Streamlining Bacillus Strain Selection Against Listeria monocytogenes Using a Fluorescence-Based Infection Assay Integrated into a Multi-Tiered Validation Pipeline
Abstract
1. Introduction
2. Results
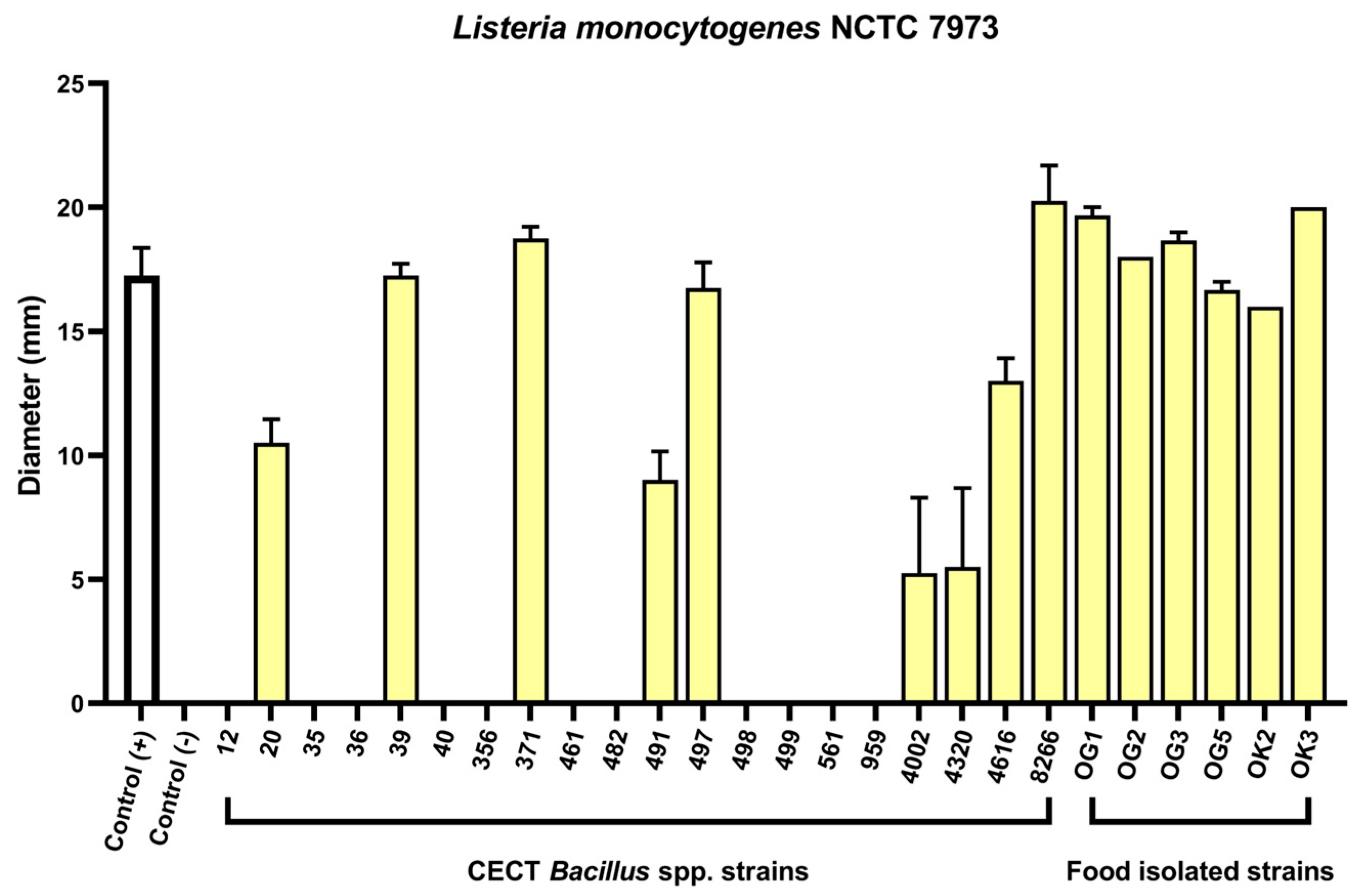
2.1. Agar Well Diffusion Screening of Bacillus spp. Reveals Anti-Listeria Activity
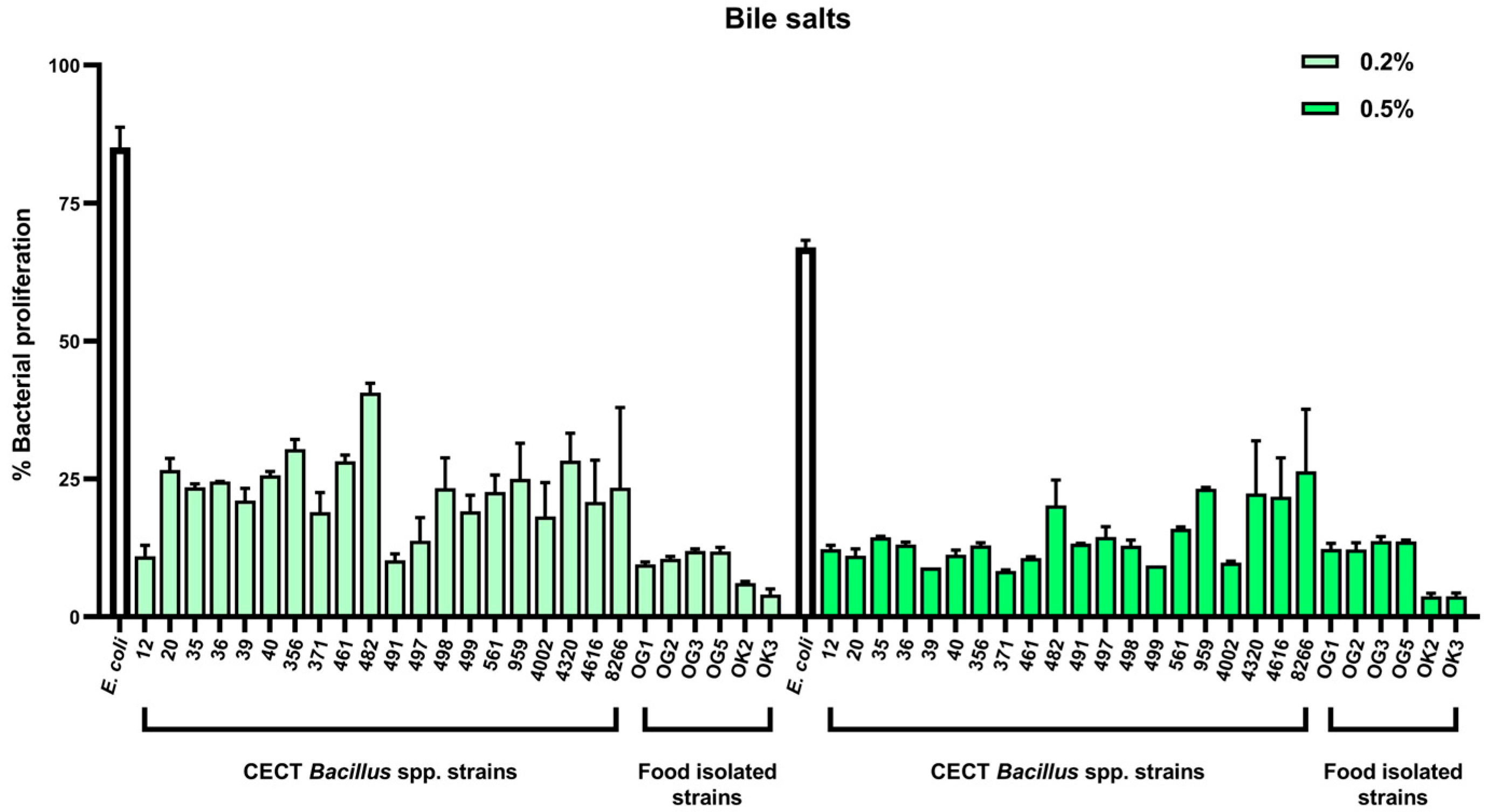
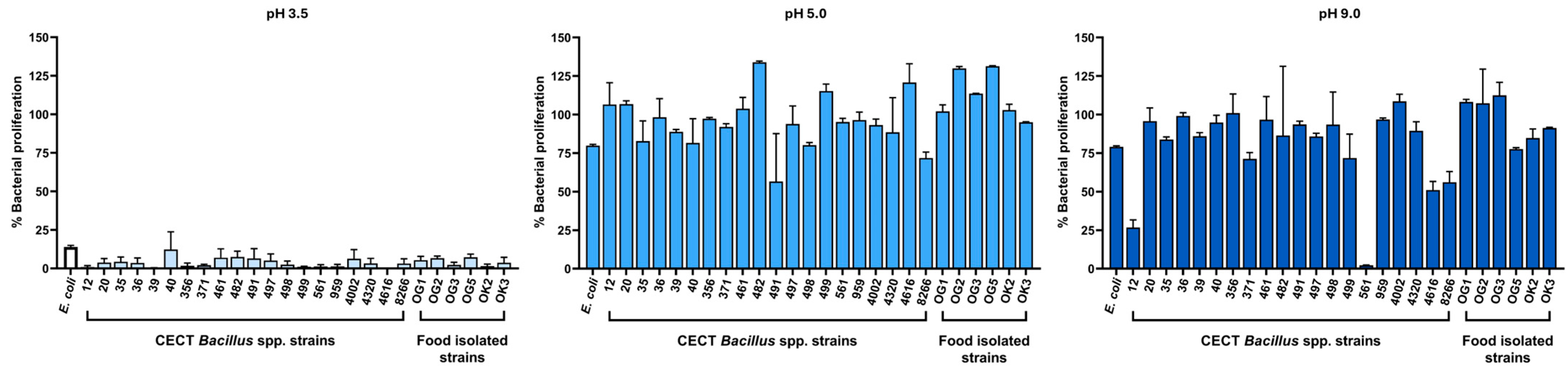
2.2. Tolerance to Gastrointestinal Stressors: Bile Salts and pH
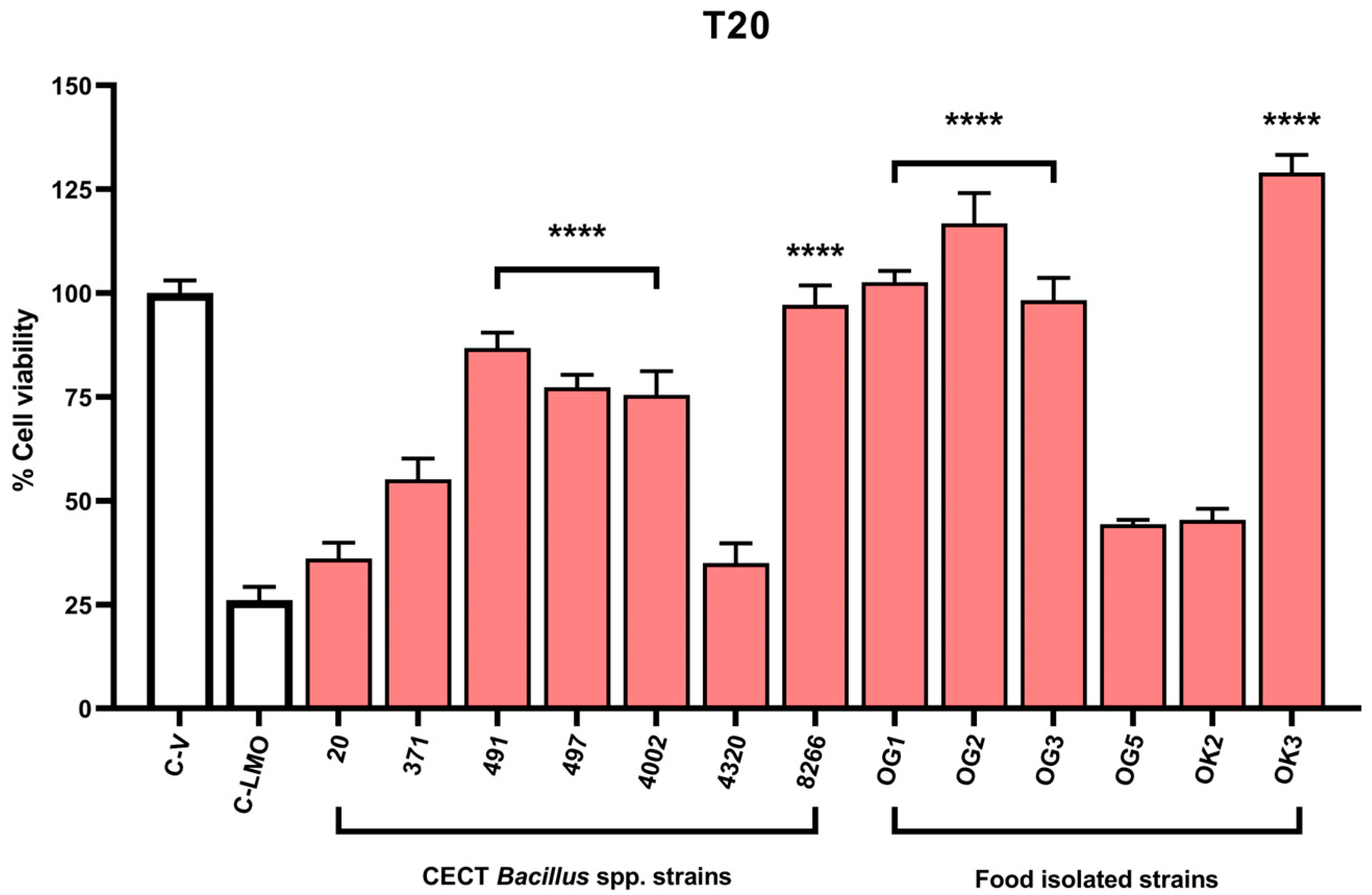
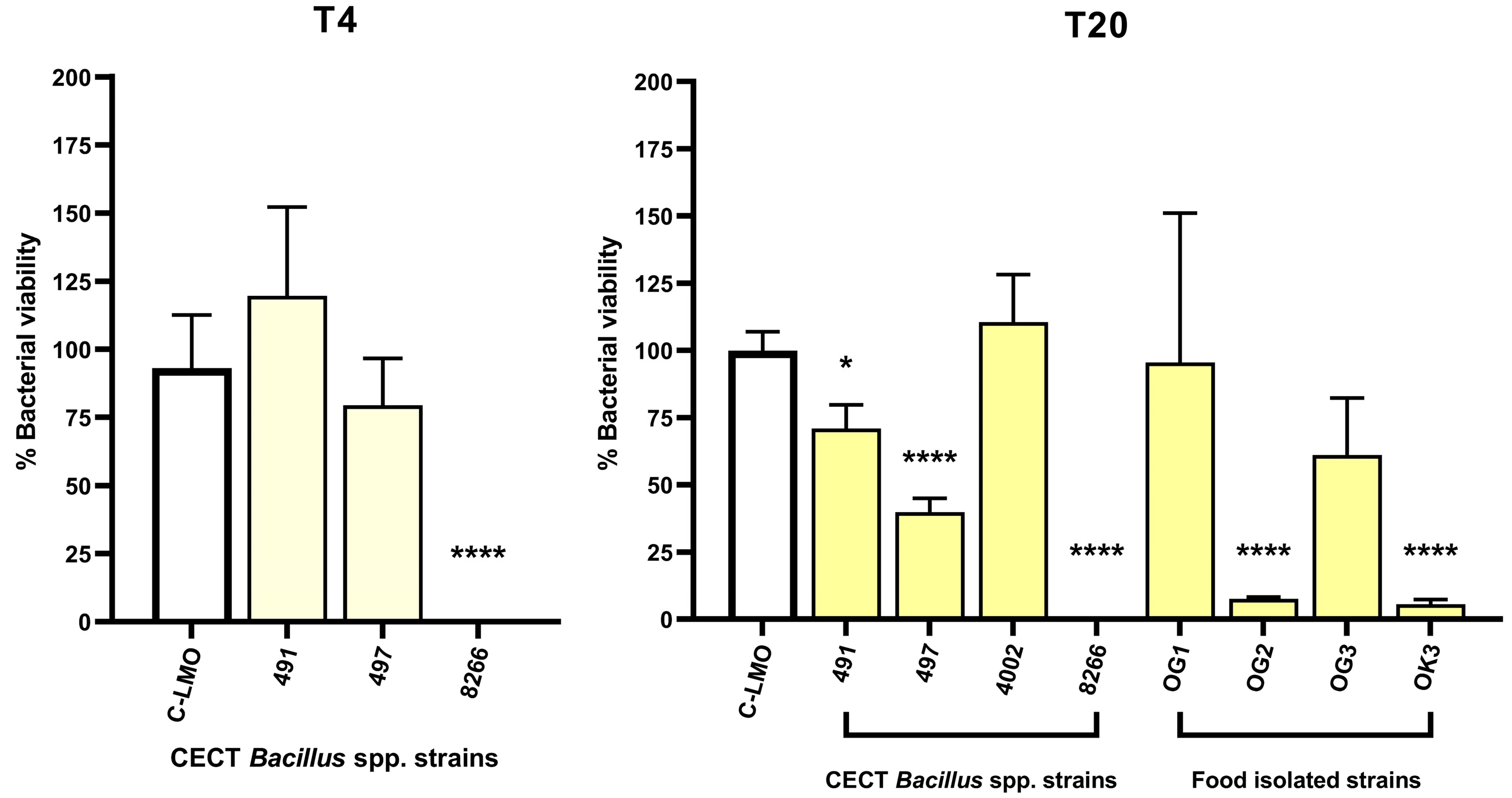
2.3. A Fluorescence-Based Infection Assay Determines Host Cell Protection Against L. monocytogenes
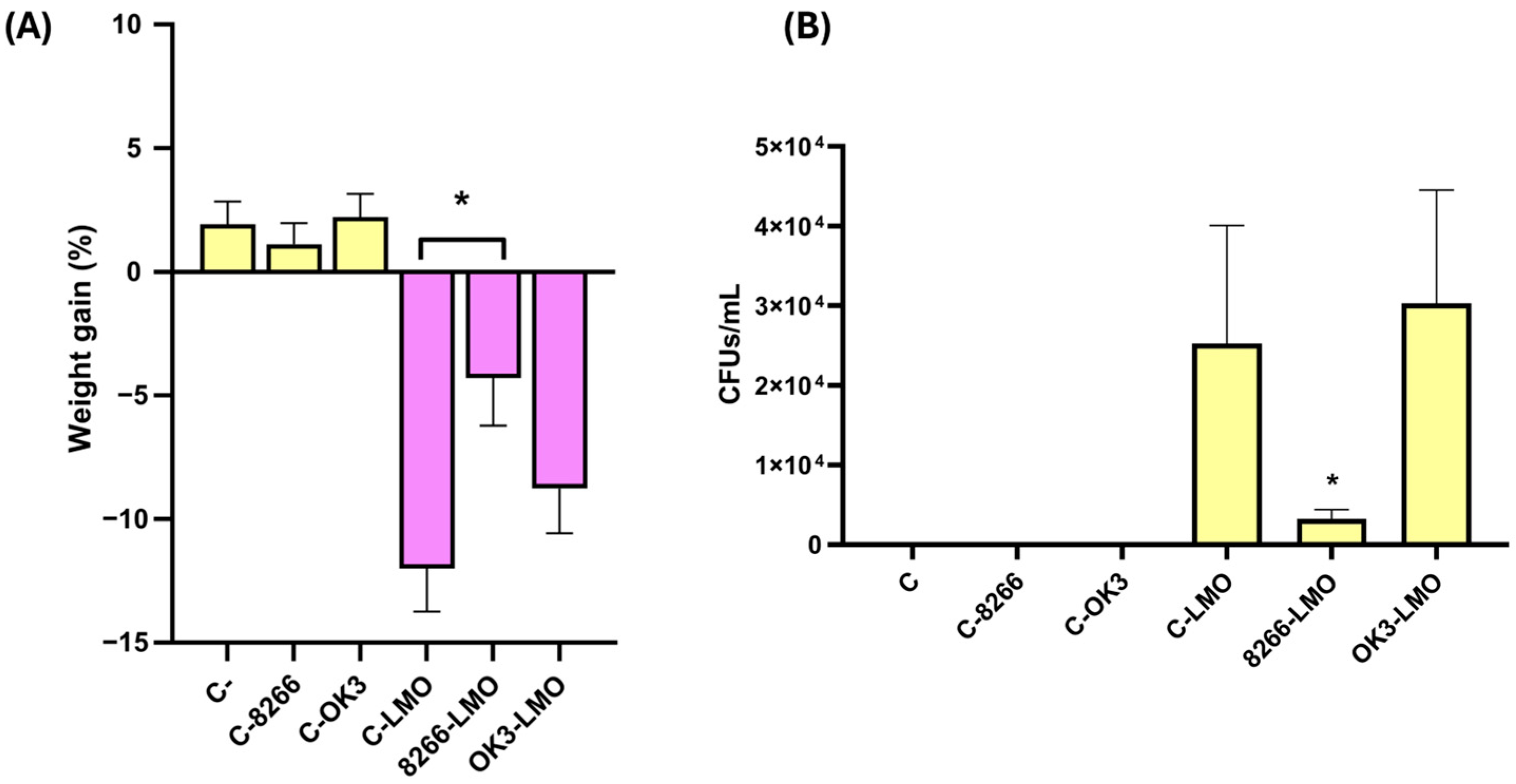
2.4. In Vivo Validation of Protective Strains in a Murine Model of Listeriosis
2.5. Host Cell Adhesion and Growth Dynamics
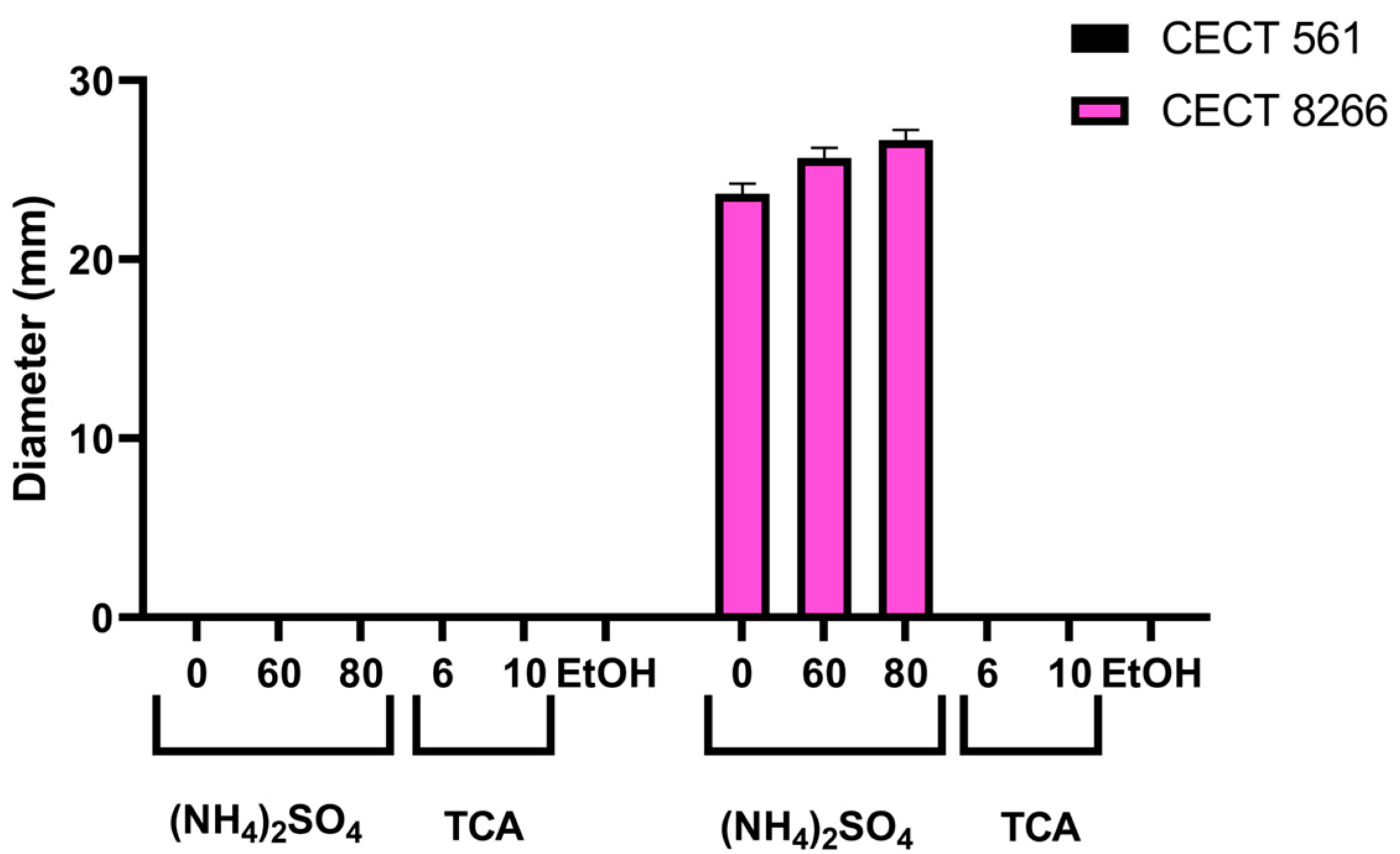
2.6. Evidence of Bacteriocin-Mediated Antimicrobial Activity in B. subtilis CECT 8266
2.7. Genomic Analysis of B. subtilis CECT 8266 Reveals Bacteriocin-Encoding Genes
2.8. Antibiotic Resistance
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Agar-Well Diffusion Tests and Determination of the Minimum Inhibitory Concentrations
4.3. Biliary Salts and pH Tests
4.4. Cellular Lines and Culture Conditions
4.5. Cell Viability Assay
4.6. Infection Assay
4.7. Adhesion Assay
4.8. Evaluation of Protective Effects of Bacillus Strains in a Mouse Model of L. monocytogenes Infection
4.9. Purification of Bacteriocins
4.10. DNA Sequencing, Annotation and Analysis
4.11. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McLauchlin, J.; Mitchell, R.T.; Smerdon, W.J.; Jewell, K. Listeria monocytogenes and Listeriosis: A Review of Hazard Characterisation for Use in Microbiological Risk Assessment of Foods. Int. J. Food Microbiol. 2004, 92, 15–33. [Google Scholar] [CrossRef]
- Rajabi, S.; Darban, D.; Tabatabaei, R.R.; Hosseini, F. Antimicrobial Effect of Spore-Forming Probiotics Bacillus laterosporus and Bacillus megaterium against Listeria monocytogenes. Arch. Microbiol. 2020, 202, 2791–2797. [Google Scholar] [CrossRef]
- Buchanan, R.L.; Gorris, L.G.M.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C. A Review of Listeria monocytogenes: An Update on Outbreaks, Virulence, Dose-Response, Ecology, and Risk Assessments. Food Control 2017, 75, 1–13. [Google Scholar] [CrossRef]
- Lungu, B.; O’Bryan, C.A.; Muthaiyan, A.; Milillo, S.R.; Johnson, M.G.; Crandall, P.G.; Ricke, S.C. Listeria monocytogenes: Antibiotic Resistance in Food Production. Foodborne Pathog. Dis. 2011, 8, 569–578. [Google Scholar] [CrossRef]
- Alonso-Hernando, A.; Prieto, M.; García-Fernández, C.; Alonso-Calleja, C.; Capita, R. Increase over Time in the Prevalence of Multiple Antibiotic Resistance among Isolates of Listeria monocytogenes from Poultry in Spain. Food Control 2012, 23, 37–41. [Google Scholar] [CrossRef]
- Halimi, B.; Dortu, C.; Arguelles-Arias, A.; Thonart, P.; Joris, B.; Fickers, P. Antilisterial Activity on Poultry Meat of Amylolysin, a Bacteriocin from Bacillus amyloliquefaciens GA1. Probiotics Antimicrob. Proteins 2010, 2, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, B.; Gerner-Smidt, P. The Epidemiology of Human Listeriosis. Microbes Infect. 2007, 9, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Gray, J.; Scott Chandry, P.; Fox, E.M. Phenotypic and Genotypic Analysis of Antimicrobial Resistance among Listeria monocytogenes Isolated from Australian Food Production Chains. Genes 2018, 9, 80. [Google Scholar] [CrossRef]
- Amrouche, T.; Noll, K.S.; Wang, Y.; Huang, Q.; Chikindas, M.L. Antibacterial Activity of Subtilosin Alone and Combined with Curcumin, Poly-Lysine and Zinc Lactate against Listeria monocytogenes Strains. Probiotics Antimicrob. Proteins 2010, 2, 250–257. [Google Scholar] [CrossRef]
- Jung, S.; Woo, C.; Fugaban, J.I.I.; Vazquez Bucheli, J.E.; Holzapfel, W.H.; Todorov, S.D. Bacteriocinogenic Potential of Bacillus amyloliquefaciens Isolated from Kimchi, a Traditional Korean Fermented Cabbage. Probiotics Antimicrob. Proteins 2021, 13, 1195–1212. [Google Scholar] [CrossRef]
- Thomas, J.; Govender, N.; McCarthy, K.M.; Erasmus, L.K.; Doyle, T.J.; Allam, M.; Ismail, A.; Ramalwa, N.; Sekwadi, P.; Ntshoe, G.; et al. Outbreak of Listeriosis in South Africa Associated with Processed Meat. N. Engl. J. Med. 2020, 382, 632–643. [Google Scholar] [CrossRef]
- Anupama, A.; Pattapulavar, V.; Christopher, J.G. The Past, Present, Future of Listeria monocytogenes: Understanding the Molecular Pathways, Antibiotic Resistance and Public Health Implications. Med. Microecol. 2025, 25, 100127. [Google Scholar] [CrossRef]
- Drolia, R.; Amalaradjou, M.A.R.; Ryan, V.; Tenguria, S.; Liu, D.; Bai, X.; Xu, L.; Singh, A.K.; Cox, A.D.; Bernal-Crespo, V.; et al. Receptor-Targeted Engineered Probiotics Mitigate Lethal Listeria Infection. Nat. Commun. 2020, 11, 6344. [Google Scholar] [CrossRef] [PubMed]
- Abriouel, H.; Franz, C.M.A.P.; Omar, N.B.; Galvez, A. Diversity and Applications of Bacillus Bacteriocins. FEMS Microbiol. Rev. 2011, 35, 201–232. [Google Scholar] [CrossRef] [PubMed]
- Khochamit, N.; Siripornadulsil, S.; Sukon, P.; Siripornadulsil, W. Antibacterial Activity and Genotypic-Phenotypic Characteristics of Bacteriocin-Producing Bacillus subtilis KKU213: Potential as a Probiotic Strain. Microbiol. Res. 2015, 170, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Settanni, L.; Corsetti, A. Application of Bacteriocins in Vegetable Food Biopreservation. Int. J. Food Microbiol. 2008, 121, 123–138. [Google Scholar] [CrossRef]
- Shelburne, C.E.; An, F.Y.; Dholpe, V.; Ramamoorthy, A.; Lopatin, D.E.; Lantz, M.S. The Spectrum of Antimicrobial Activity of the Bacteriocin Subtilosin A. J. Antimicrob. Chemother. 2007, 59, 297–300. [Google Scholar] [CrossRef]
- Willey, J.M.; Van Der Donk, W.A. Lantibiotics: Peptides of Diverse Structure and Function. Annu. Rev. Microbiol. 2007, 61, 477–501. [Google Scholar] [CrossRef]
- Elshaghabee, F.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus as Potential Probiotics: Status, Concerns, and Future Perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef]
- Choyam, S.; Jain, P.M.; Kammara, R. Characterization of a Potent New-Generation Antimicrobial Peptide of Bacillus. Front. Microbiol. 2021, 12, 710741. [Google Scholar] [CrossRef]
- Deegan, L.H.; Cotter, P.D.; Hill, C.; Ross, P. Bacteriocins: Biological Tools for Bio-Preservation and Shelf-Life Extension. Int. Dairy J. 2006, 16, 1058–1071. [Google Scholar] [CrossRef]
- Alvarez-Ordóñez, A.; Begley, M.; Clifford, T.; Deasy, T.; Considine, K.; O’Connor, P.; Paul Ross, R.; Hill, C. Investigation of the Antimicrobial Activity of Bacillus licheniformis Strains Isolated from Retail Powdered Infant Milk Formulae. Probiotics Antimicrob. Proteins 2014, 6, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Begley, M.; Cotter, P.D.; Hill, C.; Ross, R.P. Identification of a Novel Two-Peptide Lantibiotic, Lichenicidin, Following Rational Genome Mining for LanM Proteins. Appl. Environ. Microbiol. 2009, 75, 5451–5460. [Google Scholar] [CrossRef] [PubMed]
- Dischinger, J.; Josten, M.; Szekat, C.; Sahl, H.G.; Bierbaum, G. Production of the Novel Two-Peptide Lantibiotic Lichenicidin by Bacillus licheniformis DSM 13. PLoS ONE 2009, 4, e6788. [Google Scholar] [CrossRef] [PubMed]
- Saggese, A.; De Luca, Y.; Baccigalupi, L.; Ricca, E. An Antimicrobial Peptide Specifically Active against Listeria monocytogenes Is Secreted by Bacillus pumilus SF214. BMC Microbiol. 2022, 22, 3. [Google Scholar] [CrossRef]
- Rani, R.P.; Anandharaj, M.; Hema, S.; Deepika, R.; Ravindran, A.D. Purification of Antilisterial Peptide (Subtilosin A) from Novel Bacillus tequilensis FR9 and Demonstrate Their Pathogen Invasion Protection Ability Using Human Carcinoma Cell Line. Front. Microbiol. 2016, 7, 1910. [Google Scholar] [CrossRef]
- Obafemi, Y.D.; Oranusi, S.U.; Ajanaku, K.O.; Akinduti, P.A.; Leech, J.; Cotter, P.D. African Fermented Foods: Overview, Emerging Benefits, and Novel Approaches to Microbiome Profiling. NPJ Sci. Food 2022, 6, 15. [Google Scholar] [CrossRef]
- Bernardeau, M.; Lehtinen, M.J.; Forssten, S.D.; Nurminen, P. Importance of the Gastrointestinal Life Cycle of Bacillus for Probiotic Functionality. J. Food Sci. Technol. 2017, 54, 2570–2584. [Google Scholar] [CrossRef]
- Mackie, A.; Mulet-Cabero, A.I.; Torcello-Gomez, A. Simulating Human Digestion: Developing Our Knowledge to Create Healthier and More Sustainable Foods. Food Funct. 2020, 11, 9397–9431. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef]
- Van den Bossche, S.; Vandeplassche, E.; Ostyn, L.; Coenye, T.; Crabbé, A. Bacterial Interference with Lactate Dehydrogenase Assay Leads to an Underestimation of Cytotoxicity. Front. Cell Infect. Microbiol. 2020, 10, 494. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Shan, C.; Zhang, L.; Ge, D.; Wang, Y.; Xia, X.; Liu, X.; Zhou, J. A Novel Subtilin-like Lantibiotics Subtilin JS-4 Produced by Bacillus subtilis JS-4, and Its Antibacterial Mechanism against Listeria monocytogenes. LWT 2021, 142, 110993. [Google Scholar] [CrossRef]
- Xin, B.; Xu, H.; Liu, H.; Liu, S.; Wang, J.; Xue, J.; Zhang, F.; Deng, S.; Zeng, H.; Zeng, X.; et al. Identification and Characterization of a Novel Circular Bacteriocin, Bacicyclicin XIN-1, from Bacillus Sp. Xin1. Food Control 2021, 121, 107696. [Google Scholar] [CrossRef]
- Wang, J.; Xu, H.; Liu, S.; Song, B.; Liu, H.; Li, F.; Deng, S.; Wang, G.; Zeng, H.; Zeng, X.; et al. Toyoncin, a Novel Leaderless Bacteriocin That Is Produced by Bacillus toyonensis XIN-YC13 and Specifically Targets B. cereus and Listeria monocytogenes. Appl. Environ. Microbiol. 2021, 87, e00185-21. [Google Scholar] [CrossRef]
- Lin, L.Z.; Zheng, Q.W.; Wei, T.; Zhang, Z.Q.; Zhao, C.F.; Zhong, H.; Xu, Q.Y.; Lin, J.F.; Guo, L.Q. Isolation and Characterization of Fengycins Produced by Bacillus amyloliquefaciens JFL21 and Its Broad-Spectrum Antimicrobial Potential Against Multidrug-Resistant Foodborne Pathogens. Front. Microbiol. 2020, 11, 579621. [Google Scholar] [CrossRef]
- Sabaté, D.C.; Audisio, M.C. Inhibitory Activity of Surfactin, Produced by Different Bacillus subtilis subsp. subtilis Strains, against Listeria monocytogenes Sensitive and Bacteriocin-Resistant Strains. Microbiol. Res. 2013, 168, 125–129. [Google Scholar] [CrossRef]
- Deng, S.; Liu, S.; Li, X.; Liu, H.; Li, F.; Liu, K.; Zeng, H.; Zeng, X.; Xin, B. Thuricins: Novel Leaderless Bacteriocins with Potent Antimicrobial Activity Against Gram-Positive Foodborne Pathogens. J. Agric. Food Chem. 2022, 70, 9990–9999. [Google Scholar] [CrossRef]
- Hong, S.W.; Kim, J.H.; Cha, H.A.; Chung, K.S.; Bae, H.J.; Park, W.S.; Ham, J.S.; Park, B.Y.; Oh, M.H. Identification and Characterization of a Bacteriocin from the Newly Isolated Bacillus subtilis HD15 with Inhibitory Effects against Bacillus cereus. J. Microbiol. Biotechnol. 2022, 32, 1462–1470. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Z.; Zhang, S.; Li, P. Complete Genome Sequencing Revealed the Potential Application of a Novel Weizmannia coagulans PL-W Production with Promising Bacteriocins in Food Preservative. Foods 2023, 12, 216. [Google Scholar] [CrossRef]
- Zhang, X.; Xin, N.; Zhu, Z.; Li, X.; Dai, D.; Pan, C.; Peng, D.; Sun, M. Three Novel Leaderless Bacteriocins Have Antimicrobial Activity against Gram-Positive Bacteria to Serve as Promising Food Biopreservative. Microb. Cell Fact. 2022, 21, 194. [Google Scholar] [CrossRef]
- Goyal, C.; Malik, R.K.; Pradhan, D. Purification and Characterization of a Broad Spectrum Bacteriocin Produced by a Selected Lactococcus lactis Strain 63 Isolated from Indian Dairy Products. J. Food Sci. Technol. 2018, 55, 3683–3692. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Yang, H.; Bu, Y.; Yi, H.; Zhang, L.; Han, X.; Ai, L. Purification and Partial Characterization of Bacteriocin Lac-B23, a Novel Bacteriocin Production by Lactobacillus plantarum J23, Isolated from Chinese Traditional Fermented Milk. Front. Microbiol. 2018, 9, 2165. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, B.; Urdaci, M.C. Extracellular Proteins from Lactobacillus plantarum BMCM12 Prevent Adhesion of Enteropathogens to Mucin. Curr. Microbiol. 2012, 64, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Parente, E.; Ricciardi, A. Production, Recovery and Purification of Bacteriocins from Lactic Acid Bacteria. Appl. Microbiol. Biotechnol. 1999, 52, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Aseri, G.; Bhagwat, P.; Jain, N.; Ranveer, R.C. Production, Purification And Characterization Of A Novel Bacteriocin Produced By Bacillus subtilis Vs Isolated From Mango (Mangifera Indica L.). Braz. Arch. Biol. Technol. 2021, 64, e21190749. [Google Scholar] [CrossRef]
- de Araujo, L.V.; Abreu, F.; Lins, U.; de Melo Santa Anna, L.M.; Nitschke, M.; Freire, D.M.G. Rhamnolipid and Surfactin Inhibit Listeria monocytogenes Adhesion. Food Res. Int. 2011, 44, 481–488. [Google Scholar] [CrossRef]
- Ongena, M.; Jacques, P. Bacillus Lipopeptides: Versatile Weapons for Plant Disease Biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef]
- Nalli, Y.; Singh, S.; Gajjar, A.; Mahizhaveni, B.; Dusthackeer, V.N.A.; Shinde, P.B. Bacillibactin Class Siderophores Produced by the Endophyte Bacillus subtilis NPROOT3 as Antimycobacterial Agents. Lett. Appl. Microbiol. 2023, 76, ovac026. [Google Scholar] [CrossRef]
- Miao, S.; Liang, J.; Xu, Y.; Yu, G.; Shao, M. Bacillaene, Sharp Objects Consist in the Arsenal of Antibiotics Produced by Bacillus. J. Cell Physiol. 2023, 239, e30974. [Google Scholar] [CrossRef]
- Yuan, S.; Yong, X.; Zhao, T.; Li, Y.; Liu, J. Research Progress of the Biosynthesis of Natural Bio-Antibacterial Agent Pulcherriminic Acid in Bacillus. Molecules 2020, 25, 5611. [Google Scholar] [CrossRef]
- Islam, T.; Rabbee, M.F.; Choi, J.; Baek, K.H. Biosynthesis, Molecular Regulation, and Application of Bacilysin Produced by Bacillus Species. Metabolites 2022, 12, 397. [Google Scholar] [CrossRef]
- Grahovac, J.; Pajčin, I.; Vlajkov, V. Bacillus VOCs in the Context of Biological Control. Antibiotics 2023, 12, 581. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Guidance on the Assessment of Bacterial Susceptibility to Antimicrobials of Human and Veterinary Importance. EFSA J. 2012, 10, 2740. [Google Scholar] [CrossRef]
- Popoola, O.O.; Adepitan, D.S.; Adeyemi, A.S.; Oladeru, O.F.; Yusuff, S.I. A National Survey of the Antibiotic Use, Self-Medication Practices, and Knowledge of Antibiotic Resistance among Graduates of Tertiary Institutions in Nigeria. Sci. Afr. 2024, 23, e01978. [Google Scholar] [CrossRef]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial Competition: Surviving and Thriving in the Microbial Jungle. Nat. Rev. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef]
- Gray, J.; Chandry, P.S.; Kaur, M.; Kocharunchitt, C.; Fanning, S.; Bowman, J.P.; Fox, E.M. Colonisation Dynamics of Listeria monocytogenes Strains Isolated from Food Production Environments. Sci. Rep. 2021, 11, 12195. [Google Scholar] [CrossRef]
- Wang, H.; Huang, J.; Jiang, X. Perspectives on Using a Competitive Exclusion Approach to Control Listeria monocytogenes in Biological Soil Amendments of Animal Origin (BSAAO): A Review. Appl. Microbiol. 2023, 3, 786–804. [Google Scholar] [CrossRef]
- Akinsemolu, A.A.; Onyeaka, H.; Odion, S.; Adebanjo, I. Exploring Bacillus subtilis: Ecology, Biotechnological Applications, and Future Prospects. J. Basic Microbiol. 2024, 64, e2300614. [Google Scholar] [CrossRef]
- Vivant, A.L.; Garmyn, D.; Piveteau, P. Listeria monocytogenes, a down-to-Earth Pathogen. Front. Cell Infect. Microbiol. 2013, 4, 87. [Google Scholar] [CrossRef]
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the Antimicrobial Compounds Produced by Members of the Bacillus subtilis Group. Front. Microbiol. 2019, 10, 302. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, M.; Zheng, J.; Gänzle, M.G. Bacillus species in Food Fermentations: An Underappreciated Group of Organisms for Safe Use in Food Fermentations. Curr. Opin. Food Sci. 2023, 50, 101007. [Google Scholar] [CrossRef]
- Meade, E.; Slattery, M.A.; Garvey, M. Bacteriocins, Potent Antimicrobial Peptides and the Fight against Multi Drug Resistant Species: Resistance Is Futile? Antibiotics 2020, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, G.; Gueimonde, M.; Gomez-Gallego, C.; Delfederico, L.; Salminen, S. Correlation between in Vitro and in Vivo Assays in Selection of Probiotics from Traditional Species of Bacteria. Trends Food Sci. Technol. 2017, 68, 83–90. [Google Scholar] [CrossRef]
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, Natural Antimicrobials for Food Preservation. Int. J. Food Microbiol. 2001, 71, 1–20. [Google Scholar] [CrossRef]
- Sánchez, B.; Bressollier, P.; Urdaci, M.C. Exported Proteins in Probiotic Bacteria: Adhesion to Intestinal Surfaces, Host Immunomodulation and Molecular Cross-Talking with the Host. FEMS Immunol. Med. Microbiol. 2008, 54, 1–17. [Google Scholar] [CrossRef]
- Lewis, D.I. Animal Experimentation: Implementation and Application of the 3Rs. Emerg. Top. Life Sci. 2019, 3, 675–679. [Google Scholar] [CrossRef]
- Hoelzer, K.; Pouillot, R.; Dennis, S. Animal Models of Listeriosis: A Comparative Review of the Current State of the Art and Lessons Learned. Vet. Res. 2012, 43, 18. [Google Scholar] [CrossRef]
- Deekshit, V.K.; Srikumar, S. ‘To Be, or Not to Be’—The Dilemma of ‘Silent’ Antimicrobial Resistance Genes in Bacteria. J. Appl. Microbiol. 2022, 133, 2902–2914. [Google Scholar] [CrossRef]
- Jeżewska-Frąckowiak, J.; Seroczyńska, K.; Banaszczyk, J.; Jedrzejczak, G.; Żylicz-Stachula, A.; Skowron, P.M. The Promises and Risks of Probiotic Bacillus Species. Acta Biochim. Pol. 2018, 65, 509–519. [Google Scholar] [CrossRef]
- Marseglia, G.L.; Tosca, M.; Cirillo, I.; Licari, A.; Leone, M.; Marseglia, A.; Castellazzi, A.M.; Ciprandi, G. Efficacy of Bacillus clausii Spores in the Prevention of Recurrent Respiratory Infections in Children: A Pilot Study. Ther. Clin. Risk Manag. 2007, 3, 13–17. [Google Scholar] [CrossRef]
- Lefevre, M.; Racedo, S.M.; Ripert, G.; Housez, B.; Cazaubiel, M.; Maudet, C.; Jüsten, P.; Marteau, P.; Urdaci, M.C. Probiotic Strain Bacillus subtilis CU1 Stimulates Immune System of Elderly during Common Infectious Disease Period: A Randomized, Double-Blind Placebo-Controlled Study. Immun. Ageing 2015, 12, 24. [Google Scholar] [CrossRef]
- Bravo-Santano, N.; Boll, E.J.; Capern, L.C.; Cieplak, T.M.; Keleszade, E.; Letek, M.; Costabile, A. Comparative Evaluation of the Antimicrobial and Mucus Induction Properties of Selected Bacillus Strains against Enterotoxigenic Escherichia coli. Antibiotics 2020, 9, 849. [Google Scholar] [CrossRef] [PubMed]
- Humphries, R.M.; Ambler, J.; Mitchell, S.L.; Castanheira, M.; Dingle, T.; Hindler, J.A.; Koeth, L.; Sei, K. CLSI Methods Development and Standardization Working Group Best Practices for Evaluation of Antimicrobial Susceptibility Tests. J. Clin. Microbiol. 2018, 56, e01934-17. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Santano, N.; Ellis, J.K.; Mateos, L.M.; Calle, Y.; Keun, H.C.; Behrends, V.; Letek, M. Intracellular Staphylococcus aureus Modulates Host Central Carbon Metabolism to Activate Autophagy. mSphere 2018, 3, e00374-18. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Santano, N.; Stölting, H.; Cooper, F.; Bileckaja, N.; Majstorovic, A.; Ihle, N.; Mateos, L.M.; Calle, Y.; Behrends, V.; Letek, M. Host-Directed Kinase Inhibitors Act as Novel Therapies against Intracellular Staphylococcus aureus. Sci. Rep. 2019, 9, 4876. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving Bacterial Genome Assemblies from Short and Long Sequencing Reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-Scale Protein Function Classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for Taxonomy-Based Analysis of Pathways and Genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 Improves Signal Peptide Predictions Using Deep Neural Networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Kahsay, R.Y.; Gao, G.; Liao, L. An Improved Hidden Markov Model for Transmembrane Protein Detection and Topology Prediction and Its Applications to Complete Genomes. Bioinformatics 2005, 21, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; Van Wezel, G.P.; Medema, M.H.; Weber, T. AntiSMASH 6.0: Improving Cluster Detection and Comparison Capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef] [PubMed]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.L.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic Resistome Surveillance with the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef]
- da Silva Filho, A.C.; Raittz, R.T.; Guizelini, D.; De Pierri, C.R.; Augusto, D.W.; dos Santos-Weiss, I.C.R.; Marchaukoski, J.N. Comparative Analysis of Genomic Island Prediction Tools. Front. Genet. 2018, 9, 619. [Google Scholar] [CrossRef]
- Hart, T.; Moffat, J. BAGEL: A Computational Framework for Identifying Essential Genes from Pooled Library Screens. BMC Bioinform. 2016, 17, 164. [Google Scholar] [CrossRef]
- Letek, M.; Ordóñez, E.; Fernández-Natal, I.; Gil, J.A.; Mateos, L.M. Identification of the Emerging Skin Pathogen Corynebacterium amycolatum Using PCR-Amplification of the Essential divIVA Gene as a Target. FEMS Microbiol. Lett. 2006, 265, 256–263. [Google Scholar] [CrossRef]
- Xu, X.; Nielsen, L.J.D.; Song, L.; Maróti, G.; Strube, M.L.; Kovács, Á.T. Enhanced Specificity of Bacillus Metataxonomics Using a tuf-Targeted Amplicon Sequencing Approach. ISME Commun. 2023, 3, 126. [Google Scholar] [CrossRef]
- Rozewicki, J.; Li, S.; Amada, K.M.; Standley, D.M.; Katoh, K. MAFFT-DASH: Integrated Protein Sequence and Structural Alignment. Nucleic Acids Res. 2019, 47, W5–W10. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (ITOL) v5: An Online Tool for Phylogenetic Tree Display and Annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorente-Torres, B.; Castañera, P.; Ferrero, H.Á.; Fernández-Martínez, S.; Adejoh Ocholi, S.; Llano-Verdeja, J.; Javadimarand, F.; Carnicero-Mayo, Y.; Herrero-González, A.; Puente-Sanz, A.; et al. Streamlining Bacillus Strain Selection Against Listeria monocytogenes Using a Fluorescence-Based Infection Assay Integrated into a Multi-Tiered Validation Pipeline. Antibiotics 2025, 14, 765. https://doi.org/10.3390/antibiotics14080765
Lorente-Torres B, Castañera P, Ferrero HÁ, Fernández-Martínez S, Adejoh Ocholi S, Llano-Verdeja J, Javadimarand F, Carnicero-Mayo Y, Herrero-González A, Puente-Sanz A, et al. Streamlining Bacillus Strain Selection Against Listeria monocytogenes Using a Fluorescence-Based Infection Assay Integrated into a Multi-Tiered Validation Pipeline. Antibiotics. 2025; 14(8):765. https://doi.org/10.3390/antibiotics14080765
Chicago/Turabian StyleLorente-Torres, Blanca, Pablo Castañera, Helena Á. Ferrero, Sergio Fernández-Martínez, Suleiman Adejoh Ocholi, Jesús Llano-Verdeja, Farzaneh Javadimarand, Yaiza Carnicero-Mayo, Amanda Herrero-González, Alba Puente-Sanz, and et al. 2025. "Streamlining Bacillus Strain Selection Against Listeria monocytogenes Using a Fluorescence-Based Infection Assay Integrated into a Multi-Tiered Validation Pipeline" Antibiotics 14, no. 8: 765. https://doi.org/10.3390/antibiotics14080765
APA StyleLorente-Torres, B., Castañera, P., Ferrero, H. Á., Fernández-Martínez, S., Adejoh Ocholi, S., Llano-Verdeja, J., Javadimarand, F., Carnicero-Mayo, Y., Herrero-González, A., Puente-Sanz, A., Sainz Machín, I., Voigt, I. K., Guerrero Villanueva, S., López García, Á., Martín Gómez, E., Ogbonna, J. C., Gonzalo-Orden, J. M., Aparicio, J. F., Mateos, L. M., ... Letek, M. (2025). Streamlining Bacillus Strain Selection Against Listeria monocytogenes Using a Fluorescence-Based Infection Assay Integrated into a Multi-Tiered Validation Pipeline. Antibiotics, 14(8), 765. https://doi.org/10.3390/antibiotics14080765








