Characterization of Antibiotic Administration Factors Associated with Microbiome Disruption and Subsequent Antibiotic-Resistant Infection and Colonization Events in Acute Myeloid Leukemia Patients Receiving Chemotherapy
Abstract
1. Introduction
2. Results
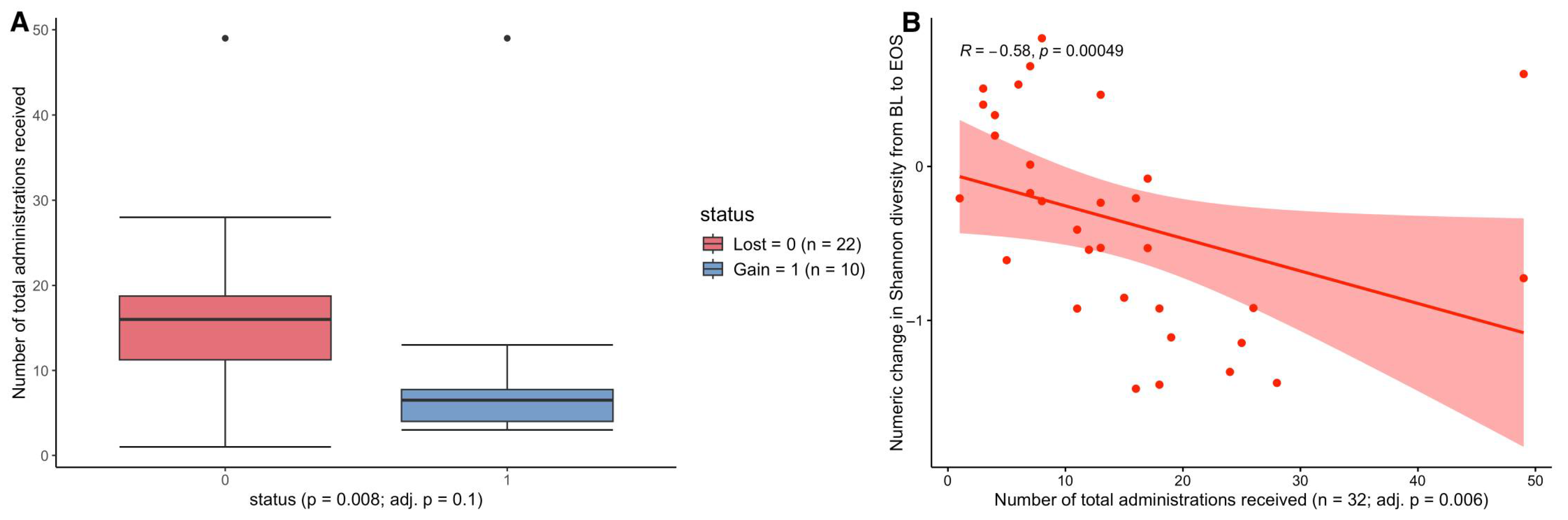
2.1. The Impact of Total Antimicrobial Administrations on Patient AR-Threat Outcomes, Resistome, and Microbial Diversity
2.2. The Impact of the Number of Unique Antibiotics on Patient Outcomes, ARGs, and Diversity
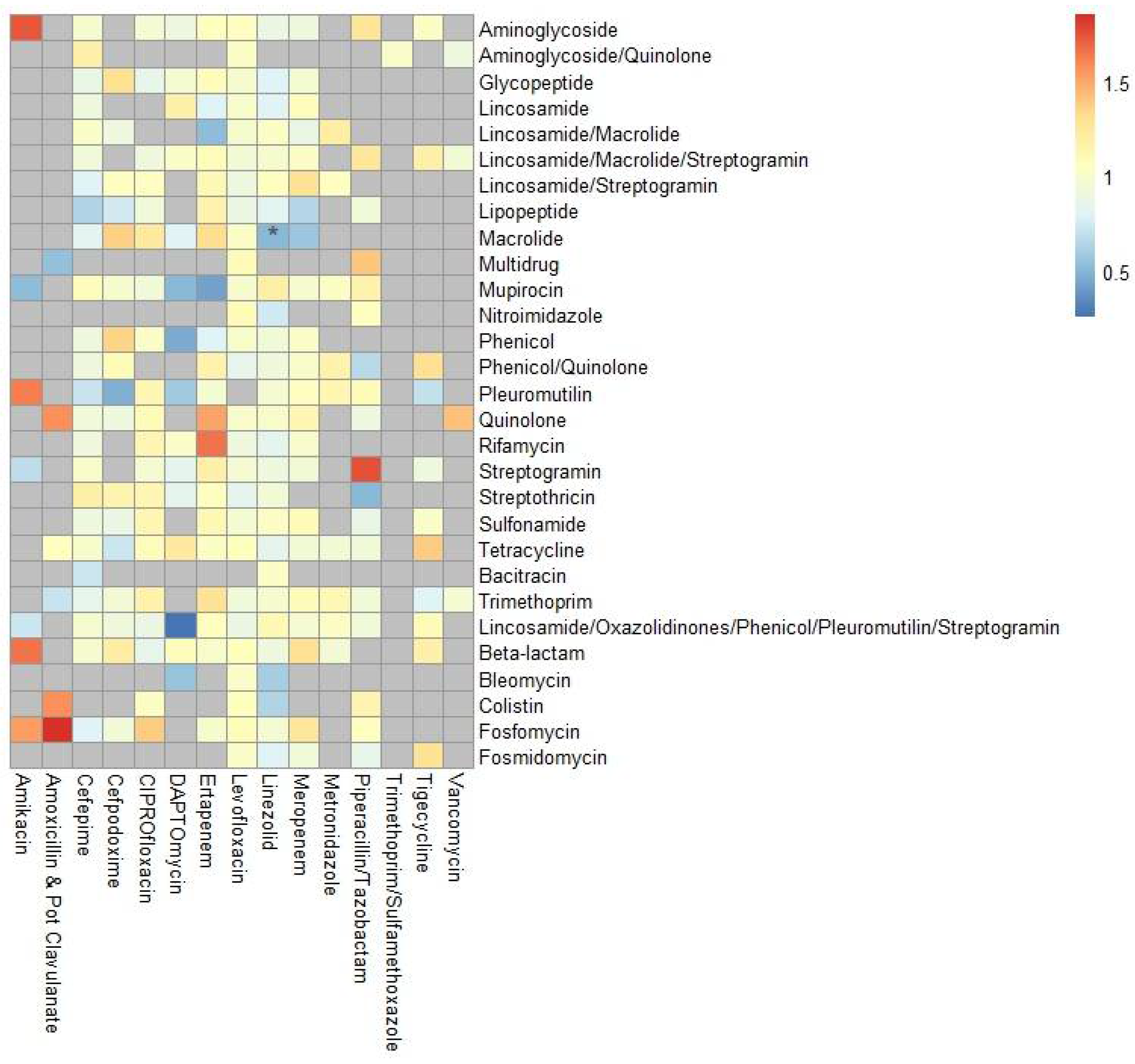
2.3. Antibiotic Exposure and AR-Threat or ARG Acquisition Risk
3. Discussion
4. Materials and Methods
4.1. Study Design, Patients, and Sample Collection
4.2. 16S Amplicon Sequencing and Identification of Potential Samples Colonized with Antibiotic-Resistant CDC-Threat Pathogens
4.3. Determination of Infection with an Antibiotic-Resistant CDC-Threat Pathogen
4.4. Whole Genome Sequencing of AR-Colonization and AR-Infection Bacterial Isolates
4.5. Shotgun Metagenomic Sequencing of Baseline and End of Study Samples
4.6. Resistome Analyses
4.7. Antibiotic Prescribing Practices and Antibiotic Administration Analyses
4.8. Statistical Analyses
= binomial (link = “logit”), data = data)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AML | acute myeloid leukemia |
| AMR | antimicrobial resistance |
| AR | Antibiotic resistant |
| RIC | remission induction chemotherapy |
| ARI | antibiotic resistant infection |
| ARC | antibiotic resistant colonization |
| BL | baseline |
| EOS | end of study |
| ARG | antimicrobial resistance genes |
| CDC | Center for Disease Prevention |
| MDRP | multidrug resistant Pseudomonas aeruginosa |
| ESBL | extended-spectrum beta-lactamase producing Enterobacteriaceae |
| MRSA | methicillin-resistant Staphylococcus aureus |
| VRE | vancomycin-resistant Enterococcus |
| CRE | carbapenem-resistant Enterobacteriaceae |
| MGE | mobile genetic element |
| ANI | absolute nucleotide identity |
| NA | not applicable |
| CI | confidence interval |
References
- World Health Organization. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 30 August 2024).
- Walsh, T.R.; Gales, A.C.; Laxminarayan, R.; Dodd, P.C. Antimicrobial resistance: Addressing a global threat to humanity. PLoS Med. 2023, 20, e1004264. [Google Scholar] [CrossRef]
- Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef]
- Gedik, H.; Şimşek, F.; Kantürk, A.; Yildirmak, T.; Arica, D.; Aydin, D.; Demirel, N.; Yokuş, O. Bloodstream infections in patients with hematological malignancies: Which is more fatal–cancer or resistant pathogens? Ther. Clin. Risk Manag. 2014, 10, 743–752. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freifeld, A.G.; Bow, E.J.; Sepkowitz, K.A.; Boeckh, M.J.; Ito, J.I.; Mullen, C.A.; Raad, I.I.; Rolston, K.V.; Young, J.-A.H.; Wingard, J.R. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2011, 52, e56–e93. [Google Scholar] [CrossRef] [PubMed]
- Hughes, W.T.; Pizzo, P.A.; Wade, J.C.; Armstrong, D.; Webb, C.D.; Young, L.S. Evaluation of new anti-infective drugs for the treatment of febrile episodes in neutropenic patients. Clin. Infect. Dis. 1992, 15 (Suppl. S1), S206–S215. [Google Scholar] [CrossRef] [PubMed]
- Nobel, Y.R.; Cox, L.M.; Kirigin, F.F.; Bokulich, N.A.; Yamanishi, S.; Teitler, I.; Chung, J.; Sohn, J.; Barber, C.M.; Goldfarb, D.S. Metabolic and metagenomic outcomes from early-life pulsed antibiotic treatment. Nat. Commun. 2015, 6, 7486. [Google Scholar] [CrossRef]
- Jutkina, J.; Rutgersson, C.; Flach, C.-F.; Larsson, D.J. An assay for determining minimal concentrations of antibiotics that drive horizontal transfer of resistance. Sci. Total Environ. 2016, 548, 131–138. [Google Scholar] [CrossRef]
- Doan, T.; Worden, L.; Hinterwirth, A.; Arzika, A.M.; Maliki, R.; Abdou, A.; Zhong, L.; Chen, C.; Cook, C.; Lebas, E. Macrolide and nonmacrolide resistance with mass azithromycin distribution. N. Engl. J. Med. 2020, 383, 1941–1950. [Google Scholar] [CrossRef]
- Van Vliet, M.J.; Harmsen, H.J.; de Bont, E.S.; Tissing, W.J. The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog. 2010, 6, e1000879. [Google Scholar] [CrossRef]
- Wong, M.; Barqasho, B.; Öhrmalm, L.; Tolfvenstam, T.; Nowak, P. Microbial translocation contribute to febrile episodes in adults with chemotherapy-induced neutropenia. PLoS ONE 2013, 8, e68056. [Google Scholar] [CrossRef]
- Craig, M. CDC’s Antibiotic Resistance Threats Report, 2019; Extended Spectrum β-Lactamase (ESBL)-Producing Enterobacteriaceae; Centers for Disease Control and Prevention: Atlanta, Georgia, 2019.
- Hansen, B.-A.; Wendelbo, Ø.; Bruserud, Ø.; Hemsing, A.L.; Mosevoll, K.A.; Reikvam, H. Febrile neutropenia in acute leukemia. Epidemiology, etiology, pathophysiology and treatment. Mediterr. J. Hematol. Infect. Dis. 2019, 12, e2020009. [Google Scholar] [CrossRef]
- Erdem, H.; Kocoglu, E.; Ankarali, H.; El-Sokkary, R.; Hakamifard, A.; Karaali, R.; Kulzhanova, S.; El-Kholy, A.; Tehrani, H.A.; Khedr, R. Prospective analysis of febrile neutropenia patients with bacteraemia: The results of an international ID–IRI study. Int. J. Antimicrob. Agents 2023, 62, 106919. [Google Scholar] [CrossRef]
- Zimmer, A.J.; Stohs, E.; Meza, J.; Arnold, C.; Baddley, J.W.; Chandrasekar, P.; El Boghdadly, Z.; Gomez, C.A.; Maziarz, E.K.; Montoya, J.G. Bloodstream infections in hematologic malignancy patients with fever and neutropenia: Are empirical antibiotic therapies in the United States still effective? Open Forum Infect. Dis. 2022, 9, ofac240. [Google Scholar] [CrossRef] [PubMed]
- Gustinetti, G.; Mikulska, M. Bloodstream infections in neutropenic cancer patients: A practical update. Virulence 2016, 7, 280–297. [Google Scholar] [CrossRef]
- Carvalho, A.S.; Lagana, D.; Catford, J.; Shaw, D.; Bak, N. Bloodstream infections in neutropenic patients with haematological malignancies. Infect. Dis. Health 2020, 25, 22–29. [Google Scholar] [CrossRef]
- Gibson, M.K.; Pesesky, M.W.; Dantas, G. The yin and yang of bacterial resilience in the human gut microbiota. J. Mol. Biol. 2014, 426, 3866–3876. [Google Scholar] [CrossRef]
- Shono, Y.; Docampo, M.D.; Peled, J.U.; Perobelli, S.M.; Velardi, E.; Tsai, J.J.; Slingerland, A.E.; Smith, O.M.; Young, L.F.; Gupta, J. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci. Transl. Med. 2016, 8, 339ra71. [Google Scholar] [CrossRef]
- Tibayrenc, M. Genetics and Evolution of Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Gibson, M.K.; Forsberg, K.J.; Dantas, G. Improved annotation of antibiotic resistance determinants reveals microbial resistomes cluster by ecology. ISME J. 2015, 9, 207–216. [Google Scholar] [CrossRef]
- Forslund, K.; Sunagawa, S.; Kultima, J.R.; Mende, D.R.; Arumugam, M.; Typas, A.; Bork, P. Country-specific antibiotic use practices impact the human gut resistome. Genome Res. 2013, 23, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Pehrsson, E.C.; Forsberg, K.J.; Gibson, M.K.; Ahmadi, S.; Dantas, G. Novel resistance functions uncovered using functional metagenomic investigations of resistance reservoirs. Front. Microbiol. 2013, 4, 145. [Google Scholar] [CrossRef] [PubMed]
- Khedkar, S.; Smyshlyaev, G.; Letunic, I.; Maistrenko, O.M.; Coelho, L.P.; Orakov, A.; Forslund, S.K.; Hildebrand, F.; Luetge, M.; Schmidt, T.S. Landscape of mobile genetic elements and their antibiotic resistance cargo in prokaryotic genomes. Nucleic Acids Res. 2022, 50, 3155–3168. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.L.; Olsen, M.H.; Pallejá, A.; Ebdrup, S.R.; Sørensen, N.; Lukjancenko, O.; Marvig, R.L.; Møller, K.; Frimodt-Møller, N.; Hertz, F.B. Microbiome compositions and resistome levels after antibiotic treatment of critically ill patients: An observational cohort study. Microorganisms 2021, 9, 2542. [Google Scholar] [CrossRef]
- Klastersky, J. Management of fever in neutropenic patients with different risks of complications. Clin. Infect. Dis. 2004, 39, S32–S37. [Google Scholar] [CrossRef]
- Galloway-Peña, J.R.; Smith, D.P.; Sahasrabhojane, P.; Ajami, N.J.; Wadsworth, W.D.; Daver, N.G.; Chemaly, R.F.; Marsh, L.; Ghantoji, S.S.; Pemmaraju, N. The role of the gastrointestinal microbiome in infectious complications during induction chemotherapy for acute myeloid leukemia. Cancer 2016, 122, 2186–2196. [Google Scholar] [CrossRef]
- Galloway-Peña, J.R.; Smith, D.P.; Sahasrabhojane, P.; Wadsworth, W.D.; Fellman, B.M.; Ajami, N.J.; Shpall, E.J.; Daver, N.; Guindani, M.; Petrosino, J.F. Characterization of oral and gut microbiome temporal variability in hospitalized cancer patients. Genome Med. 2017, 9, 21. [Google Scholar] [CrossRef]
- Averbuch, D.; Orasch, C.; Cordonnier, C.; Livermore, D.M.; Mikulska, M.; Viscoli, C.; Gyssens, I.C.; Kern, W.V.; Klyasova, G.; Marchetti, O. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: Summary of the 2011 4th European Conference on Infections in Leukemia. Haematologica 2013, 98, 1826. [Google Scholar] [CrossRef]
- Keenan, J.D.; Chin, S.A.; Amza, A.; Kadri, B.; Nassirou, B.; Cevallos, V.; Cotter, S.Y.; Zhou, Z.; West, S.K.; Bailey, R.L. The effect of antibiotic selection pressure on the nasopharyngeal macrolide resistome: A cluster-randomized trial. Clin. Infect. Dis. 2018, 67, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Davey, P.; Marwick, C.A.; Scott, C.L.; Charani, E.; McNeil, K.; Brown, E.; Gould, I.M.; Ramsay, C.R.; Michie, S. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2017, 2, CD003543. [Google Scholar] [CrossRef]
- Ballo, O.; Tarazzit, I.; Stratmann, J.; Reinheimer, C.; Hogardt, M.; Wichelhaus, T.A.; Kempf, V.; Serve, H.; Finkelmeier, F.; Brandts, C. Colonization with multidrug resistant organisms determines the clinical course of patients with acute myeloid leukemia undergoing intensive induction chemotherapy. PLoS ONE 2019, 14, e0210991. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Covington, A.; Pamer, E.G. The intestinal microbiota: Antibiotics, colonization resistance, and enteric pathogens. Immunol. Rev. 2017, 279, 90–105. [Google Scholar] [CrossRef]
- Heston, S.M.; Young, R.R.; Jenkins, K.; Martin, P.L.; Stokhuyzen, A.; Ward, D.V.; Bhattarai, S.K.; Bucci, V.; Arshad, M.; Chao, N.J. The effects of antibiotic exposures on the gut resistome during hematopoietic cell transplantation in children. Gut Microbes 2024, 16, 2333748. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Nadal, G.; Puerta-Alcalde, P.; Gudiol, C.; Cardozo, C.; Albasanz-Puig, A.; Marco, F.; Laporte-Amargós, J.; Moreno-García, E.; Domingo-Doménech, E.; Chumbita, M. Inappropriate empirical antibiotic treatment in high-risk neutropenic patients with bacteremia in the era of multidrug resistance. Clin. Infect. Dis. 2020, 70, 1068–1074. [Google Scholar] [CrossRef]
- Riaño, A.F.F.; Castro, O.J.R.; Ospina, S.; Ramírez-Sánchez, I.C. Association between inappropriate empirical antimicrobial therapy and mortality in gram-negative bloodstream infections in patients with febrile neutropenia and hematological malignancy. J. Infect. Chemother. 2025, 31, 102538. [Google Scholar] [CrossRef]
- Galloway-Peña, J.R.; Shi, Y.; Peterson, C.B.; Sahasrabhojane, P.; Gopalakrishnan, V.; Brumlow, C.E.; Daver, N.G.; Alfayez, M.; Boddu, P.C.; Khan, M.A.W. Gut microbiome signatures are predictive of infectious risk following induction therapy for acute myeloid leukemia. Clin. Infect. Dis. 2020, 71, 63–71. [Google Scholar] [CrossRef]
- Rashidi, A.; Kaiser, T.; Graiziger, C.; Holtan, S.G.; Rehman, T.U.; Weisdorf, D.J.; Khoruts, A.; Staley, C. Specific gut microbiota changes heralding bloodstream infection and neutropenic fever during intensive chemotherapy. Leukemia 2020, 34, 312–316. [Google Scholar] [CrossRef]
- Rattanathammethee, T.; Tuitemwong, P.; Thiennimitr, P.; Sarichai, P.; Na Pombejra, S.; Piriyakhuntorn, P.; Hantrakool, S.; Chai-Adisaksopha, C.; Rattarittamrong, E.; Tantiworawit, A. Gut microbiota profiles of treatment-naïve adult acute myeloid leukemia patients with neutropenic fever during intensive chemotherapy. PLoS ONE 2020, 15, e0236460. [Google Scholar] [CrossRef]
- Curran, J.; Lo, J.; Leung, V.; Brown, K.; Schwartz, K.L.; Daneman, N.; Garber, G.; Wu, J.H.; Langford, B.J. Estimating daily antibiotic harms: An umbrella review with individual study meta-analysis. Clin. Microbiol. Infect. 2022, 28, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Walker, M.J.; De Oliveira, D.M. Vancomycin resistance in Enterococcus and Staphylococcus aureus. Microorganisms 2022, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Binda, E.; Marinelli, F.; Marcone, G.L. Old and new glycopeptide antibiotics: Action and resistance. Antibiotics 2014, 3, 572–594. [Google Scholar] [CrossRef] [PubMed]
- Navidifar, T.; Zare Banadkouki, A.; Parvizi, E.; Mofid, M.; Golab, N.; Beig, M.; Sholeh, M. Global prevalence of macrolide-resistant Staphylococcus spp.: A comprehensive systematic review and meta-analysis. Front. Microbiol. 2025, 16, 1524452. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, J.C.; Jørgensen, M.; Moestrup, K.S.; Ilett, E.E.; Zucco, A.G.; Marandi, R.Z.; Julian, M.N.; Paredes, R.; Lundgren, J.D.; Sengeløv, H. Impact of antibiotic treatment on the gut microbiome and its resistome in hematopoietic stem cell transplant recipients. J. Infect. Dis. 2023, 228, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Willmann, M.; Vehreschild, M.J.; Biehl, L.M.; Vogel, W.; Dörfel, D.; Hamprecht, A.; Seifert, H.; Autenrieth, I.B.; Peter, S. Distinct impact of antibiotics on the gut microbiome and resistome: A longitudinal multicenter cohort study. BMC Biol. 2019, 17, 76. [Google Scholar] [CrossRef] [PubMed]
- Palleja, A.; Mikkelsen, K.H.; Forslund, S.K.; Kashani, A.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Liang, S.; Feng, Q.; Zhang, C. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat. Microbiol. 2018, 3, 1255–1265. [Google Scholar] [CrossRef]
- Margolis, E.B.; Hakim, H.; Dallas, R.H.; Allison, K.J.; Ferrolino, J.; Sun, Y.; Pui, C.-H.; Yao, J.; Chang, T.-C.; Hayden, R.T. Antibiotic prophylaxis and the gastrointestinal resistome in paediatric patients with acute lymphoblastic leukaemia: A cohort study with metagenomic sequencing analysis. Lancet Microbe 2021, 2, e159–e167. [Google Scholar] [CrossRef]


| Patient Characteristics | Total | Paired a |
|---|---|---|
| Patient count | 119 | 46 |
| Sex, N (%) | ||
| Female | 56 (47.06) | 18 (39.13) |
| Male | 57 (47.9) | 25 (54.35) |
| Unavailable information | 6 (5.04) | 3 (6.52) |
| Chemotherapy intensity, N (%) | ||
| High | 66 (55.46) | 19 (41.3) |
| Low | 47 (39.5) | 24 (52.17) |
| Unavailable information | 6 (5.04) | 3 (6.52) |
| No. of patients with confirmed ARI, N (%) b | 8 (6.72) | 4 (8.7) |
| No. of ARI isolates | 12 | 6 |
| Pseudomonas aeruginosa | 6 | 2 |
| Staphylococcus aureus | 1 | 0 |
| Escherichia coli | 5 | 4 |
| No. of patients with confirmed ARC, N (%) c | 14 (11.76) | 7 (15.22) |
| No. of ARC isolates | 18 | 9 |
| Enterobacter cloacae | 1 | 1 |
| Klebsiella pneumoniae | 3 | 1 |
| Pseudomonas aeruginosa | 1 | 1 |
| Enterococcus faecium | 6 | 2 |
| Escherichia coli | 3 | 2 |
| Staphylococcus aureus | 4 | 2 |
| Antibiotic Administration, N (Total Administrations) | BL to Event or 56 days | BL to EOS |
| Aminoglycosides | 22 (59) | 10 (26) |
| Carbapenems | 59 (1299) | 22 (650) |
| Cephalosporins | 88 (2088) | 31 (804) |
| Fluoroquinolones | 101 (1318) | 41 (663) |
| Glycopeptide | 7 (89) | 5 (147) |
| Lincosamides | 4 (63) | 0 (0) |
| Lipopeptide | 35 (199) | 13 (71) |
| Macrolides | 6 (37) | 2 (17) |
| Monobactam | 12 (194) | 4 (37) |
| Nitroimidazole | 22 (296) | 8 (118) |
| Oxazolidinone | 90 (1181) | 32 (473) |
| Penicillins | 39 (835) | 16 (213) |
| Sulfonamides | 11 (180) | 3 (105) |
| Tetracyclines | 30 (372) | 13 (103) |
| Hazard Ratios, Confidence Intervals, and p-Values a | |||
|---|---|---|---|
| Antibiotic | ARI | ARC | Any AR-Threat Event |
| Amikacin | 0.937 (0.324–2.707) [0.905] | 0.933 (0.272–3.207) [0.913] | 0.634 (0.193–2.081) [0.452] |
| Amoxicillin and clavulanate | NA b | 0.375 (0.119–1.171) [0.091] | 0.272 (0.072–1.024) [0.054] |
| Azithromycin | NA | NA | 0.6841 (0.37–1.973) [0.483] |
| Cefepime HCL | 0.997 (0.499–1.994) [0.994] | 0.687 (0.272–1.73) [0.425] | 0.846 (0.449–1.593) [0.604] |
| Cefpodoxime | 0.890 (0.385–2.056) [0.785] | 1.256 (0.608–2.597) [0.538] | 1.305 (0.729–2.330) [0.37] |
| Ciprofloxacin | 0.718 (0.206–2.508) [0.603] | 0.573 (0.163–2.011) [0.384] | 0.466 (0.157–1.376) [0.167] |
| Daptomycin | 1.315 (0.431–4.011) [0.631] | 0.510 (0.120–1.857) [0.307] | 0.923 (0.373–2.280) [0.862] |
| Ertapenem Sodium | 0.657 (0.115–3.768) [0.638] | NA | 0.596 (0.101–3.504) [0.567] |
| Levofloxacin | 0.832 (0.392–1.766) [0.631] | 0.719 (0.450–1.148) [0.167] | 0.719 (0.443–1.166) [0.181] |
| Linezolid | 0.565 (0.214–1.491) [0.249] | 0.663 (0.364–0.992) [0.047] * | 0.662 (0.402–1.09) [0.105] |
| Meropenem | 3.411 (0.0753–1.141) [0.077] | 0.881 (0.436–1.777) [0.722] | 0.640 (0.291–1.405) [0.266] |
| Metronidazole | 0.523 (0.239–1.144) [0.104] | 1.563 (0.912–2.677) [0.104] | 0.912 (0.440–1.89) [0.805] |
| Minocycline | NA | 1.229 (0.368–4.108) [0.737] | 2.736 (1.333–5.614) [0.006] * |
| Piperacillin/Tazobactam | 1.022 (0.439–2.377) [0.959] | 0.883 (0.311–2.51) [0.816] | 1.211 (0.607–2.414) [0.587] |
| Tigecycline | 1.295 (0.391–4.285) [0.672] | NA | 1.413 (0.588–3.397) [0.440] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franklin, S.; Ramont, C.; Batool, M.; McMahon, S.; Sahasrabhojane, P.; Blazier, J.C.; Kontoyiannis, D.P.; Ni, Y.; Galloway-Peña, J. Characterization of Antibiotic Administration Factors Associated with Microbiome Disruption and Subsequent Antibiotic-Resistant Infection and Colonization Events in Acute Myeloid Leukemia Patients Receiving Chemotherapy. Antibiotics 2025, 14, 770. https://doi.org/10.3390/antibiotics14080770
Franklin S, Ramont C, Batool M, McMahon S, Sahasrabhojane P, Blazier JC, Kontoyiannis DP, Ni Y, Galloway-Peña J. Characterization of Antibiotic Administration Factors Associated with Microbiome Disruption and Subsequent Antibiotic-Resistant Infection and Colonization Events in Acute Myeloid Leukemia Patients Receiving Chemotherapy. Antibiotics. 2025; 14(8):770. https://doi.org/10.3390/antibiotics14080770
Chicago/Turabian StyleFranklin, Samantha, Corina Ramont, Maliha Batool, Stephanie McMahon, Pranoti Sahasrabhojane, John C. Blazier, Dimitrios P. Kontoyiannis, Yang Ni, and Jessica Galloway-Peña. 2025. "Characterization of Antibiotic Administration Factors Associated with Microbiome Disruption and Subsequent Antibiotic-Resistant Infection and Colonization Events in Acute Myeloid Leukemia Patients Receiving Chemotherapy" Antibiotics 14, no. 8: 770. https://doi.org/10.3390/antibiotics14080770
APA StyleFranklin, S., Ramont, C., Batool, M., McMahon, S., Sahasrabhojane, P., Blazier, J. C., Kontoyiannis, D. P., Ni, Y., & Galloway-Peña, J. (2025). Characterization of Antibiotic Administration Factors Associated with Microbiome Disruption and Subsequent Antibiotic-Resistant Infection and Colonization Events in Acute Myeloid Leukemia Patients Receiving Chemotherapy. Antibiotics, 14(8), 770. https://doi.org/10.3390/antibiotics14080770









