Ceftazidime-Avibactam for the Treatment of Carbapenem-Resistant Organisms: A Prospective, Observational, Single-Center Study
Abstract
1. Introduction
2. Results
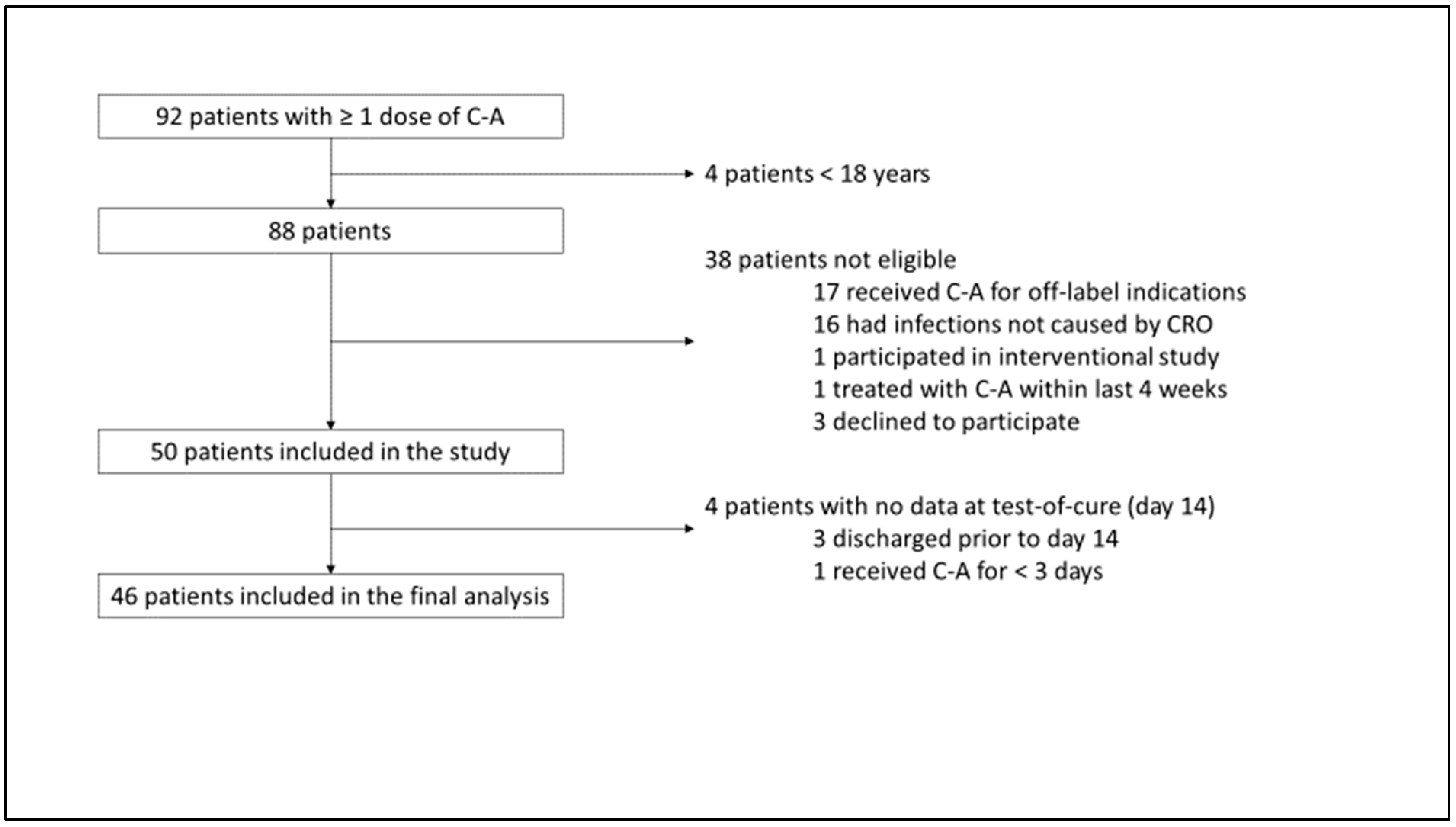
2.1. Inclusion
2.2. Baseline Characteristics
2.3. Infection
2.4. Primary Outcome
2.5. Secondary Outcomes
3. Discussion
4. Methods
4.1. Outcomes
4.2. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Ten Threats to Global Health in 2019. 2019. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 13 September 2021).
- World Health Organization. WHO Bacterial Priority Pathogens List, 2024. 2024. Available online: https://iris.who.int/bitstream/handle/10665/376776/9789240093461-eng.pdf?sequence=1 (accessed on 4 October 2024).
- Shields, R.K.; Nguyen, M.H.; Chen, L.; Press, E.G.; Potoski, B.A.; Marini, R.V.; Doi, Y.; Kreiswirth, B.N.; Clancy, C.J. Ceftazidime-Avibactam Is Superior to Other Treatment Regimens against Carbapenem-Resistant Klebsiella pneumoniae Bacteremia. Antimicrob. Agents Chemother. 2017, 61, e00883-17. [Google Scholar] [CrossRef] [PubMed]
- Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/zavicefta-epar-product-information_en.pdf (accessed on 10 September 2021).
- Sternbach, N.; Weissman, Y.L.; Avni, T.; Yahav, D. Efficacy and safety of ceftazidime/avibactam: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2018, 73, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Potoski, B.A.; Haidar, G.; Hao, B.; Doi, Y.; Chen, L.; Press, E.G.; Kreiswirth, B.N.; Clancy, C.J.; Nguyen, M.H. Clinical Outcomes, Drug Toxicity, and Emergence of Ceftazidime-Avibactam Resistance Among Patients Treated for Carbapenem-Resistant Enterobacteriaceae Infections. Clin. Infect. Dis. 2016, 63, 1615–1618. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, D.R.; Marelli, C.; Cattardico, G.; Fanelli, C.; Signori, A.; Di Meco, G.; Di Pilato, V.; Mikulska, M.; Mazzitelli, M.; Cattelan, A.M.; et al. Mortality in KPC-producing Klebsiella pneumoniae bloodstream infections: A changing landscape. J. Antimicrob. Chemother. 2023, 78, 2505–2514. [Google Scholar] [CrossRef] [PubMed]
- van Duin, D.; Lok, J.J.; Earley, M.; Cober, E.; Richter, S.S.; Perez, F.; Salata, R.A.; Kalayjian, R.C.; Watkins, R.R.; Doi, Y.; et al. Colistin Versus Ceftazidime-Avibactam in the Treatment of Infections Due to Carbapenem-Resistant Enterobacteriaceae. Clin. Infect. Dis. 2018, 66, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Horcajada, J.P.; Kamat, S.; Irani, P.M.; Tawadrous, M.; Welte, T. Ceftazidime-Avibactam in the Treatment of Patients with Bacteremia or Nosocomial Pneumonia: A Systematic Review and Meta-analysis. Infect. Dis. Ther. 2024, 13, 1639–1664. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Daikos, G.L.; Durante-Mangoni, E.; Yahav, D.; Carmeli, Y.; Benattar, Y.D.; Skiada, A.; Andini, R.; Eliakim-Raz, N.; Nutman, A.; et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: An open-label, randomised controlled trial. Lancet Infect. Dis. 2018, 18, 391–400. [Google Scholar] [CrossRef]
- Ackley, R.; Roshdy, D.; Meredith, J.; Minor, S.; Anderson, W.E.; Capraro, G.A.; Polk, C. Meropenem-Vaborbactam versus Ceftazidime-Avibactam for Treatment of Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob. Agents Chemother. 2020, 64, e02313-19. [Google Scholar] [CrossRef]
- Karaiskos, I.; Daikos, G.L.; Gkoufa, A.; Adamis, G.; Stefos, A.; Symbardi, S.; Chrysos, G.; Filiou, E.; Basoulis, D.; Mouloudi, E.; et al. Ceftazidime/Avibactam Registry Study, Ceftazidime/avibactam in the era of carbapenemase-producing Klebsiella pneumoniae: Experience from a national registry study. J. Antimicrob. Chemother. 2021, 76, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, H.; Alghamdi, A.; Alobaidallah, N.; Alfayez, A.; Almousa, R.; Albagli, R.; Shamas, N.; Farahat, F.; Mahmoud, E.; Bosaeed, M.; et al. Evaluation of ceftazidime/avibactam for treatment of carbapenemase-producing carbapenem-resistant Enterobacterales with OXA-48 and/or NDM genes with or without combination therapy. JAC Antimicrob. Resist. 2022, 4, dlac104. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Heil, E.L.; Justo, J.A.; Mathers, A.J.; Satlin, M.J.; Bonomo, R.A. Infectious Diseases Society of America 2024 Guidance on the Treatment of Antimicrobial-Resistant Gram-Negative Infections. Clin. Infect. Dis. 2024. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Nguyen, M.H.; Chen, L.; Press, E.G.; Kreiswirth, B.N.; Clancy, C.J. Pneumonia and Renal Replacement Therapy Are Risk Factors for Ceftazidime-Avibactam Treatment Failures and Resistance among Patients with Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob. Agents Chemother. 2018, 62, e02497-17. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Abbo, L.M.; Ackley, R.; Aitken, S.L.; Albrecht, B.; Babiker, A.; Burgoon, R.; Cifuentes, R.; Claeys, K.C.; Curry, B.N.; et al. Effectiveness of ceftazidime-avibactam versus ceftolozane-tazobactam for multidrug-resistant Pseudomonas aeruginosa infections in the USA (CACTUS): A multicentre, retrospective, observational study. Lancet Infect. Dis. 2025, 25, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Pfennigwerth, N.; Cremanns, M.; Eisfeld, J.; Hans, J.; Anders, A.; Gatermann, S.G. Bericht des Nationalen Referenzzentrums für gramnegative Krankenhauserreger. Epid Bull. 2023, 27, 3–10. [Google Scholar]
- Sandfort, M.; Hans, J.B.; Fischer, M.A.; Reichert, F.; Cremanns, M.; Eisfeld, J.; Pfeifer, Y.; Heck, A.; Eckmanns, T.; Werner, G.; et al. Increase in NDM-1 and NDM-1/OXA-48-producing Klebsiella pneumoniae in Germany associated with the war in Ukraine, 2022. Euro. Surveill. 2022, 27, 2200926. [Google Scholar] [CrossRef] [PubMed]
- Emblaveo (Aztreonam/Avibactam). 2024. Available online: https://www.ema.europa.eu/en/documents/overview/emblaveo-epar-medicine-overview_en.pdf (accessed on 30 December 2024).
- GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- Vasikasin, V.; Rawson, T.M.; Holmes, A.H.; Otter, J. Can precision antibiotic prescribing help prevent the spread of carbapenem-resistant organisms in the hospital setting? JAC Antimicrob. Resist. 2023, 5, dlad036. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Vinau, T.; Penalva, G.; Garcia-Martinez, L.; Caston, J.J.; Munoz-Rosa, M.; Cano, A.; Recio, M.; Cisneros, J.M.; Perez-Nadales, E.; Aguirre, J.R.; et al. Impact of an Antimicrobial Stewardship Program on the Incidence of Carbapenem Resistant Gram-Negative Bacilli: An Interrupted Time-Series Analysis. Antibiotics 2021, 10, 586. [Google Scholar] [CrossRef] [PubMed]
| Demographics and Background | No of Patients (%) If Not Specified Otherwise | |
|---|---|---|
| Patients analyzed | 50 | |
| Female | 15 (30) | |
| Age [y], median (IQR) | 58 (51–71) | |
| Charlson Comorbidity Index, median (IQR) | 5.5 (3–8) | |
| Type of ward at day 1 | ||
| General medical ward | 9 (18) | |
| General surgical ward | 2 (4) | |
| Medical ICU | 29 (57) | |
| Surgical ICU | 10 (20) | |
| Main comorbidities | ||
| Cardiac | 32 (64) | |
| Chronic pulmonary disease | 31 (62) | |
| Malignancy | 14 (28) | |
| Chronic kidney disease | 16 (32) | |
| Diabetes | 13 (26) | |
| Chronic liver disease | 7 (14) | |
| Solid organ transplantation | 5 (10) | |
| HIV | 2 (4) | |
| Immunosuppression | ||
| Prednisolone equivalent ≥ 20 mg/d for ≥14 d within last mth | 8 (16) | |
| Other immunosuppressants for ≥1 mth within last 6 mths | 9 (18) | |
| Chemotherapy within last 6 mths | 4 (8) | |
| Neutropenia within 7 d prior to study inclusion | 1 (2) | |
| Invasive ventilation within 7 d prior to study inclusion | 38 (76) | |
| Surgery within last mth prior to study inclusion | 26 (52) | |
| Status at day 1 | Status at day 14 | |
| Septic shock | 3 (6) | - |
| Invasive ventilation | 32 (64) | 24 (52) |
| Non-invasive ventilation | 9 (18) | 10 (22) |
| ECMO | 2 (4) | 1 (3) |
| Intermittent hemodialysis | 4 (8) | 4 (8) |
| Continuous veno-venous hemodiafiltration | 12 (24) | 7 (15) |
| SOFA score, median (IQR) | 7 (4–11) | 5 (2–8) |
| Systolic blood pressure [mmHg], median (IQR) | 115 (110–122) | 115 (110–120) |
| Oxygenation index, median (IQR) | 286 (205–393) | 344 (288–424) |
| Creatinine [mg/dL], median (IQR) | 1.0 (0.7–1.7) | 1.0 (0.6–1.7) |
| Total bilirubine [mg/dL], median (IQR) | 0.8 (0.4–2.0) | 0.5 (0.3–1.2) |
| ALT [U/L], median (IQR) | 37 (22–67) | 31 (14–58) |
| Leukocytes [/nL], median (IQR) | 14 (9–18) | 10 (8–14) |
| CrP [mg/L], median (IQR) | 155 (107–188) | 90 (35–148) |
| PCT [µg/L], median (IQR) | 1.0 (0.4–2.4) | 0.8 (0.3–1.7) |
| No of Patients (%) If Not Specified Otherwise | No of Isolates with Carbapenemases | |
|---|---|---|
| Type of infection * | ||
| Hospital acquired pneumonia | 25 (50) | |
| Abdominal infection | 14 (28) | |
| Blood stream infection | 8 (16) | |
| Skin and soft tissue infection | 3 (6) | |
| Complicated urinary tract infection | 1 (2) | |
| Bone infection | 1 (2) | |
| Type of specimen # | ||
| Respiratory secretion | 24 (48) | |
| Intraoperative sample | 15 (30) | |
| Blood culture | 11 (22) | |
| Urin | 1 (2) | |
| Other | 1 (2) | |
| Carbapenem-resistant pathogens | Carbapenemases | |
| P. aeruginosa | 24 (48) | n.a. |
| K. pneumoniae | 14 (28) | OXA-48 (10), KPC (3) |
| K. aerogenes | 4 (8) | KPC (1) |
| E. coli | 3 (6) | OXA-48 (3) |
| E. cloacae complex | 3 (6) | OXA-48 (1) |
| S. maltophilia | 1 (2) | n.a. |
| C. freundii | 1 (2) | KPC (1) |
| S. marcescens | 1 (2) | None |
| Other pathogens in polymicrobial infection | Candida spp. 14 E. faecium 11 Coagulase-negative staphylococci 10 P. aeruginosa 4 S. maltophilia 4 K. pneumoniae 3 E. coli 3 Proteus spp. 3 K. aerogenes 2 E. cloacae 2 E. faecalis 1 R. planticola 1 C. koseri 1 S. marcescens 1 E. durans 1 S. aureus 1 P. distasonis 1 A. flavus 1 Fusarium sp. 1 | |
| Treatment | ||
| Treatment duration with C-A [d], median (IQR) | 13 (10–18) | |
| Combination antibiotic therapy | 23 (45) | |
| Intravenous partner drugs | Colistin 4 Ciprofloxacin 4 Gentamicin 4 Tigecycline 1 Aztreonam 1 Fosfomycin 1 Cotrimoxazole 1 | |
| Nebulized partner drugs | Colistin 10 Tobramycin 3 Gentamycin 2 | |
| Multidrug therapy | 11 (22) | |
| Antibacterial drugs in multidrug regimens | Linezolid 12 Vancomycin 10 Metronidazole 8 Meropenem 7 Cotrimoxazole 6 Ampicillin-sulbactam 4 Ampicillin 4 Daptomycin 3 Imipenem 2 Ciprofloxacin 2 Tigecycline 2 Rifampicin 1 Teicoplanin 1 Fosfomycin 1 | |
| Antifungal drugs in multidrug regimens | Caspofungin 15 Liposomal amphotericin B 3 Anidulafungin 2 Voriconazole 2 Fluconazole 1 |
| No of Patients (%) If Not Specified Otherwise | |
|---|---|
| Primary outcome (day 14) | |
| Clinical cure | 27 (59) |
| Reasons for clinical failure | Death 3 (7) Vasopressors 2 (4) CROs after treatment ≥ 7 d 2 (4) Oxygenation index not improved 2 (4) SOFA not improved 10 (22) |
| Secondary outcomes (day 30) | |
| All-cause mortality at day 30 | 4 (9) |
| Length of stay in ICU [d], median (IQR) | 14 (2–25) |
| Detection of CROs after treatment ≥ 7 d | 2 (4) |
| Resistance to C-A | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfäfflin, F.; Theloe, A.; Stegemann, M.S.; Leistner, R.; Sander, L.E.; Kurth, F.; Achterberg, S. Ceftazidime-Avibactam for the Treatment of Carbapenem-Resistant Organisms: A Prospective, Observational, Single-Center Study. Antibiotics 2025, 14, 773. https://doi.org/10.3390/antibiotics14080773
Pfäfflin F, Theloe A, Stegemann MS, Leistner R, Sander LE, Kurth F, Achterberg S. Ceftazidime-Avibactam for the Treatment of Carbapenem-Resistant Organisms: A Prospective, Observational, Single-Center Study. Antibiotics. 2025; 14(8):773. https://doi.org/10.3390/antibiotics14080773
Chicago/Turabian StylePfäfflin, Frieder, Anja Theloe, Miriam Songa Stegemann, Rasmus Leistner, Leif Erik Sander, Florian Kurth, and Stephan Achterberg. 2025. "Ceftazidime-Avibactam for the Treatment of Carbapenem-Resistant Organisms: A Prospective, Observational, Single-Center Study" Antibiotics 14, no. 8: 773. https://doi.org/10.3390/antibiotics14080773
APA StylePfäfflin, F., Theloe, A., Stegemann, M. S., Leistner, R., Sander, L. E., Kurth, F., & Achterberg, S. (2025). Ceftazidime-Avibactam for the Treatment of Carbapenem-Resistant Organisms: A Prospective, Observational, Single-Center Study. Antibiotics, 14(8), 773. https://doi.org/10.3390/antibiotics14080773





