Methicillin-Resistant Staphylococcus aureus (MRSA): Resistance, Prevalence, and Coping Strategies
Abstract
1. Introduction
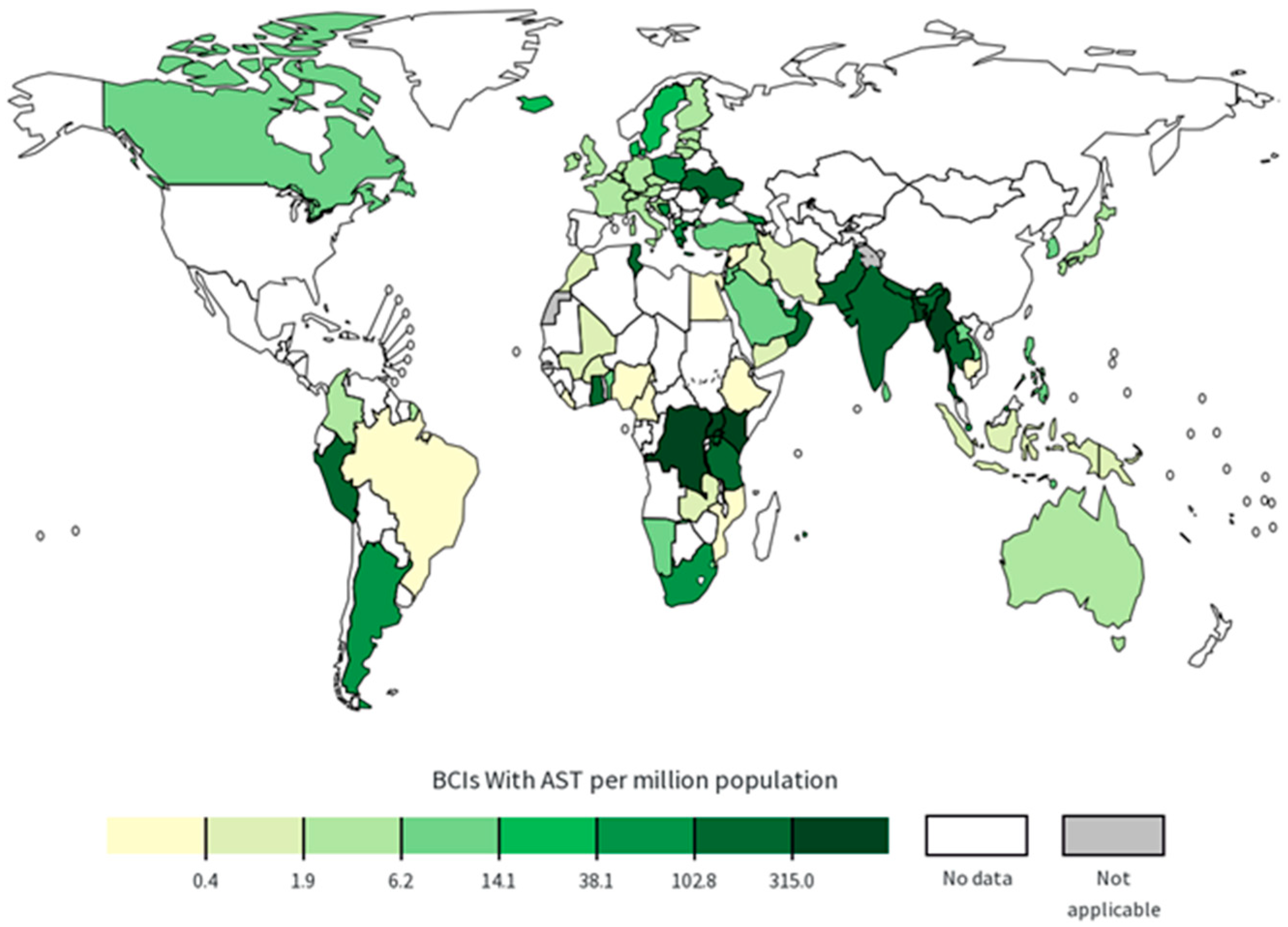
2. MRSA Types, Prevalence, and Spread
2.1. HA-MRSA
2.2. CA-MRSA
2.3. LA-MRSA
3. MRSA Resistance Mechanisms
3.1. Inherent Resistance Mechanisms
3.1.1. SCCmec Element and PBP2a Protein
3.1.2. β-Lactamase
3.1.3. Efflux Pumping System
3.1.4. Alterations in Cell Membrane Permeability
3.1.5. Alterations in Cell Wall Permeability
3.1.6. Auxiliary Gene Regulation
3.2. Acquired Resistance Mechanisms
3.2.1. Antibiotic Target Modification
3.2.2. Biofilm Formation and Persistent Infection
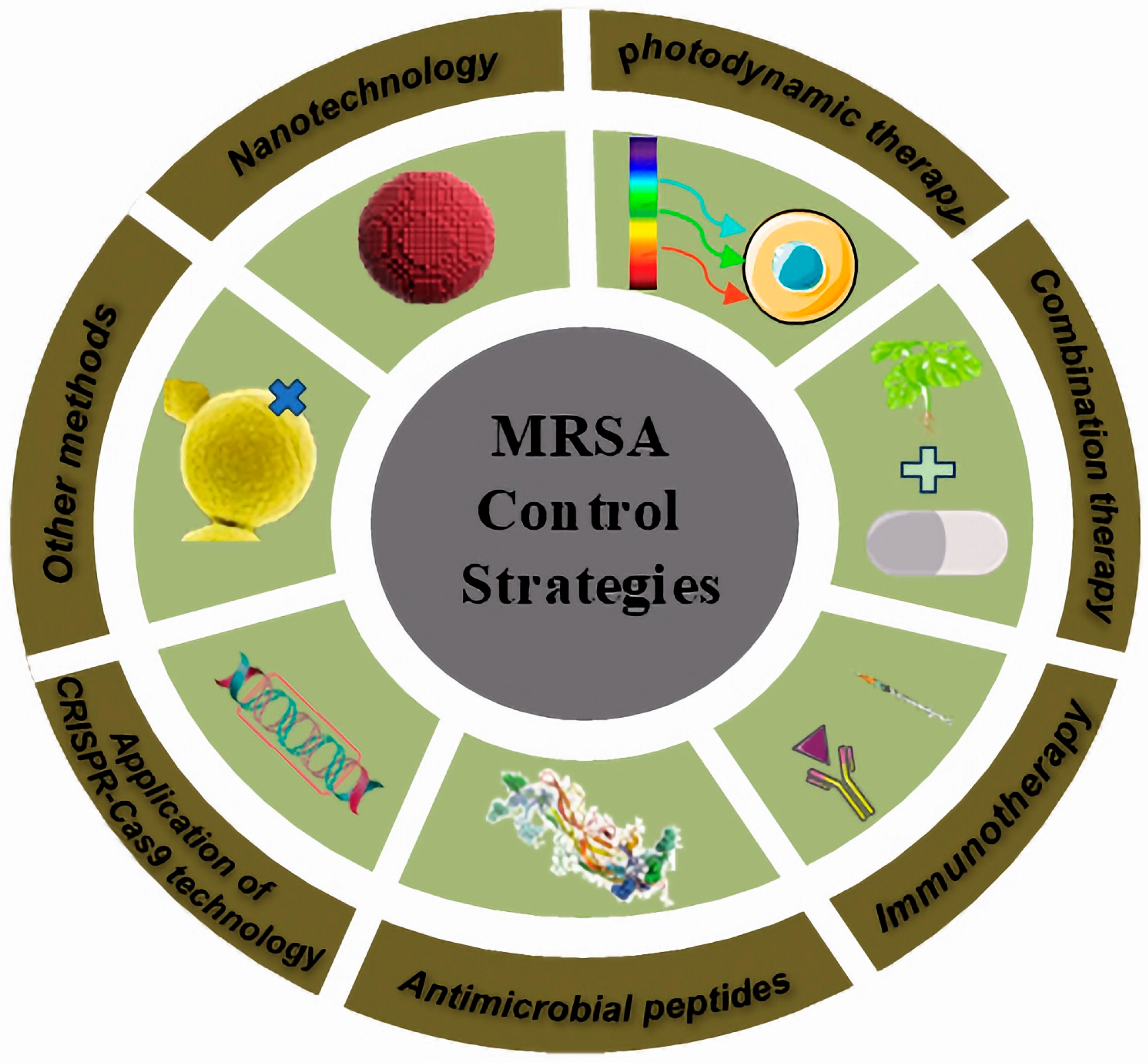
4. MRSA Control Strategies
4.1. Nanotechnology
4.2. Photodynamic Therapy
4.3. Combination Therapy
4.4. Immunotherapy
4.5. Antimicrobial Peptides
4.6. Application of CRISPR-Cas9 Technology
4.7. Other Methods
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larsen, J.; Raisen, C.L.; Ba, X.; Sadgrove, N.J.; Padilla-González, G.F.; Simmonds, M.S.J.; Loncaric, I.; Kerschner, H.; Apfalter, P.; Hartl, R.; et al. Emergence of methicillin resistance predates the clinical use of antibiotics. Nature 2022, 602, 135–141. [Google Scholar] [CrossRef]
- Jevons, M.P. “Celbenin”—Resistant Staphylococci. Br. Med. J. 1961, 1, 124–125. [Google Scholar] [CrossRef]
- Wang, L.; Haq, S.U.; Shoaib, M.; He, J.; Guo, W.; Wei, X.; Zheng, X. Subclinical Mastitis in Small-Holder Dairy Herds of Gansu Province, Northwest China: Prevalence, Bacterial Pathogens, Antimicrobial Susceptibility, and Risk Factor Analysis. Microorganisms 2024, 12, 2643. [Google Scholar] [CrossRef]
- Zhou, F.; Gu, X.; Wang, W.; Lin, M.; Wang, L. Advancements in MRSA treatment: The role of berberine in enhancing antibiotic therapy. BMC Microbiol. 2024, 24, 540. [Google Scholar] [CrossRef]
- Kourtis, A.P. Vital Signs: Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections—United States. MMWR Morb Mortal Wkly. Rep. 2019, 68, 214–219. [Google Scholar] [CrossRef]
- Fukunaga, B.T.; Sumida, W.K.; Taira, D.A.; Davis, J.W.; Seto, T.B. Hospital-Acquired Methicillin-resistant Staphylococcus aureus Bacteremia Related to Medicare Antibiotic Prescriptions: A State-Level Analysis. Hawaii J. Med. Public Health 2016, 75, 303–309. [Google Scholar]
- Lammie, S.L.; Hughes, J.M. Antimicrobial Resistance, Food Safety, and One Health: The Need for Convergence. Annu. Rev. Food Sci. Technol. 2016, 7, 287–312. [Google Scholar] [CrossRef]
- Spagnolo, A.M.; Orlando, P.; Panatto, D.; Amicizia, D.; Perdelli, F.; Cristina, M.L. Staphylococcus aureus with reduced susceptibility to vancomycin in healthcare settings. J. Prev. Med. Hyg. 2014, 55, 137–144. [Google Scholar]
- Burford-Gorst, C.M.; Kidd, S.P. Phenotypic Variation in Staphylococcus aureus during Colonisation Involves Antibiotic-Tolerant Cell Types. Antibiotics 2024, 13, 845. [Google Scholar] [CrossRef]
- Heidarian, S.; Guliaev, A.; Nicoloff, H.; Hjort, K.; Andersson, D.I. High prevalence of heteroresistance in Staphylococcus aureus is caused by a multitude of mutations in core genes. PLoS Biol. 2024, 22, e3002457. [Google Scholar] [CrossRef]
- Johnson, A.P.; Uttley, A.H.; Woodford, N.; George, R. Resistance to vancomycin and teicoplanin: An emerging clinical problem. Clin. Microbiol. Rev. 1990, 3, 280–291. [Google Scholar] [CrossRef]
- Lakhundi, S.; Zhang, K. Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef]
- Shoaib, M.; Aqib, A.I.; Ali, M.M.; Ijaz, M.; Sattar, H.; Ghaffar, A.; Sajid Hasni, M.; Bhutta, Z.A.; Ashfaq, K.; Kulyar, M.F.; et al. Tracking Infection and Genetic Divergence of Methicillin-Resistant Staphylococcus aureus at Pets, Pet Owners, and Environment Interface. Front. Vet. Sci. 2022, 9, 900480. [Google Scholar] [CrossRef]
- Stefani, S.; Chung, D.R.; Lindsay, J.A.; Friedrich, A.W.; Kearns, A.M.; Westh, H.; Mackenzie, F.M. Meticillin-resistant Staphylococcus aureus (MRSA): Global epidemiology and harmonisation of typing methods. Int. J. Antimicrob. Agents 2012, 39, 273–282. [Google Scholar] [CrossRef]
- Saïd-Salim, B.; Mathema, B.; Kreiswirth, B.N. Community-acquired methicillin-resistant Staphylococcus aureus: An emerging pathogen. Infect. Control Hosp. Epidemiol. 2003, 24, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Millar, B.C.; Loughrey, A.; Elborn, J.S.; Moore, J.E. Proposed definitions of community-associated meticillin-resistant Staphylococcus aureus (CA-MRSA). J. Hosp. Infect. 2007, 67, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Moreno, F.; Crisp, C.; Jorgensen, J.H.; Patterson, J.E. Methicillin-resistant Staphylococcus aureus as a community organism. Clin. Infect. Dis. 1995, 21, 1308–1312. [Google Scholar] [CrossRef]
- Cefai, C.; Ashurst, S.; Owens, C. Human carriage of methicillin-resistant Staphylococcus aureus linked with pet dog. Lancet 1994, 344, 539–540. [Google Scholar] [CrossRef]
- Saravolatz, L.D.; Markowitz, N.; Arking, L.; Pohlod, D.; Fisher, E. Methicillin-resistant Staphylococcus aureus. Epidemiologic observations during a community-acquired outbreak. Ann. Intern. Med. 1982, 96, 11–16. [Google Scholar] [CrossRef]
- Köck, R.; Becker, K.; Cookson, B.; van Gemert-Pijnen, J.E.; Harbarth, S.; Kluytmans, J.; Mielke, M.; Peters, G.; Skov, R.L.; Struelens, M.J.; et al. Methicillin-resistant Staphylococcus aureus (MRSA): Burden of disease and control challenges in Europe. Eurosurveillance 2010, 15, 19688. [Google Scholar] [CrossRef]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef]
- Liu, L.; Peng, H.; Zhang, N.; Li, M.; Chen, Z.; Shang, W.; Hu, Z.; Wang, Y.; Yang, Y.; Wang, D.; et al. Genomic Epidemiology and Phenotypic Characterization of Staphylococcus aureus from a Tertiary Hospital in Tianjin Municipality, Northern China. Microbiol. Spectr. 2023, 11, e0420922. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, X.; Wang, B.; Shen, L.; Rao, L.; Wang, X.; Zhang, J.; Xiao, Y.; Xu, Y.; Yu, J.; et al. Phenotypic and genomic analysis of the hypervirulent ST22 methicillin-resistant Staphylococcus aureus in China. mSystems 2023, 8, e0124222. [Google Scholar] [CrossRef]
- Gostev, V.; Ivanova, K.; Kruglov, A.; Kalinogorskaya, O.; Ryabchenko, I.; Zyryanov, S.; Kalisnikova, E.; Likholetova, D.; Lobzin, Y.; Sidorenko, S. Comparative genome analysis of global and Russian strains of community-acquired methicillin-resistant Staphylococcus aureus ST22, a “Gaza clone”. Int. J. Antimicrob. Agents 2021, 57, 106264. [Google Scholar] [CrossRef]
- Abrudan, M.I.; Shamanna, V.; Prasanna, A.; Underwood, A.; Argimón, S.; Nagaraj, G.; Di Gregorio, S.; Govindan, V.; Vasanth, A.; Dharmavaram, S.; et al. Novel multidrug-resistant sublineages of Staphylococcus aureus clonal complex 22 discovered in India. mSphere 2023, 8, e0018523. [Google Scholar] [CrossRef]
- Global Antimicrobial Resistance and Use Surveillance System (GLASS). Available online: https://www.who.int/initiatives/glass (accessed on 28 April 2025).
- Shoaib, M.; Aqib, A.I.; Muzammil, I.; Majeed, N.; Bhutta, Z.A.; Kulyar, M.F.E.A.; Fatima, M.; Zaheer, C.N.F.; Muneer, A.; Murtaza, M.; et al. MRSA compendium of epidemiology, transmission, pathophysiology, treatment, and prevention within one health framework. Front. Microbiol. 2022, 13, 1067284. [Google Scholar] [CrossRef]
- Liu, M.J.; Zhang, Y.L.; Wan, X.Y. Research progress on methicillin-resistant Staphylococcus aureus biofilm. Zhonghua Nei Ke Za Zhi 2020, 59, 473–476. [Google Scholar]
- Sisodia, K.; Anveshi, A.K.; Ranga, D.; Kumar, D.; Gaind, R. Phenotypic and genotypic characterization of MRSA isolated from health care workers in tertiary care hospital. Indian J. Med. Microbiol. 2025, 56, 100871. [Google Scholar] [CrossRef]
- Bravo-Queipo-de-Llano, B.; Jiménez, B.; García-Lorenzo, M.; Melendo, S.; Carrasco-Colom, J.; Olteanu-Olteanu, F.C.; Ramos Lacuey, B.; Calle-Miguel, L.; Harillo, L.; Falces-Romero, I.; et al. Community-acquired Methicillin-resistant Staphylococcus aureus Infection in Children: Key Features and Comparison to Colonization. Pediatr. Infect. Dis. J. 2025. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Otter, J.A.; French, G.L. Molecular epidemiology of community-associated meticillin-resistant Staphylococcus aureus in Europe. Lancet Infect. Dis. 2010, 10, 227–239. [Google Scholar] [CrossRef]
- Silva, V.; Monteiro, A.; Pereira, J.E.; Maltez, L.; Igrejas, G.; Poeta, P. MRSA in Humans, Pets and Livestock in Portugal: Where We Came from and Where We Are Going. Pathogens 2022, 11, 1110. [Google Scholar] [CrossRef]
- van Duijkeren, E.; Hengeveld, P.; Zomer, T.P.; Landman, F.; Bosch, T.; Haenen, A.; van de Giessen, A. Transmission of MRSA between humans and animals on duck and turkey farms. J. Antimicrob. Chemother. 2016, 71, 58–62. [Google Scholar] [CrossRef]
- Vittorakis, E.; Vica, M.L.; Zervaki, C.O.; Vittorakis, E.; Maraki, S.; Mavromanolaki, V.E.; Schürger, M.E.; Neculicioiu, V.S.; Papadomanolaki, E.; Junie, L.M. A Comparative Analysis of MRSA: Epidemiology and Antibiotic Resistance in Greece and Romania. Int. J. Mol. Sci. 2024, 25, 7535. [Google Scholar] [CrossRef]
- Strommenger, B.; Kehrenberg, C.; Kettlitz, C.; Cuny, C.; Verspohl, J.; Witte, W.; Schwarz, S. Molecular characterization of methicillin-resistant Staphylococcus aureus strains from pet animals and their relationship to human isolates. J. Antimicrob. Chemother. 2006, 57, 461–465. [Google Scholar] [CrossRef]
- Shoaib, M. Diversified Epidemiological Pattern and Antibiogram of mecA Gene in Staphylococcus aureus Isolates of Pets, Pet Owners and Environment. Pak. Vet. J. 2020, 40, 331–336. [Google Scholar]
- Haenni, M.; Châtre, P.; Dupieux-Chabert, C.; Métayer, V.; Bes, M.; Madec, J.Y.; Laurent, F. Molecular Epidemiology of Methicillin-Resistant Staphylococcus aureus in Horses, Cats, and Dogs over a 5-Year Period in France. Front. Microbiol. 2017, 8, 2493. [Google Scholar] [CrossRef]
- Drougka, E.; Foka, A.; Koutinas, C.K.; Jelastopulu, E.; Giormezis, N.; Farmaki, O.; Sarrou, S.; Anastassiou, E.D.; Petinaki, E.; Spiliopoulou, I. Interspecies spread of Staphylococcus aureus clones among companion animals and human close contacts in a veterinary teaching hospital. A cross-sectional study in Greece. Prev. Vet. Med. 2016, 126, 190–198. [Google Scholar] [CrossRef]
- Kinross, P.; Petersen, A.; Skov, R.; Van Hauwermeiren, E.; Pantosti, A.; Laurent, F.; Voss, A.; Kluytmans, J.; Struelens, M.J.; Heuer, O.; et al. Livestock-associated meticillin-resistant Staphylococcus aureus (MRSA) among human MRSA isolates, European Union/European Economic Area countries, 2013. Eurosurveillance 2017, 22, 16–00696. [Google Scholar] [CrossRef]
- Smith, T.C.; Male, M.J.; Harper, A.L.; Kroeger, J.S.; Tinkler, G.P.; Moritz, E.D.; Capuano, A.W.; Herwaldt, L.A.; Diekema, D.J. Methicillin-resistant Staphylococcus aureus (MRSA) strain ST398 is present in midwestern U.S. swine and swine workers. PLoS ONE 2009, 4, e4258. [Google Scholar] [CrossRef]
- Golding, G.R.; Bryden, L.; Levett, P.N.; McDonald, R.R.; Wong, A.; Wylie, J.; Graham, M.R.; Tyler, S.; Van Domselaar, G.; Simor, A.E.; et al. Livestock-associated Methicillin-Resistant Staphylococcus aureus Sequence Type 398 in Humans, Canada. Emerg. Infect. Dis. 2010, 16, 587–594. [Google Scholar] [CrossRef]
- Chen, C.J.; Lauderdale, T.L.Y.; Lu, C.T.; Chuang, Y.Y.; Yang, C.C.; Wu, T.S.; Lee, C.Y.; Lu, M.C.; Ko, W.C.; Huang, Y.C. Clinical and molecular features of MDR livestock-associated MRSA ST9 with staphylococcal cassette chromosome mecXII in humans. J. Antimicrob. Chemother. 2018, 73, 33–40. [Google Scholar] [CrossRef]
- Gopal, S.; Divya, K.C. Can methicillin-resistant Staphylococcus aureus prevalence from dairy cows in India act as potential risk for community-associated infections?: A review. Vet. World. 2017, 10, 311–318. [Google Scholar] [CrossRef][Green Version]
- Abebe, A.A.; Birhanu, A.G. Methicillin Resistant Staphylococcus aureus: Molecular Mechanisms Underlying Drug Resistance Development and Novel Strategies to Combat. Infect. Drug Resist. 2023, 16, 7641–7662. [Google Scholar] [CrossRef]
- Ambade, S.S.; Gupta, V.K.; Bhole, R.P.; Khedekar, P.B.; Chikhale, R.V. A Review on Five and Six-Membered Heterocyclic Compounds Targeting the Penicillin-Binding Protein 2 (PBP2A) of Methicillin-Resistant Staphylococcus aureus (MRSA). Molecules 2023, 28, 7008. [Google Scholar] [CrossRef]
- Hou, Z.; Liu, L.; Wei, J.; Xu, B. Progress in the Prevalence, Classification and Drug Resistance Mechanisms of Methicillin-Resistant Staphylococcus aureus. Infect. Drug Resist. 2023, 16, 3271–3292. [Google Scholar] [CrossRef]
- Rimi, S.S.; Ashraf, M.N.; Sigma, S.H.; Ahammed, M.T.; Siddique, M.P.; Zinnah, M.A.; Rahman, M.T.; Islam, M.S. Biofilm formation, agr typing and antibiotic resistance pattern in methicillin-resistant Staphylococcus aureus isolated from hospital environments. PLoS ONE 2024, 19, e0308282. [Google Scholar] [CrossRef]
- Uehara, Y. Current Status of Staphylococcal Cassette Chromosome mec (SCCmec). Antibiotics 2022, 11, 86. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Ono, D.; Sato, A. Staphylococcal Cassette Chromosome mec (SCCmec) Analysis of MRSA. Methods Mol. Biol. 2020, 2069, 59–78. [Google Scholar]
- Maree, M.; Thi Nguyen, L.T.; Ohniwa, R.L.; Higashide, M.; Msadek, T.; Morikawa, K. Natural transformation allows transfer of SCCmec-mediated methicillin resistance in Staphylococcus aureus biofilms. Nat. Commun. 2022, 13, 2477. [Google Scholar] [CrossRef]
- Monecke, S.; Boswihi, S.; Braun, S.D.; Diezel, C.; Müller, E.; Reinicke, M.; Udo, E.; Ehricht, R. Sequencing a CC239-MRSA-III with a novel composite SCC mec element from Kuwait. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 1761–1775. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. Epidemiology of β-Lactamase-Producing Pathogens. Clin. Microbiol. Rev. 2020, 33, e00047-19. [Google Scholar] [CrossRef]
- Fuda, C.C.S.; Fisher, J.F.; Mobashery, S. Beta-lactam resistance in Staphylococcus aureus: The adaptive resistance of a plastic genome. Cell. Mol. Life Sci. 2005, 62, 2617–2633. [Google Scholar] [CrossRef]
- Lade, H.; Kim, J.-S. Molecular Determinants of β-Lactam Resistance in Methicillin-Resistant Staphylococcus aureus (MRSA): An Updated Review. Antibiotics 2023, 12, 1362. [Google Scholar] [CrossRef]
- Dwivedi, G.R.; Tyagi, R.; Sanchita, null; Tripathi, S.; Pati, S.; Srivastava, S.K.; Darokar, M.P.; Sharma, A. Antibiotics potentiating potential of catharanthine against superbug Pseudomonas aeruginosa. J. Biomol. Struct. Dyn. 2018, 36, 4270–4284. [Google Scholar] [CrossRef]
- Silva, K.P.T.; Sundar, G.; Khare, A. Efflux pump gene amplifications bypass necessity of multiple target mutations for resistance against dual-targeting antibiotic. Nat. Commun. 2023, 14, 3402. [Google Scholar] [CrossRef]
- Foster, T.J. The remarkably multifunctional fibronectin binding proteins of Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1923–1931. [Google Scholar] [CrossRef]
- Noguchi, N.; Suwa, J.; Narui, K.; Sasatsu, M.; Ito, T.; Hiramatsu, K.; Song, J.H. Susceptibilities to antiseptic agents and distribution of antiseptic-resistance genes qacA/B and smr of methicillin-resistant Staphylococcus aureus isolated in Asia during 1998 and 1999. J. Med. Microbiol. 2005, 54 Pt 6, 557–565. [Google Scholar] [CrossRef]
- Mo, X.; Li, J.; Tang, Y.; Zhang, Y.; Zhu, J.; Tan, S.; Lu, J. The action of active efflux system on multi-drug resistance in methicillin resistant Staphylococcus aureus. Zhonghua Jie He He Hu Xi Za Zhi 2007, 30, 40–43. [Google Scholar]
- Li, M.; Jian, Q.; Ye, X.; Jing, M.; Wu, J.; Wu, Z.; Ruan, Y.; Long, X.; Zhang, R.; Ren, H.; et al. Mechanisms of mepA Overexpression and Membrane Potential Reduction Leading to Ciprofloxacin Heteroresistance in a Staphylococcus aureus Isolate. Int. J. Mol. Sci. 2025, 26, 2372. [Google Scholar] [CrossRef]
- Li, N.; Luo, M.; Fu, Y.; Zu, Y.; Wang, W.; Zhang, L.; Yao, L.; Zhao, C.; Sun, Y. Effect of corilagin on membrane permeability of Escherichia coli, Staphylococcus aureus and Candida albicans. Phytother. Res. 2013, 27, 1517–1523. [Google Scholar] [CrossRef]
- Mlynarczyk-Bonikowska, B.; Kowalewski, C.; Krolak-Ulinska, A.; Marusza, W. Molecular Mechanisms of Drug Resistance in Staphylococcus aureus. Int. J. Mol. Sci. 2022, 23, 8088. [Google Scholar] [CrossRef]
- Egan, A.J.F.; Cleverley, R.M.; Peters, K.; Lewis, R.J.; Vollmer, W. Regulation of bacterial cell wall growth. FEBS J. 2017, 284, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Romaniuk, J.A.H.; Cegelski, L. Bacterial cell wall composition and the influence of antibiotics by cell-wall and whole-cell NMR. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20150024. [Google Scholar] [CrossRef]
- Renzoni, A.; Kelley, W.L.; Vaudaux, P.; Cheung, A.L.; Lew, D.P. Exploring innate glycopeptide resistance mechanisms in Staphylococcus aureus. Trends Microbiol. 2010, 18, 55–56. [Google Scholar] [CrossRef][Green Version]
- Hawkey, P.M. Low-level glycopeptide resistance in methicillin-resistant Staphylococcus aureus and how to test it. Clin. Microbiol. Infect. 2009, 15, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Jama-Kmiecik, A.; Mączyńska, B.; Frej-Mądrzak, M.; Choroszy-Król, I.; Dudek-Wicher, R.; Piątek, D.; Kujawa, K.; Sarowska, J. The Changes in the Antibiotic Resistance of Staphylococcus aureus, Streptococcus pneumoniae, Enterococcus faecalis and Enterococcus faecium in the Clinical Isolates of a Multiprofile Hospital over 6 Years (2017–2022). J. Clin. Med. 2025, 14, 332. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, H.; Mo, Z.; Yu, M.; Liang, Z. Cell wall thickness and the molecular mechanism of heterogeneous vancomycin-intermediate Staphylococcus aureus. Lett. Appl. Microbiol. 2021, 72, 604–609. [Google Scholar] [CrossRef]
- Malachowa, N.; Sturdevant, D.E.; Porter, A.R.; Martin, G.; Martens, C.; Nair, V.; Hansen, B.; Ricklefs, S.; Jenkins, S.G.; Chen, L.; et al. Insights into the molecular basis of reduced vancomycin susceptibility among three prominent Staphylococcus aureus clonal complexes. Microbiol. Spectr. 2024, 12, e00486-24. [Google Scholar] [CrossRef]
- Fait, A.; Silva, S.F.; Abrahamsson, J.Å.H.; Ingmer, H. Staphylococcus Aureus Response and Adaptation to Vancomycin. In Advances in Microbial Physiology; Poole, R.K., Kelly, D.J., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 201–258. [Google Scholar]
- Akkiraju, A.G.; Atcha, K.R.; Sagurthi, S.R. Cloning, Purification, and Biophysical Characterization of FemB Protein from Methicillin-Resistant Staphylococcus aureus and Inhibitors Screening. Appl. Biochem. Biotechnol. 2024, 196, 4974–4992. [Google Scholar] [CrossRef]
- Brahma, U.; Sharma, P.; Murthy, S.; Sharma, S.; Chakraborty, S.; Appalaraju, S.N.; Bhandari, V. Decreased expression of femXAB genes and fnbp mediated biofilm pathways in OS-MRSA clinical isolates. Sci. Rep. 2019, 9, 16028. [Google Scholar] [CrossRef]
- Monteiro, J.M.; Covas, G.; Rausch, D.; Filipe, S.R.; Schneider, T.; Sahl, H.G.; Pinho, M.G. The pentaglycine bridges of Staphylococcus aureus peptidoglycan are essential for cell integrity. Sci. Rep. 2019, 9, 5010. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Sirisarn, W.; Alharbi, O.; Alharbi, M.; Liu, H.; Nøhr-Meldgaard, K.; Mayer, K.; Vestergaard, M.; Gallagher, L.A.; Derrick, J.P.; et al. The Novel Membrane-Associated Auxiliary Factors AuxA and AuxB Modulate β-lactam Resistance in MRSA by stabilizing Lipoteichoic Acids. Int. J. Antimicrob. Agents 2021, 57, 106283. [Google Scholar] [CrossRef]
- Arredondo-Alonso, S.; Top, J.; Corander, J.; Willems, R.J.L.; Schürch, A.C. Mode and dynamics of vanA-type vancomycin resistance dissemination in Dutch hospitals. Genome Med. 2021, 13, 9. [Google Scholar] [CrossRef]
- Lee, G.Y.; Kim, G.-B.; Yang, S.-J. Co-occurrence of cfr-mediated linezolid-resistance in ST398 LA-MRSA and non-aureus staphylococci isolated from a pig farm. Vet. Microbiol. 2022, 266, 109336. [Google Scholar] [CrossRef]
- Yoo, I.Y.; Kang, O.K.; Shim, H.J.; Huh, H.J.; Lee, N.Y. Linezolid Resistance in Methicillin-Resistant Staphylococcus aureus in Korea: High Rate of False Resistance to Linezolid by the VITEK 2 System. Ann. Lab. Med. 2020, 40, 57–62. [Google Scholar] [CrossRef]
- Hernández-Cuellar, E.; Tsuchiya, K.; Valle-Ríos, R.; Medina-Contreras, O. Differences in Biofilm Formation by Methicillin-Resistant and Methicillin-Susceptible Staphylococcus aureus Strains. Diseases 2023, 11, 160. [Google Scholar] [CrossRef]
- O’Neill, E.; Pozzi, C.; Houston, P.; Smyth, D.; Humphreys, H.; Robinson, D.A.; O’Gara, J.P. Association between Methicillin Susceptibility and Biofilm Regulation in Staphylococcus aureus Isolates from Device-Related Infections. J. Clin. Microbiol. 2007, 45, 1379–1388. [Google Scholar] [CrossRef]
- Almaadhidy, R.A.K.J.; Al Meani, O.M.I.S.A.L. Estimation of Biofilm Components and Prevalence of SDR Genes among Staphylococcus aureus Isolated from Anbar Hospitals. Open Microbiol. J. 2024, 18, e18742858349916. [Google Scholar] [CrossRef]
- Yang, W.; Liu, J.; Blažeković, B.; Sun, Y.; Ma, S.; Ren, C.; Vladimir-Knežević, S.; Li, C.; Xing, Y.; Tian, G.; et al. In vitro antibacterial effects of Tanreqing injection combined with vancomycin or linezolid against methicillin-resistant Staphylococcus aureus. BMC Complement. Altern. Med. 2018, 18, 169. [Google Scholar] [CrossRef]
- Fisher, R.A.; Gollan, B.; Helaine, S. Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 2017, 15, 453–464. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Saravolatz, L.D.; Pawlak, J. In vitro activity of fosfomycin alone and in combination against Staphylococcus aureus with reduced susceptibility or resistance to methicillin, vancomycin, daptomycin or linezolid. J. Antimicrob. Chemother. 2023, 78, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Tasellari, A.; Wagner, J.L.; Scheetz, M.H. Contemporary pharmacologic treatments of MRSA for hospitalized adults: Rationale for vancomycin versus non-vancomycin therapies as first line agents. Expert Rev. Anti Infect. Ther. 2023, 21, 1309–1325. [Google Scholar] [CrossRef]
- Sindhwani, S.; Chan, W.C.W. Nanotechnology for modern medicine: Next step towards clinical translation. J. Intern. Med. 2021, 290, 486–498. [Google Scholar] [CrossRef]
- Munir, M.U.; Ahmed, A.; Usman, M.; Salman, S. Recent Advances in Nanotechnology-Aided Materials in Combating Microbial Resistance and Functioning as Antibiotics Substitutes. Int. J. Nanomed. 2020, 15, 7329–7358. [Google Scholar] [CrossRef]
- Kang, Y.; Chen, S.; Chen, Y.; Tian, L.; Wu, Q.; Zheng, M.; Li, Z. Alterations of fecal antibiotic resistome in COVID-19 patients after empirical antibiotic exposure. Int. J. Hyg. Environ. Health 2022, 240, 113882. [Google Scholar] [CrossRef]
- Anwar, M.A.; Aqib, A.I.; Ashfaq, K.; Deeba, F.; Khan, M.K.; Khan, S.R.; Muzammil, I.; Shoaib, M.; Naseer, M.A.; Riaz, T.; et al. Antimicrobial resistance modulation of MDR E. coli by antibiotic coated ZnO nanoparticles. Microb. Pathog. 2020, 148, 104450. [Google Scholar] [CrossRef]
- Lodhi, F.L.; Saleem, M.I.; Aqib, A.I.; Rashid, I.; Qureshi, Z.I.; Anwar, M.A.; Ashraf, F.; Khan, S.R.; Jamil, H.; Fatima, R.; et al. Bringing resistance modulation to epidemic methicillin resistant S. aureus of dairy through antibiotics coupled metallic oxide nanoparticles. Microb. Pathog. 2021, 159, 105138. [Google Scholar] [CrossRef]
- Beha, M.J.; Ryu, J.S.; Kim, Y.S.; Chung, H.J. Delivery of antisense oligonucleotides using multi-layer coated gold nanoparticles to methicillin-resistant S. aureus for combinatorial treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 126, 112167. [Google Scholar] [CrossRef]
- Labruère, R.; Sona, A.J.; Turos, E. Anti-Methicillin-Resistant Staphylococcus aureus Nanoantibiotics. Front. Pharmacol. 2019, 10, 1121. [Google Scholar] [CrossRef]
- Andrade, S.; Ramalho, M.J.; Santos, S.B.; Melo, L.D.R.; Santos, R.S.; Guimarães, N.; Azevedo, N.F.; Loureiro, J.A.; Pereira, M.C. Fighting Methicillin-Resistant Staphylococcus aureus with Targeted Nanoparticles. Int. J. Mol. Sci. 2023, 24, 9030. [Google Scholar] [CrossRef]
- Hulme, J. Application of Nanomaterials in the Prevention, Detection, and Treatment of Methicillin-Resistant Staphylococcus aureus (MRSA). Pharmaceutics 2022, 14, 805. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, X.; Wu, Y.; Chen, X.; Feng, L.; Xie, N.; Shen, G. Nanotechnology’s frontier in combatting infectious and inflammatory diseases: Prevention and treatment. Sig. Transduct. Target. Ther. 2024, 9, 34. [Google Scholar] [CrossRef]
- Nandhini, P.; Kumar, P.; Mickymaray, S.; Alothaim, A.S.; Somasundaram, J.; Rajan, M. Recent Developments in Methicillin-Resistant Staphylococcus aureus (MRSA) Treatment: A Review. Antibiotics 2022, 11, 606. [Google Scholar] [CrossRef]
- Bispo, M.; Suhani, S.; van Dijl, J.M. Empowering antimicrobial photodynamic therapy of Staphylococcus aureus infections with potassium iodide. J. Photochem. Photobiol. B. 2021, 225, 112334. [Google Scholar] [CrossRef]
- Ashar, A.; Bhutta, Z.A.; Shoaib, M.; Alharbi, N.K.; Fakhar-e-Alam, M.; Atif, M.; Kulyar, M.F.A.; Mahfooz, A.; Boruah, P.; Eletmany, M.R.; et al. Cotton fabric loaded with ZnO nanoflowers as a photocatalytic reactor with promising antibacterial activity against pathogenic E. coli. Arab. J. Chem. 2023, 16, 105084. [Google Scholar] [CrossRef]
- Carter, S.; Miller, J.; Cramer, G.; Yuan, M.; Guzman, S.; Putt, M.E.; Cengel, K.A.; Freedman, G.M.; Busch, T.M. Adjuvant Photodynamic Therapy, Mediated via Topical Versus Systemic Administration of 5-Aminolevulinic Acid for Control of Murine Mammary Tumor after Surgical Resection. Photochem. Photobiol. 2022, 98, 117–126. [Google Scholar] [CrossRef]
- Dharmaratne, P.; Wang, B.; Wong, R.C.H.; Chan, B.C.L.; Lau, K.M.; Ke, M.R.; Lau, C.B.S.; Ng, D.K.P.; Fung, K.P.; Ip, M. Monosubstituted tricationic Zn(II) phthalocyanine enhances antimicrobial photodynamic inactivation (aPDI) of methicillin-resistant Staphylococcus aureus (MRSA) and cytotoxicity evaluation for topical applications: In vitro and in vivo study. Emerg. Microbes Infect. 2020, 9, 1628–1637. [Google Scholar] [CrossRef]
- Willis, J.A.; Cheburkanov, V.; Chen, S.; Soares, J.M.; Kassab, G.; Blanco, K.C.; Bagnato, V.S.; de Figueiredo, P.; Yakovlev, V.V. Breaking down antibiotic resistance in methicillin-resistant Staphylococcus aureus: Combining antimicrobial photodynamic and antibiotic treatments. Proc. Natl. Acad. Sci. USA 2022, 119, e2208378119. [Google Scholar] [CrossRef]
- Ribeiro, I.S.; Muniz, I.P.R.; Galantini, M.P.L.; Gonçalves, C.V.; Lima, P.H.B.; Silva, E.S.; Silva, N.R.; Rosa, F.C.S.; Rosa, L.P.; Costa, D.J.; et al. Characterization of Brazilian green propolis as a photosensitizer for LED light-induced antimicrobial photodynamic therapy (aPDT) against methicillin-resistant Staphylococcus aureus (MRSA) and Vancomycin-intermediate Staphylococcus aureus (VISA). Photochem. Photobiol. Sci. 2023, 22, 2877–2890. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, J.; Zhu, L.; Wang, D.; Song, J.; Yu, X.; Li, Y.; Tang, B.Z. Photosensitive AIEgens sensitize bacteria to oxidative damage and modulate the inflammatory responses of macrophages to salvage the photodynamic therapy against MRSA. Biomaterials 2024, 309, 122583. [Google Scholar] [CrossRef]
- Fu, X.; Fang, Y.; Yao, M. Antimicrobial Photodynamic Therapy for Methicillin-Resistant Staphylococcus aureus Infection. Biomed. Res. Int. 2013, 2013, 159157. [Google Scholar] [CrossRef]
- Roa-Tort, K.; Saavedra, Y.; Villanueva-Martínez, A.; Ganem-Rondero, A.; Pérez-Carranza, L.A.; de la Rosa-Vázquez, J.M.; Ugalde-Femat, G.; Molina-Alejandre, O.; Becerril-Osnaya, A.A.; Rivera-Fernández, J.D. In Vitro Antimicrobial Photodynamic Therapy for Pseudomonas aeruginosa (P. aeruginosa) and methicillin-resistant Staphylococcus aureus (MRSA) Inhibition Using a Green Light Source. Pharmaceutics 2024, 16, 518. [Google Scholar] [CrossRef]
- Dai, T.; Tegos, G.P.; Zhiyentayev, T.; Mylonakis, E.; Hamblin, M.R. Photodynamic Therapy for Methicillin-Resistant Staphylococcus aureus Infection in a Mouse Skin Abrasion Model. Lasers Surg. Med. 2010, 42, 38. [Google Scholar] [CrossRef]
- Bao, M.; Zhang, L.; Liu, B.; Li, L.; Zhang, Y.; Zhao, H.; Ji, X.; Chen, Q.; Hu, M.; Bai, J.; et al. Synergistic effects of anti-MRSA herbal extracts combined with antibiotics. Future Microbiol. 2020, 15, 1265–1276. [Google Scholar] [CrossRef]
- Ahmad, S.; Aqib, A.I.; Ghafoor, M.; Shoaib, M.; Haq, S.U.; Ataya, F.S.; Li, J.X. Drug Resistance Modulation of Dairy MRSA through Berberine, Artesunate and Quercetin in Combination with β-Lactams. Pak. Vet. J. 2024, 44, 510–516. [Google Scholar]
- Lan, J.E.; Li, X.J.; Zhu, X.F.; Sun, Z.L.; He, J.M.; Zloh, M.; Gibbons, S.; Mu, Q. Flavonoids from Artemisia rupestris and their synergistic antibacterial effects on drug-resistant Staphylococcus aureus. Nat. Prod. Res. 2021, 35, 1881–1886. [Google Scholar] [CrossRef]
- Gupta, V.K.; Tiwari, N.; Gupta, P.; Verma, S.; Pal, A.; Srivastava, S.K.; Darokar, M.P. A clerodane diterpene from Polyalthia longifolia as a modifying agent of the resistance of methicillin resistant Staphylococcus aureus. Phytomedicine 2016, 23, 654–661. [Google Scholar] [CrossRef]
- Rattanakiat, S.; Kaewchang, K.; Thongsang, S.; Jaruchotikamol, A.; Pulbutr, P. Synergistic Activity of Lupinifolin in Combinations with Antibiotics Against Staphylococcus aureus. Pak. J. Biol. Sci. 2021, 24, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Soe, W.M.; Lin, R.T.P.; Lim, C.S.; Sakharkar, K.R.; Sakharkar, M.K. In vitro drug interactions of gallates with antibiotics in Staphylococcus aureus. Front. Biosci.-Elite 2010, 2, 668–672. [Google Scholar]
- Guan, S.; Zhong, L.; Yu, H.; Wang, L.; Jin, Y.; Liu, J.; Xiang, H.; Yu, H.; Wang, L.; Wang, D. Molecular docking and proteomics reveals the synergistic antibacterial mechanism of theaflavin with β-lactam antibiotics against MRSA. Front. Microbiol. 2022, 13, 993430. [Google Scholar] [CrossRef]
- Batista de Andrade Neto, J.; Pessoa de Farias Cabral, V.; Brito Nogueira, L.F.; Rocha da Silva, C.; Gurgel do Amaral Valente Sá, L.; Ramos da Silva, A.; Barbosa da Silva, W.M.; Silva, J.; Marinho, E.S.; Cavalcanti, B.C.; et al. Anti-MRSA activity of curcumin in planktonic cells and biofilms and determination of possible action mechanisms. Microb. Pathog. 2021, 155, 104892. [Google Scholar] [CrossRef]
- Wang, M.; Ma, B.; Ni, Y.; Xue, X.; Li, M.; Meng, J.; Luo, X.; Fang, C.; Hou, Z. Restoration of the Antibiotic Susceptibility of Methicillin-Resistant Staphylococcus aureus and Extended-Spectrum β-Lactamases Escherichia coli Through Combination with Chelerythrine. Microb. Drug Resist. 2021, 27, 337–341. [Google Scholar] [CrossRef]
- Catteau, L.; Reichmann, N.T.; Olson, J.; Pinho, M.G.; Nizet, V.; Van Bambeke, F.; Quetin-Leclercq, J. Synergy between Ursolic and Oleanolic Acids from Vitellaria paradoxa Leaf Extract and β-Lactams against Methicillin-Resistant Staphylococcus aureus: In Vitro and In Vivo Activity and Underlying Mechanisms. Molecules 2017, 22, 2245. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Dai, Y.; Ouyang, P.; Rehman, T.; Hussain, S.; Zhang, T.; Yin, Z.; Fu, H.; Lin, J.; He, C.; et al. Thymol Inhibits Biofilm Formation, Eliminates Pre-Existing Biofilms, and Enhances Clearance of Methicillin-Resistant Staphylococcus aureus (MRSA) in a Mouse Peritoneal Implant Infection Model. Microorganisms 2020, 8, 99. [Google Scholar] [CrossRef]
- Li, J.G.; Chen, X.F.; Lu, T.Y.; Zhang, J.; Dai, S.H.; Sun, J.; Liu, Y.H.; Liao, X.P.; Zhou, Y.F. Increased Activity of β-Lactam Antibiotics in Combination with Carvacrol against MRSA Bacteremia and Catheter-Associated Biofilm Infections. ACS Infect. Dis. 2023, 9, 2482–2493. [Google Scholar] [CrossRef] [PubMed]
- Harro, C.D.; Betts, R.F.; Hartzel, J.S.; Onorato, M.T.; Lipka, J.; Smugar, S.S.; Kartsonis, N.A. The immunogenicity and safety of different formulations of a novel Staphylococcus aureus vaccine (V710): Results of two Phase I studies. Vaccine 2012, 30, 1729–1736. [Google Scholar] [CrossRef] [PubMed]
- Skaar, E.P.; Schneewind, O. Iron-regulated surface determinants (Isd) of Staphylococcus aureus: Stealing iron from heme. Microbes Infect. 2004, 6, 390–397. [Google Scholar] [CrossRef]
- Nissen, M.; Marshall, H.; Richmond, P.; Shakib, S.; Jiang, Q.; Cooper, D.; Rill, D.; Baber, J.; Eiden, J.; Gruber, W.; et al. A randomized phase I study of the safety and immunogenicity of three ascending dose levels of a 3-antigen Staphylococcus aureus vaccine (SA3Ag) in healthy adults. Vaccine 2015, 33, 1846–1854. [Google Scholar] [CrossRef]
- GlaxoSmithKline. A Partially Blind Study to Evaluate the Safety, Reactogenicity and Immunogenicity of GSK Biologicals’ Staphylococcal 4-Component Investigational Vaccine (GSK2392102A) in Healthy Adults. Clinical Trial Registration NCT01160172. Clinicaltrials.gov; 2017. Available online: https://clinicaltrials.gov/study/NCT01160172 (accessed on 27 July 2025).
- Henry, M. Jackson Foundation for the Advancement of Military Medicine. A Randomized, Multi-Center Trial to Evaluate the Safety & Immunogenicity of Staphylococcus aureus Toxoids, rAT and rLukS-PV, in Healthy Volunteers. Clinical Trial Registration. clinicaltrials.gov. 2023. Available online: https://www.clinicaltrials.gov/study/NCT01011335 (accessed on 27 July 2025).
- LimmaTech: First Patients Vaccinated Against Staphylococcus aureus. Swiss Biotech. Available online: https://www.swissbiotech.org/listing/limmatech-vaccinates-first-participants-in-phase-1-study-of-staphylococcus-aureus-vaccine-candidate-lbt-sa7/ (accessed on 22 June 2025).
- Oesterreich, B.; Lorenz, B.; Schmitter, T.; Kontermann, R.; Zenn, M.; Zimmermann, B.; Haake, M.; Lorenz, U.; Ohlsen, K. Characterization of the biological anti-staphylococcal functionality of hUK-66 IgG1, a humanized monoclonal antibody as substantial component for an immunotherapeutic approach. Hum. Vaccin. Immunother. 2014, 10, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Weisman, L.E.; Thackray, H.M.; Garcia-Prats, J.A.; Nesin, M.; Schneider, J.H.; Fretz, J.; Kokai-Kun, J.F.; Mond, J.J.; Kramer, W.G.; Fischer, G.W. Phase 1/2 double-blind, placebo-controlled, dose escalation, safety, and pharmacokinetic study of pagibaximab (BSYX-A110), an antistaphylococcal monoclonal antibody for the prevention of staphylococcal bloodstream infections, in very-low-birth-weight neonates. Antimicrob. Agents Chemother. 2009, 53, 2879–2886. [Google Scholar]
- Ragle, B.E.; Bubeck Wardenburg, J. Anti-alpha-hemolysin monoclonal antibodies mediate protection against Staphylococcus aureus pneumonia. Infect. Immun. 2009, 77, 2712–2718. [Google Scholar] [CrossRef] [PubMed]
- Gan, B.H.; Gaynord, J.; Rowe, S.M.; Deingruber, T.; Spring, D.R. The multifaceted nature of antimicrobial peptides: Current synthetic chemistry approaches and future directions. Chem. Soc. Rev. 2021, 50, 7820–7880, Erratum in Chem. Soc. Rev. 2022, 51, 792. [Google Scholar] [CrossRef] [PubMed]
- Pirnay, J.-P.; Djebara, S.; Steurs, G.; Griselain, J.; Cochez, C.; De Soir, S.; Glonti, T.; Spiessens, A.; Vanden Berghe, E.; Green, S.; et al. Personalized bacteriophage therapy outcomes for 100 consecutive cases: A multicentre, multinational, retrospective observational study. Nat. Microbiol. 2024, 9, 1434–1453. [Google Scholar] [CrossRef]
- Shi, J.; Chen, C.; Kong, P.; Yu, F.; Lv, Q.; Wang, Z.; Liu, Y. Non-Membrane Active Peptide Resensitizes MRSA to β-Lactam Antibiotics and Inhibits S. aureus Virulence. Adv. Sci. 2025, 12, e2416260. [Google Scholar] [CrossRef]
- Awdhesh Kumar Mishra, R.; Kodiveri Muthukaliannan, G. In-silico and in-vitro study of novel antimicrobial peptide AM1 from Aegle marmelos against drug-resistant Staphylococcus aureus. Sci. Rep. 2024, 14, 25822. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, W.; Liu, S.; Li, B.; Jiang, X. Delivery of CRISPR/Cas9 by Novel Strategies for Gene Therapy. Chembiochem 2019, 20, 634–643. [Google Scholar] [CrossRef]
- Ates, A.; Tastan, C.; Ermertcan, S. CRISPR-Cas9-Mediated Targeting of Multidrug Resistance Genes in Methicillin-Resistant Staphylococcus aureus. CRISPR J. 2024, 7, 374–384. [Google Scholar] [CrossRef]
- Shimamori, Y.; Tan, X.E.; Li, F.Y.; Nishikawa, Y.; Watanabe, S.; Sasahara, T.; Miyanaga, K.; Aiba, Y.; Veeranarayanan, S.; Thitiananpakorn, K.; et al. Efficient synthesis of CRISPR-Cas13a-antimicrobial capsids against MRSA facilitated by silent mutation incorporation. Sci. Rep. 2024, 14, 16225. [Google Scholar] [CrossRef]
- Sewell, E.W.C.; Brown, E.D. Taking aim at wall teichoic acid synthesis: New biology and new leads for antibiotics. J. Antibiot. 2014, 67, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, F.; Zhao, Q.; Cao, Q.; Chen, R.; Pan, H.; Wang, Y.; Huang, H.; Huang, R.; Liu, Q.; et al. Modulation of MRSA virulence gene expression by the wall teichoic acid enzyme TarO. Nat. Commun. 2023, 14, 1594. [Google Scholar] [CrossRef] [PubMed]
- Aires-de-Sousa, M. Methicillin-resistant Staphylococcus aureus among animals: Current overview. Clin. Microbiol. Infect. 2017, 23, 373–380. [Google Scholar] [CrossRef] [PubMed]
| Structure Type | Plant Extracts | Antibiotics | FIC Index | MRSA Source (Strains Tested) | Mechanism of Action | References |
|---|---|---|---|---|---|---|
| Phenolic compounds | Lupinifolin | Ampicillin | <0.5625 | DMST 20645 | Cell wall, cell membrane | [112] |
| Cloxacillin | <5156 | |||||
| Chrysoeriol | Norfloxacin | <0.266 | SA1199B | NorA efflux pump | [110] | |
| Ciprofloxacin | <0.024 | |||||
| Oxacillin | <0.375 | |||||
| Epigallocatechin gallate | Tetracycline | 0.5 | Clinical MRSA (C1, ATCC 43300) | Cell membrane, β-lactamase, biofilm, virulence factors | [113] | |
| Mupirocin | 0.5 | |||||
| Fusidic Acid | 0.5 | |||||
| Ethyl gallate | Tetracycline | 0.5 | ||||
| Mupirocin | 0.5 | |||||
| Fusidic Acid | 0.5 | |||||
| Theaflavin | Ceftiofur | 0.1875 | MRSA (USA300) | Cell wall | [114] | |
| Cefoxitin | 0.3125 | |||||
| Latamoxef | 0.3125 | |||||
| Ceftazidime | 0.3125 | |||||
| Oxacillin | 0.3125 | |||||
| Ampicillin | 0.3125 | |||||
| Curcumin | Oxacillin | Clinical MRSA (7 strains) | Cell membrane, biofilm, | [115] | ||
| Alkaloids | Berberine | Gentamicin | 0.53–1.06 | Clinical MRSA (50 strains) | Membrane permeability, cell wall | [4] |
| Levofloxacin | 0.62–1.5 | |||||
| Amikacin | 0.16–1.25 | |||||
| Chelerythrine | Oxacillin | 0.5 | MRSA (ATCC 43300, ATCC 700699) | Cell wall, cell membrane | [116] | |
| Terpenoids | Clerodane diterpene 16 α-hydroxycleroda-3,13(14)-Z-dien-15,16-olide | Norfloxacin | 0.315–0.5 | Clinical MRSA (9 strains) | Efflux pump | [111] |
| Oxfloxacin | 0.324 | Clinical MRSA (8 strains) | ||||
| Ciprofloxacin | 0.324 | Clinical MRSA (6 strains) | ||||
| Ursolic acid | Tetracycline | 0.28 | MRSA (ATCC 43300) | Cell wall, PBP2a protein, β-lactamase | [117] | |
| Cleanolic acid | Oxacillin | 0.5 | MRSA (ATCC 43300) | Cell wall, PBP2a protein, β-lactamase | [117] | |
| Essential oils | Thymol | Vancomycin | 1.0 | MRSA (TCH1516) | Biofilm | [118] |
| Carvacrol | Cefotaxime | 0.28–0.5 | MRSA (SA-70, SA-372, 161402) | Biofilm | [119] | |
| Oxacillin | 0.28–0.5 | |||||
| Ampicillin | 0.375–0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Cheng, F.; Wei, X.; Bai, Y.; Wang, Q.; Li, B.; Zhou, Y.; Zhai, B.; Zhou, X.; Wang, W.; et al. Methicillin-Resistant Staphylococcus aureus (MRSA): Resistance, Prevalence, and Coping Strategies. Antibiotics 2025, 14, 771. https://doi.org/10.3390/antibiotics14080771
Li J, Cheng F, Wei X, Bai Y, Wang Q, Li B, Zhou Y, Zhai B, Zhou X, Wang W, et al. Methicillin-Resistant Staphylococcus aureus (MRSA): Resistance, Prevalence, and Coping Strategies. Antibiotics. 2025; 14(8):771. https://doi.org/10.3390/antibiotics14080771
Chicago/Turabian StyleLi, Jiajing, Fusheng Cheng, Xiaojuan Wei, Yubin Bai, Qing Wang, Bing Li, Yaxin Zhou, Bintao Zhai, Xuzheng Zhou, Weiwei Wang, and et al. 2025. "Methicillin-Resistant Staphylococcus aureus (MRSA): Resistance, Prevalence, and Coping Strategies" Antibiotics 14, no. 8: 771. https://doi.org/10.3390/antibiotics14080771
APA StyleLi, J., Cheng, F., Wei, X., Bai, Y., Wang, Q., Li, B., Zhou, Y., Zhai, B., Zhou, X., Wang, W., & Zhang, J. (2025). Methicillin-Resistant Staphylococcus aureus (MRSA): Resistance, Prevalence, and Coping Strategies. Antibiotics, 14(8), 771. https://doi.org/10.3390/antibiotics14080771







