4]. Requires quantification of EPI and antibiotic inside bacterial cell and analyses of other mechanisms of resistance [4]. | Reduces predictive value of in vitro results. |
| Stability in patient’s body and in infectious site | Different tissues present unique chemical and physiological conditions and drug EPIs may undergo degradation or inactivation in specific biological environments [4]. | Decreases the available dose of EPI in the infectious site. |
| Potential toxicity | EPIs may interact with human transporters. | Complicates clinical use due to side effects. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rouvier, F.; Brunel, J.-M.; Pagès, J.-M.; Vergalli, J. Efflux-Mediated Resistance in Enterobacteriaceae: Recent Advances and Ongoing Challenges to Inhibit Bacterial Efflux Pumps. Antibiotics 2025, 14, 778. https://doi.org/10.3390/antibiotics14080778
Rouvier F, Brunel J-M, Pagès J-M, Vergalli J. Efflux-Mediated Resistance in Enterobacteriaceae: Recent Advances and Ongoing Challenges to Inhibit Bacterial Efflux Pumps. Antibiotics. 2025; 14(8):778. https://doi.org/10.3390/antibiotics14080778
Chicago/Turabian StyleRouvier, Florent, Jean-Michel Brunel, Jean-Marie Pagès, and Julia Vergalli. 2025. "Efflux-Mediated Resistance in Enterobacteriaceae: Recent Advances and Ongoing Challenges to Inhibit Bacterial Efflux Pumps" Antibiotics 14, no. 8: 778. https://doi.org/10.3390/antibiotics14080778
APA StyleRouvier, F., Brunel, J.-M., Pagès, J.-M., & Vergalli, J. (2025). Efflux-Mediated Resistance in Enterobacteriaceae: Recent Advances and Ongoing Challenges to Inhibit Bacterial Efflux Pumps. Antibiotics, 14(8), 778. https://doi.org/10.3390/antibiotics14080778








