Advancing Nigerian Indigenous Poultry Health and Production, Use of Probiotics as Viable Alternatives to Antibiotics: A Review
Abstract
1. Introduction
2. An Overview of the Indigenous Poultry Industry in Nigeria
Antimicrobial Resistance
3. Biotics
3.1. Prebiotics
3.2. Synbiotics
3.3. Postbiotics
3.4. Probiotics
3.4.1. Characteristics of a Good Probiotic
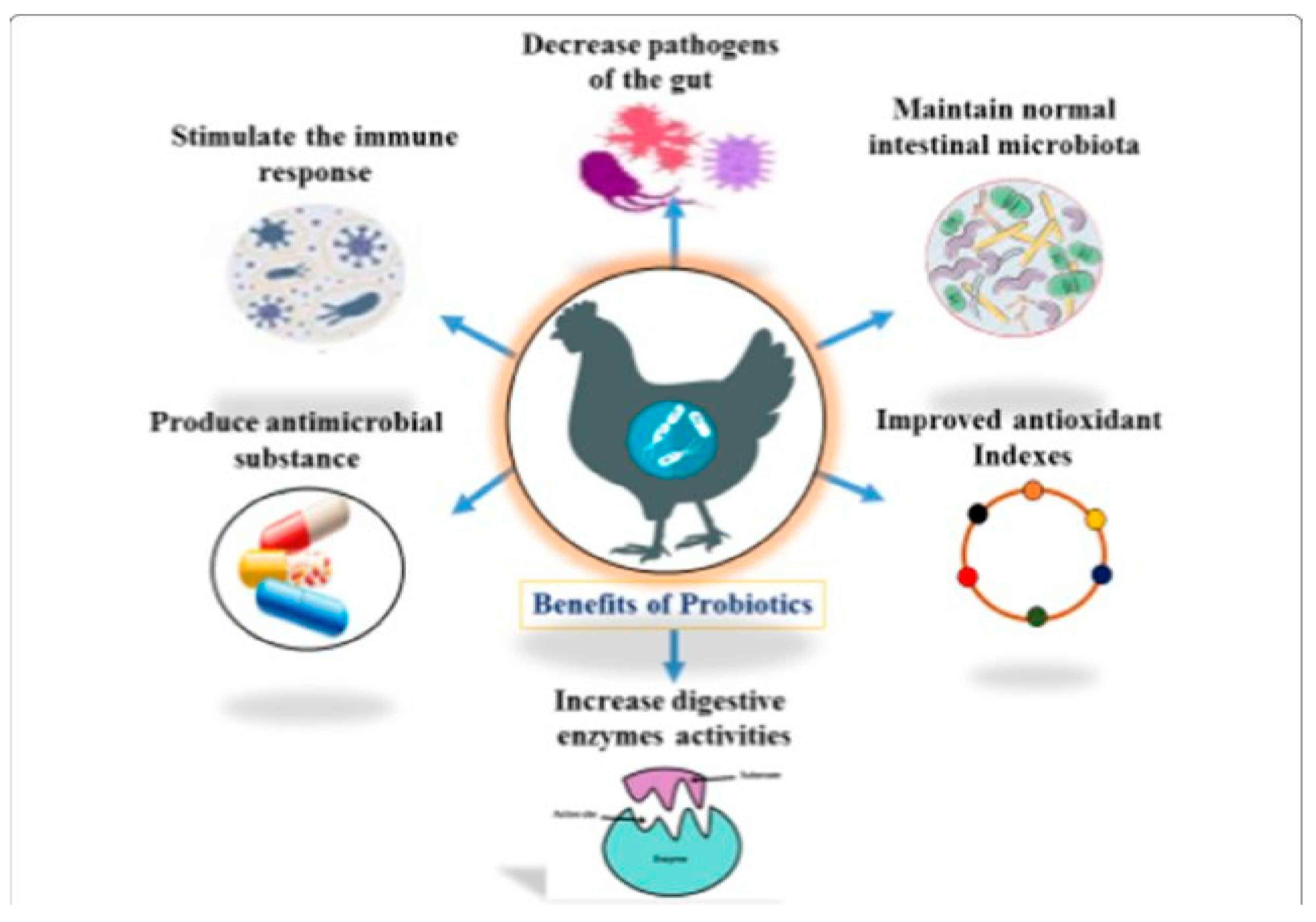
3.4.2. Role of Probiotics in Augmenting a Balanced Gut Microbiome and the Immune System
3.4.3. Role of Probiotics in Promoting Growth and Production Performance
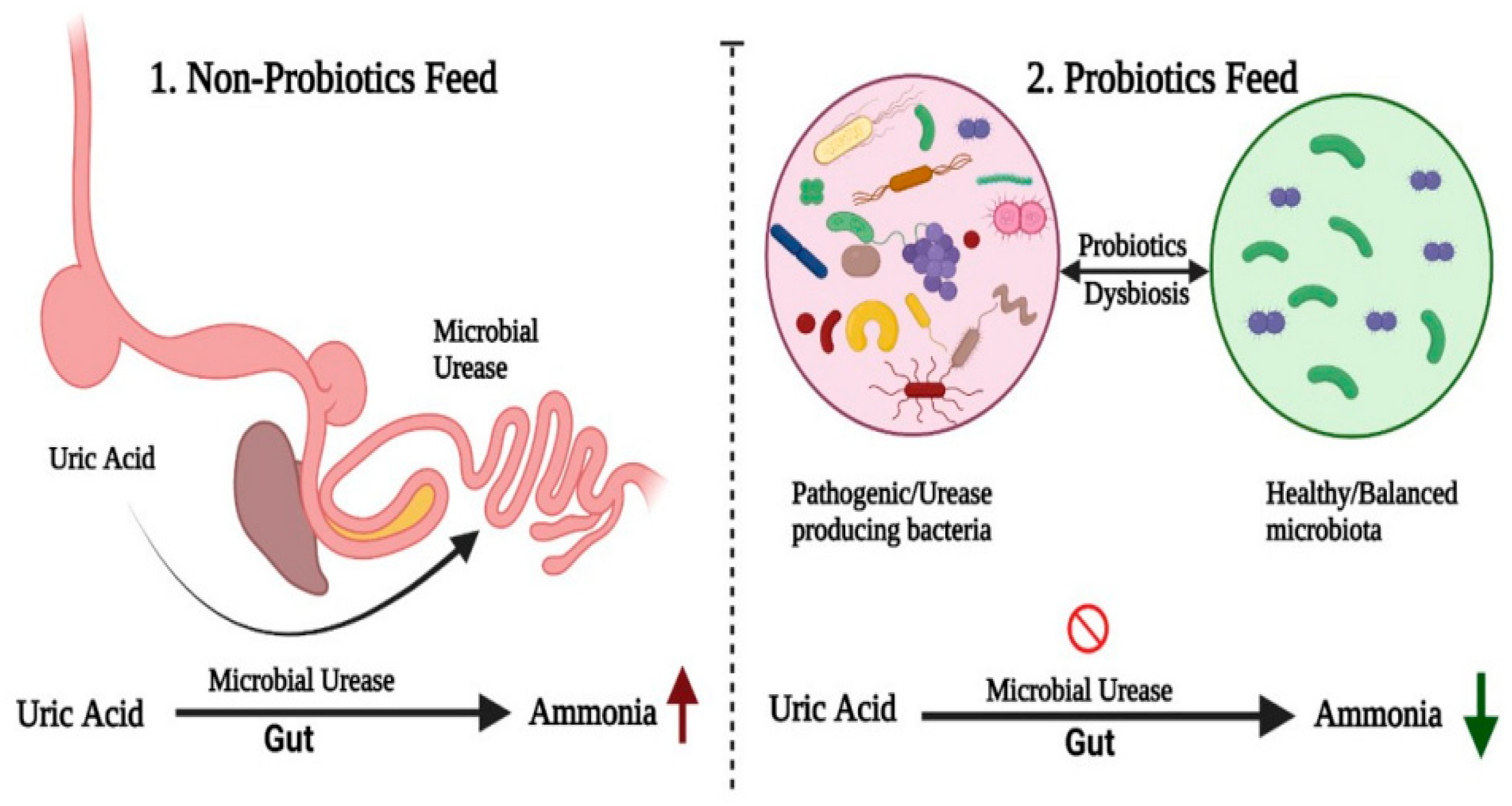
3.4.4. The Role of Probiotics in Reducing Ammonia, Hydrogen Sulphide, and Greenhouse Gas Emissions
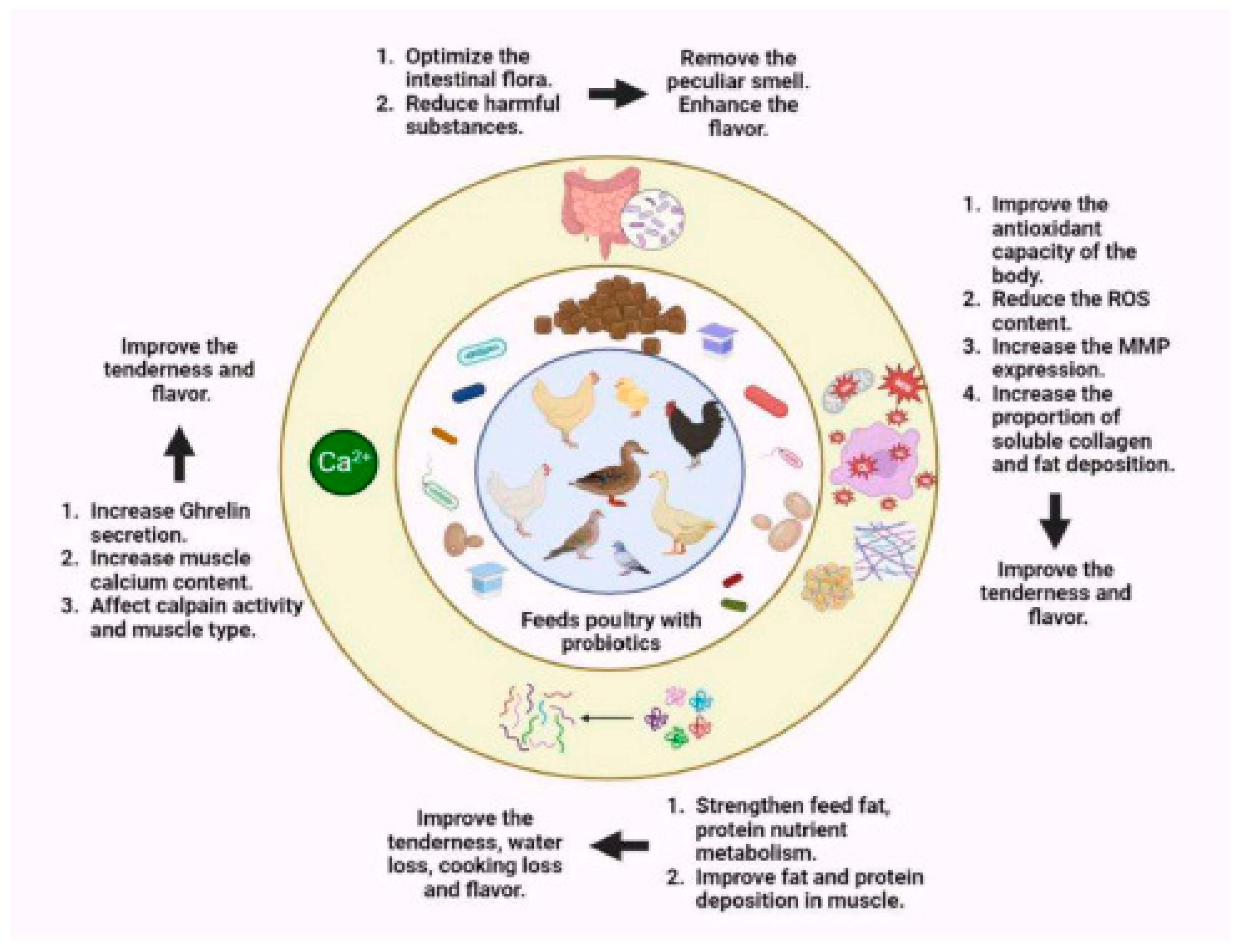
3.4.5. Meat Quality
3.4.6. Impact of Probiotics on Stress Management in Poultry
4. Challenges, Specific Knowledge Gaps, and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, P.; Vidyarthi, V.K. Impact of microplastic intake via poultry products: Environmental toxicity and human health. J. Hazard. Mater. Adv. 2024, 14, 100426. [Google Scholar] [CrossRef]
- Mnisi, C.M.; Mlambo, V.; Montso, P.K.; Manyeula, F.; Kumanda, C.; Moreki, J.C. Nutraceuticals as components of sustainable poultry production systems for food and nutrition security in Africa: A review. Agric. Food Secur. 2024, 13, 24. [Google Scholar] [CrossRef]
- Djikeng, A.; Olori, V.E.; Houaga, I.; Aggrey, S.E.; Mwai, O.; Ibeagha-Awemu, E.M.; Mrode, R.; Chagunda, M.G.G.; Tiambo, C.K.; Rekaya, R.; et al. The African Animal Breeding Network as a pathway towards genetic improvement of livestock. Nat. Genet. 2025, 57, 498–504. [Google Scholar] [CrossRef]
- United Nations. 9.7 Billion on Earth by 2050, But Growth Rate Slowing, Says New UN Population Report|UN News. 2019. Available online: https://news.un.org/en/story/2019/06/1040621 (accessed on 6 April 2025).
- Ponnampalam, E.N.; Holman, B.W.B. Sustainability II: Sustainable animal production and meat processing. In Lawrie’s Meat Science; Elsevier: Amsterdam, The Netherlands, 2023; pp. 727–798. [Google Scholar] [CrossRef]
- Galal, S. Livestock Population of Major Species in Africa as of 2022. Available online: https://www.statista.com/statistics/1290023/livestock-population-in-africa/ (accessed on 30 May 2025).
- Manyelo, T.G.; Selaledi, L.; Hassan, Z.M.; Mabelebele, M. Local Chicken Breeds of Africa: Their Description, Uses and Conservation Methods. Animals 2020, 10, 2257. [Google Scholar] [CrossRef]
- Rachman, M.P.; Bamidele, O.; Dessie, T.; Smith, J.; Hanotte, O.; Gheyas, A.A. Genomic analysis of Nigerian indigenous chickens reveals their genetic diversity and adaptation to heat-stress. Sci. Rep. 2024, 14, 2209. [Google Scholar] [CrossRef] [PubMed]
- Pewan, S.B.; Yibis, G.G.; Danbirni, S.; Nyam, P.P.; Tahir, I.; Mbap, S.T. Effects of seasons on haematologic and serum biochemical profiles of indigenous chickens in Shendam, Plateau State, Nigeria. Niger. J. Anim. Sci. 2019, 21, 77–84. [Google Scholar]
- Ikpeme, E.; Ekerette, E.E.; Job, I.E.; Umoyen, A.J.; Osim, P.B.; Ozoje, M.O. Genetic relationship among three Nigerian chicken (Gallus gallus) genotypes based on cytochrome-b of mitochondrial DNA. Asian J. Anim. Sci. 2020, 15, 35–42. [Google Scholar] [CrossRef]
- Kasimanickam, V.; Kasimanickam, M.; Kasimanickam, R. Antibiotics use in food animal production: Escalation of antimicrobial resistance: Where are we now in combating AMR? Med. Sci. 2021, 9, 14. [Google Scholar] [CrossRef]
- Dokuta, S.; Yadoung, S.; Hongjaisee, S.; Khamnoi, P.; Manochomphu, S.; Chuttong, B.; Hongsibsong, S. Seasonal Determination of Antibiotic-Resistant Microorganisms and Ciprofloxacin Residues in Pork and Chicken Meats Collected from Fresh Markets in Chiang Mai, Northern Thailand. Foods 2025, 14, 174. [Google Scholar] [CrossRef]
- Cortés-Avizanda, A.; Blanco, G.; De Vault, T.L.; Markandya, A.; Virani, M.Z.; Donázar, J.A. Supplementary feeding and endangered avian scavengers: Benefits, caveats and controversies. Front. Ecol. Environ. 2016, 14, 191–199. [Google Scholar] [CrossRef]
- Liu, T.; Shao, Y.; Pang, X.; Liu, Y.; Mo, X.; Chen, Z.; Lu, X. Intestinal microbiota and high-risk antibiotic resistance genes in wild birds with varied ecological traits: Insights from opportunistic direct sampling in Tianjin, China. Environ. Res. 2023, 263, 120040. [Google Scholar] [CrossRef] [PubMed]
- Ndahi, M.D.; Hendriksen, R.; Helwigh, B.; Card, R.M.; Fagbamila, I.O.; Abiodun-Adewusi, O.O.; Ekeng, E.; Adetunji, V.; Adebiyi, I.; Andersen, J.K. Determination of antimicrobial use in commercial poultry farms in Plateau and Oyo States, Nigeria. Antimicrob. Resist. Infect. Control. 2023, 12, 30. [Google Scholar] [CrossRef]
- Cullen, L.; Neale, D.; Kinchin, H. Exploring perceptions of bacteriophage use in the UK across the One Health spectrum: A roundtable discussion. Sustain. Microbiol. 2024, 1, qvae030. [Google Scholar] [CrossRef]
- Tran, C.; Horyanto, D.; Stanley, D.; Cock, I.E.; Chen, X.; Feng, Y. Antimicrobial Properties of Bacillus Probiotics as Animal Growth Promoters. Antibiotics 2023, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Li, L.; Hao, F.; Fang, Z.; Zhong, R.; Wu, J.; Fang, X. Improving quality of poultry and its meat products with probiotics, prebiotics, and phytoextracts. Poult. Sci. 2024, 103, 103287. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Afzal, Z.; Afzal, F.; Khan, R.U.; Elnesr, S.S.; Alagawany, M.; Chen, H. Use of postbiotic as growth promoter in poultry industry: A review of current knowledge and future prospects. Food Sci. Anim. Resour. 2023, 43, 1111. [Google Scholar] [CrossRef]
- Obianwuna, U.E.; Chang, X.; Oleforuh-Okoleh, V.U.; Onu, P.N.; Zhang, H.; Qiu, K.; Wu, S. Phytobiotics in poultry: Revolutionizing broiler chicken nutrition with plant-derived gut health enhancers. J. Anim. Sci. Biotechnol. 2024, 15, 169. [Google Scholar] [CrossRef]
- Olorundare, O.O.; Zrelovs, N.; Kabantiyok, D.; Svanberga, K.; Jansons, J.; Kazaks, A.; Agada, G.O.; Agu, C.G.; Morenikeji, O.R.; Oluwapelumi, O.A.; et al. Isolation and Characterization of a Novel Jumbo Phage HPP-Temi Infecting Pseudomonas aeruginosa Pa9 and Increasing Host Sensitivity to Ciprofloxacin. Antibiotics 2024, 13, 1006. [Google Scholar] [CrossRef]
- Lipilkina, T.A.; Xu, C.; Barbosa, M.d.S.; Khramova, V.N.; Shebeko, S.K.; Ermakov, A.M.; Ivanova, I.V.; Todorov, S.D. Beneficial and Safety Properties of a Bacteriocinogenic and Putative Probiotic Latilactobacillus sakei subsp. sakei 2a Strain. Foods 2024, 13, 3770. [Google Scholar] [CrossRef]
- Cowieson, A.J.; Kluenter, A.M. Contribution of exogenous enzymes to potentiate the removal of antibiotic growth promoters in poultry production. Anim. Feed Sci. Technol. 2019, 250, 81–92. [Google Scholar] [CrossRef]
- Marquardt, R.R.; Li, S. Antimicrobial resistance in livestock: Advances and alternatives to antibiotics. Anim. Front. 2018, 8, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Barman, I.; Seo, H.; Kim, S.; Rahim, M.A.; Yoon, Y.; Hossain, M.S.; Shuvo, S.H.; Song, H.Y. Isolation of New Strains of Lactic Acid Bacteria from the Vaginal Microbiome of Postmenopausal Women and their Probiotic Characteristics. Curr. Microbiol. 2025, 82, 76. [Google Scholar] [CrossRef]
- Jan, T.; Negi, R.; Sharma, B.; Kour, D.; Kumar, S.; Rai, A.K.; Rustagi, S.; Singh, S.; Sheikh, M.A.; Kumar, K.J.B. Diversity, distribution and role of probiotics for human health: Current research and future challenges. Biocatal. Agric. Biotechnol. 2023, 53, 102889. [Google Scholar] [CrossRef]
- Ebeid, T.A.; Al-Homidan, I.H.; Fathi Moataz, M. Physiological and immunological benefits of probiotics and their impacts in poultry productivity. World’s Poult Sci. J. 2021, 77, 883–899. [Google Scholar] [CrossRef]
- Halder, N.; Sunder, J.; De, A.K.; Bhattacharya, D.; Joardar, S.N. Probiotics in poultry: A comprehensive review. J. Basic Appl. Zool. 2024, 85, 23. [Google Scholar] [CrossRef]
- Shehata, A.A.; Yalçın, S.; Latorre, J.D.; Basiouni, S.; Attia, Y.A.; Abd El-Wahab, A.; Visscher, C.; El-Seedi, H.R.; Huber, C.; Hafez, H.M.; et al. Probiotics, Prebiotics, and Phytogenic Substances for Optimizing Gut Health in Poultry. Microorganisms 2022, 10, 395. [Google Scholar] [CrossRef]
- Lan, R.; Tran, H.; Kim, I. Effects of probiotic supplementation in different nutrient density diets on growth performance, nutrient digestibility, blood profiles, faecal microflora and noxious gas emission in weaning pig. J. Sci. Food Agric. 2017, 97, 1335–1341. [Google Scholar] [CrossRef]
- Herich, R.; Szabóová, R.; Karaffová, V.; Racines, M.P.; Šefcová, M.A.; Larrea-Álvarez, M. A Narrative Review on the Impact of Probiotic Supplementation on Muscle Development, Metabolic Regulation, and Fiber Traits Related to Meat Quality in Broiler Chickens. Microorganisms 2025, 13, 784. [Google Scholar] [CrossRef]
- Leão, A.P.A.; Alvarenga, R.R.; Zangeronimo, M.G. In Ovo Inoculation of Probiotics for Broiler Chickens: Systematic Review and Meta-Analysis. Anim. Feed Sci. Technol. 2021, 280, 115080. [Google Scholar] [CrossRef]
- Grace, D.; Knight-Jones, T.J.D.; Melaku, A.; Alders, R.; Jemberu, W.T. The Public Health Importance and Management of Infectious Poultry Diseases in Smallholder Systems in Africa. Foods 2024, 13, 411. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.T.; de Bruyn, J.; Bagnol, B.; Grieve, H.; Li, M.; Pym, R.; Alders, R.G. Small-scale poultry and food security in resource-poor settings: A review. Glob. Food Secur. 2017, 15, 43–52. [Google Scholar] [CrossRef]
- Odah, E.O.; Daikwo, S.I.; Mbap, S.T.; Okpanachi, U. Phenotypic characterization of local chickens (gallus gallus domesticus) in Bekwarra Cross River State, Nigeria. JSM Vet. Med. Res. 2019, 2, 1–7. [Google Scholar]
- Sokoya, O.O.; Babajide, J.M.; Shittu, T.A.; Sanwo, K.A.; Adegbite, J.A. Chemical and colour characterisation of breast meat from FUNAAB indigenous and Marshall broiler chickens. Trop. Anim. Health Prod. 2019, 51, 2575–2582. [Google Scholar] [CrossRef]
- Yakubu, A.; Dahloum, L.; Gimba, E.G. Smallholder cattle farmers’ breeding practices and trait preferences in a tropical guinea savanna agro-ecological zone. Trop. Anim. Health Prod. 2019, 51, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Desta, T.T. Indigenous village chicken production: A tool for poverty alleviation, the empowerment of women, and rural development. Trop. Anim. Health Prod. 2021, 53, 337. [Google Scholar] [CrossRef]
- Adesola, R.O.; Idris, I.; Bakre, A.A. Implementation of national poultry improvement plan in poultry disease control in Africa: Current perspectives, challenges, and prospects. Bull. Natl. Res. Cent. 2025, 49, 10. [Google Scholar] [CrossRef]
- Matloa, T.; Erasmus, R.; Ledwaba, M.B.; Malatji, D.P. Avian haemosporidian parasites affecting non-descript village chickens in Africa. Trop. Anim. Health Prod. 2025, 57, 28. [Google Scholar] [CrossRef]
- Enahoro, D.; Galiè, A.; Abukari, Y.; Chiwanga, G.H.; Kelly, T.R.; Kahamba, J.; Massawe, F.A.; Mapunda, F.; Jumba, H.; Weber, C.; et al. Strategies to upgrade animal health delivery in village poultry systems: Perspectives of stakeholders from Northern Ghana and Central Zones in Tanzania. Front. Vet. Sci. 2021, 8, 611357. [Google Scholar] [CrossRef] [PubMed]
- Ouma, E.A.; Kankya, C.; Dione, M.; Kelly, T.; Enahoro, D.; Chiwanga, G.; Abukari, Y.; Msoffe, P.; Kayang, B.B.; Zhou, H. Poultry health constraints in smallholder village poultry systems in Northern Ghana and Central Tanzania. Front. Vet. Sci. 2023, 10, 1159331. [Google Scholar] [CrossRef] [PubMed]
- Agusi, E.R.; Kabantiyok, D.; Mkpuma, N.; Atai, R.B.; Okongwu-Ejike, C.; Bakare, E.L.; Budaye, J.; Sule, K.G.; Rindaps, R.J.; James, G.K.; et al. Prevalence of multidrug-resistant Escherichia coli isolates and virulence gene expression in poultry farms in Jos, Nigeria. Front. Microbiol. 2024, 15, 1298582. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, S.H.; Li, X.; Rashid, A.; Su, J.; Xu, J.; Brejnrod, A.D.; Su, J.-Q.; Wu, Y.; Zhu, Y.-G.; Zhou, S.G.; et al. Co-selection of antibiotic resistance genes, and mobile genetic elements in the presence of heavy metals in poultry farm environments. Sci. Total Environ. 2021, 755, 142702. [Google Scholar] [CrossRef] [PubMed]
- Andrew Selaledi, L.; Mohammed Hassan, Z.; Manyelo, T.G.; Mabelebele, M. The Current Status of the Alternative Use to Antibiotics in Poultry Production: An African Perspective. Antibiotics 2020, 9, 594. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Manphweli, S.; Meyer, E.; Okoh, A. Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Gadde, U.; Kim, W.H.; Oh, S.T.; Lillehoj, H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: A review. Anim. Health Res. Rev. 2017, 18, 26–45. [Google Scholar] [CrossRef]
- Rushton, J. Anti-microbial use in animals: How to assess the trade-offs. Zoonoses Public Health 2015, 62, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Van, T.T.H.; Yidana, Z.; Smooker, P.M.; Coloe, P.J. Antibiotic use in food animals in the world with focus on Africa: Pluses and minuses. J. Glob. Antimicrob. Resist. 2020, 20, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Ronquillo, M.G.; Hernandez, J.C.A. Antibiotic and synthetic growth promoters in animal diets: Review of impact and analytical methods. Food Control 2017, 72, 255–267. [Google Scholar] [CrossRef]
- Oladeji, O.M.; Mugivhisa, L.L.; Olowoyo, J.O. Antibiotic Residues in Animal Products from Some African Countries and Their Possible Impact on Human Health. Antibiotics 2025, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Lateefat, H.M.; Olaniyi, O.A.; Misbahu, G.; Raimi, O.M. A wake-up call: Determination of antibiotics residue level in raw meat in abattoir and selected slaughterhouses in five local government in Kano State, Nigeria. bioRxiv 2022. [Google Scholar] [CrossRef]
- Abbas, W.; Bi, R.; Hussain, M.D.; Tajdar, A.; Guo, F.; Guo, Y.; Wang, Z. Antibiotic Cocktail Effects on Intestinal Microbial Community, Barrier Function, and Immune Function in Early Broiler Chickens. Antibiotics 2024, 13, 413. [Google Scholar] [CrossRef]
- Ire, F.S.; Njoku, T.T.; Ahaotu, I.; Maduka, N. Bioefficacy performance and meat quality of a commercial feed supplemented with probiotics on broilers. Sci. Afr. 2024, 23, 213–230. [Google Scholar]
- Nagpala, M.J.; Mora, J.F.; Pavon, R.D.; Rivera, W.L. Genomic characterization of antimicrobial-resistant Salmonella enterica in chicken meat from wet markets in Metro Manila, Philippines. Front. Microbiol. 2025, 16, 1496685. [Google Scholar] [CrossRef]
- Danaei, B.; Sarmastzadeh, T.; Khalili, F.; Yazarlou, F.; Centis, R.; D’Ambrosio, L.; Sotgiu, G.; Migliori, G.B.; Nasiri, M.J. The battle against colistin-resistant E. coli and the need for a one health approach. New Microbes New Infect. 2023, 54, 101161. [Google Scholar] [CrossRef]
- Bacanlı, M.G. The two faces of antibiotics: An overview of the effects of antibiotic residues in foodstuffs. Arch. Toxicol. 2024, 98, 1717–1725. [Google Scholar] [CrossRef]
- Ghimpețeanu, O.M.; Pogurschi, E.N.; Popa, D.C.; Dragomir, N.; Drăgotoiu, T.; Mihai, O.D.; Petcu, C.D. Antibiotic Use in Livestock and Residues in Food—A Public Health Threat: A Review. Foods 2022, 16, 1430. [Google Scholar] [CrossRef]
- Lhermie, G.; Grohn, Y.T.; Raboisson, D. Addressing antimicrobial resistance: An overview of priority actions to prevent suboptimal antimicrobial use in food-animal production. Front. Microbiol. 2016, 7, 2114. [Google Scholar] [CrossRef]
- Sardar, D.; Afsana, S.; Habib, A.; Hossain, T. Dietary effects of multi-strain probiotics as an alternative to antibiotics on growth performance, carcass characteristics, blood profiling and meat quality of broilers. Vet. Integr. Sci. 2025, 23, 255–267. [Google Scholar] [CrossRef]
- Murungi, M.; Vudriko, P.; Ndagije, H.B.; Kesi, D.N.; Serwanga, A.; Rajab, K.; Manirakiza, L.; Waswa, J.P.; Kasujja, H.; Barigye, M.; et al. National-Level Consumption of Antimicrobials in the Veterinary Sector in Uganda: A Report on Analysis of Import Data for 2021. Antibiotics 2025, 14, 150. [Google Scholar] [CrossRef]
- Odey, T.O.J.; Tanimowo, W.O.; Afolabi, K.O.; Jahid, I.K.; Reuben, R.C. Antimicrobial use and resistance in food animal production: Food safety and associated concerns in Sub-Saharan Africa. Int. Microbiol. 2024, 27, 1–23. [Google Scholar] [CrossRef]
- Craig, J.; Hiban, K.; Frost, I.; Kapoor, G.; Alimi, Y.; Varma, J.K. Comparison of national antimicrobial treatment guidelines, African Union. Bull. World Health Org. 2022, 100, 50. [Google Scholar] [CrossRef] [PubMed]
- Gulumbe, B.H.; Haruna, U.A.; Almazan, J.; Ibrahim, I.H.; Faggo, A.A.; Bazata, A.Y. Combating the menace of antimicrobial resistance in Africa: A review on stewardship, surveillance and diagnostic strategies. Biol. Proced. Online 2022, 24, 19. [Google Scholar] [CrossRef] [PubMed]
- Tukaram, N.M.; Biswas, A.; Deo, C.; Laxman, A.J.; Monika, M.; Tiwari, A.K. Effects of paraprobiotic as replacements for antibiotic on performance, immunity, gut health and carcass characteristics in broiler chickens. Sci. Rep. 2022, 12, 22619. [Google Scholar] [CrossRef]
- Akhavan, N.; Hrynkiewicz, K.; Thiem, D.; Randazzo, C.; Walsh, A.M.; Guinan, K.J.T.J.; Stadnicka, K. Evaluation of probiotic growth stimulation using prebiotic ingredients to optimize compounds for in ovo delivery. Front. Microbiol. 2023, 14, 1242027. [Google Scholar] [CrossRef]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the future of probiotics and prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef]
- Teng, P.Y.; Kim, W.K. Roles of prebiotics in intestinal ecosystem of broilers. Front. Vet. Sci. 2018, 5, 245. [Google Scholar] [CrossRef]
- Xing, M.; Cao, Q.; Wang, Y.; Xiao, H.; Zhao, J.; Zhang, Q.; Ji, A.; Song, S. Advances in research on the bioactivity of alginate oligosaccharides. Mar. Drugs 2020, 18, 144. [Google Scholar] [CrossRef] [PubMed]
- Morgan, N.K. Advances in prebiotics for poultry: Role of the caeca and Oligosaccharides. Anim. Prod. Sci. 2023, 63, 1911–1925. [Google Scholar] [CrossRef]
- Liu, L.; Li, Q.; Yang, Y.; Guo, A. Biological Function of Short-Chain Fatty Acids and Its Regulation on Intestinal Health of Poultry. Front. Vet. Sci. 2021, 8, 736739. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Abelardo, M.; Gueimonde, M.; Reyes-Gavilán, C.G.d.L.; Salazar, N. Intestinal short-chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Shini, S.; Bryden, W.L. Probiotics and gut health: Linking gut homeostasis and poultry productivity. Anim. Prod. Sci. 2021, 62, 1090–1112. [Google Scholar] [CrossRef]
- Jiang, S.; Mohammed, A.; Jacobs, J.; Cramer, T.; Cheng, H. Effect of synbiotics on thyroid hormones, intestinal histomorphology, and heat shock protein 70 expression in broiler chickens reared under cyclic heat stress. Poult. Sci. 2020, 99, 142–150. [Google Scholar] [CrossRef]
- Hossain, H.; Nuradji, H.; Miah, M.Y.; Islam, M.N.; Siddiqui, M.S.I. Impact of synbiotic on growth performance, histo-architectural modulation of lymphoid organ, hematology, blood biochemistry and humoral immune response in naked neck chicken. Trop. Anim. Health Prod. 2025, 57, 4. [Google Scholar] [CrossRef]
- Bueno, D.J.; Latorre, J.D.; Shehata, A.A.; Eisenreich, W.; Tellez, G. Strategies to attack pathogenic avian microorganisms: From probiotics to postbiotics. Ger. J. Vet. Res. 2024, 4, 95–118. [Google Scholar] [CrossRef]
- Youssef, I.M.; Elsherbeni, A.I.; Almuraee, A.A.; Nass, N.M.; Beyari, E.A.; Alshammarii, N.M.; Abdel-Ghany, A.M.; Ahmed, E.S.G.; Nasr, S.; Youssef, K.M.; et al. Influence of using synbiotics by various routes on Mandarah male chicks: Intestinal bacterial counts, gut morphology and histological status. Poult. Sci. 2024, 103, 103601. [Google Scholar] [CrossRef]
- Sayed, Y.; Hassan, M.; Salem, H.M.; Al-Amry, K.; Eid, G.E. Probiotics/prebiotics effect on chicken gut microbiota and immunity in relation to heat-stress and climate-change mitigation. J. Therm. Biol. 2025, 129, 104097. [Google Scholar] [CrossRef]
- Olowoyeye, J.C. Biotics as Sustainable Alternatives to Antibiotics in Nigerian Poultry Farming. Asian J. Food Res. Nutr. 2025, 4, 522–533. [Google Scholar] [CrossRef]
- Hossain, M.I.; Mizan, M.F.R.; Roy, P.K.; Nahar, S.; Toushik, S.H.; Ashrafudoulla, M.; Jahid, I.K.; Lee, J.; Ha, S.D. Listeria monocytogenes biofilm inhibition on food contact surfaces by application of postbiotics from Lactobacillus curvatus B.67 and Lactobacillus plantarum M.2. Food Res. Int. 2021, 148, 110595. [Google Scholar] [CrossRef]
- Urban, J.; Kareem, K.Y.; Atanasov, A.G.; Matuszewski, A.; Bień, D.; Ciborowska, P.; Rygało-Galewska, A.; Michalczuk, M. Postbiotics, a natural feed additive for growth performance, gut microbiota and quality of poultry products. Curr. Res. Biotechnol. 2024, 8, 100247. [Google Scholar] [CrossRef]
- Chaney, W.E.; Naqvi, S.A.; Gutierrez, M.; Gernat, A.; Johnson, T.J.; Petry, D. Dietary Inclusion of a Saccharomyces cerevisiae-Derived Postbiotic Is Associated with Lower Salmonella enterica Burden in Broiler Chickens on a Commercial Farm in Honduras. Microorganisms 2022, 10, 544. [Google Scholar] [CrossRef]
- Diez-Gutiérrez, L.; San Vicente, L.; Barrón, L.J.R.; del Carmen Villarán, M.; Chávarri, M. Gamma-aminobutyric acid and probiotics: Multiple health benefits and their future in the global functional food and nutraceuticals market. J. Funct. Foods 2020, 64, 103669. [Google Scholar] [CrossRef]
- Chaluvadi, S.; Hotchkiss, A.T.; Yam, K.L. Gut microbiota: Impact of probiotics, prebiotics, synbiotics, pharmabiotics, and postbiotics on human health. In Probiotics, Prebiotics, and Synbiotics: Bioactive Foods in Health Promotion; Academic Press: Cambridge, MA, USA, 2015; pp. 515–523. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Khodaii, Z.; Ghaderian, S.M.H.; Natanzi, M.M. Probiotic Bacteria and their Supernatants Protect Enterocyte Cell Lines from Enteroinvasive Escherichia coli (EIEC) Invasion. Int. J. Mol. Cell. Med. 2017, 6, 183–189. [Google Scholar] [PubMed]
- Otto, J.R.; Mwangi, F.W.; Pewan, S.B.; Holman, B.W.B.; Malau-Aduli, A.E.O. Emerging applications of postbiotics to sustainable livestock production systems. Aust. J. Agric. Vet. Anim. Sci. 2025, 1, 100002. [Google Scholar]
- Danladi, Y.; Teck, C.H.; Hooi, L.F. Effect of supplementation of postbiotics and pararobiotics on the immune response of broiler chickens. Niger. J. Anim. Prod. 2024, 1311–1316. [Google Scholar] [CrossRef]
- Çam, G.; Akın, N.; Konak Göktepe, Ç.; Demirci, T. Pea (Pisum sativum L.) pod powder as a potential enhancer of probiotic Enterococcus faecium M74 in ice cream and its physicochemical, structural, and sensory effects. J. Sci. Food Agric. 2023, 103, 3184–3193. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef] [PubMed]
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; Philippart de Foy, J.M.; de Timary Dequenne, I.; Cani, P.D. How probiotics affect the microbiota. Front. Cell. Infect. Microbiol. 2020, 9, 454. [Google Scholar] [CrossRef]
- Rasinkangas, P.; Forssten, S.D.; Marttinen, M.; Ibarra, A.; Bothe, G.; Junnila, J.; Uebelhack, R.; Donazzolo, Y.; Ouwehand, A.C. Bifidobacterium animalis subsp. lactis Bi-07 supports lactose digestion in vitro and in randomized, placebo- and lactase-controlled clinical trials. Am. J. Clin. Nutr. 2022, 116, 1580–1594. [Google Scholar] [CrossRef]
- Sałański, P.; Kowalczyk, M.; Bardowski, J.K.; Szczepankowska, A.K. Health-Promoting nature of Lactococcus lactis IBB109 and Lactococcus lactis IBB417 strains exhibiting proliferation inhibition and stimulation of interleukin-18 expression in colorectal cancer cells. Front. Microbiol. 2022, 13, 822912. [Google Scholar] [CrossRef]
- Wang, S.; Ren, H.; Zhong, H.; Zhao, X.; Li, C.; Ma, J.; Gu, X.; Xue, Y.; Huang, S.; Yang, J.; et al. Combined berberine and probiotic treatment as an effective regimen for improving postprandial hyperlipidemia in type 2 diabetes patients: A double blinded placebo controlled randomized study. Gut Microbes 2022, 14, 2003176. [Google Scholar] [CrossRef]
- Zommiti, M.; Feuilloley, M.G.J.; Connil, N. Update of Probiotics in Human World: A Nonstop Source of Benefactions till the End of Time. Microorganisms 2020, 8, 1907. [Google Scholar] [CrossRef]
- Kabir, S.L. Dietary probiotics in poultry: A game-changer for growth, immunity, and microbiota balance. Asian J. Med. Biol. Res. 2025, 11, 1–4. [Google Scholar] [CrossRef]
- Diaz Carrasco, J.M.; Casanova, N.A.; Fernández Miyakawa, M.E. Microbiota, Gut Health and Chicken Productivity: What Is the Connection? Microorganisms 2019, 7, 374. [Google Scholar] [CrossRef]
- Krysiak, K.; Konkol, D.; Korczyński, M. Overview of the Use of Probiotics in Poultry Production. Animals 2021, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Fusco, V.; Wu, R.; Zhang, W.; Zhai, Q. Editorial: Role of probiotics and probiotics’ metabolites in food and intestine. Front. Microbiol. 2023, 14, 1183550. [Google Scholar] [CrossRef]
- Brito Sampaio, K.; Fusco, V.; de Brito Alves, J.L.; Leite de Souza, E. Chapter 1—Probiotics: Concepts, evolution, and applications. In Probiotics for Human Nutrition in Health and Disease; Leite de Souza, E., de Brito Alves, J.L., Fusco, V., Eds.; Academic Press: Cambridge, MA, USA, 2022; ISBN 9780323899086. [Google Scholar]
- Idowu, P.A.; Mpofu, T.J.; Magoro, A.M.; Modiba, M.C.; Nephawe, K.A.; Mtileni, B. Impact of probiotics on chicken gut microbiota, immunity, behavior, and productive performance—A systematic review. Front. Anim. Sci. 2025, 6, 1562527. [Google Scholar] [CrossRef]
- Al-Shawi, S.G.; Dang, D.S.; Yousif, A.Y.; Al-Younis, Z.K.; Najm, T.A.; Matarneh, S.K. The Potential Use of Probiotics to Improve Animal Health, Efficiency, and Meat Quality: A Review. Agriculture 2020, 10, 452. [Google Scholar] [CrossRef]
- Ezema, C. Probiotics in animal production: A review. J. Vet. Med. Anim. Health 2013, 5, 308–316. [Google Scholar]
- de Melo Pereira, G.V.; de Oliveira Coelho, B.; Magalhães Júnior, A.I.; Thomaz-Soccol, V.; Soccol, C.R. How to select a probiotic? A review and update of methods and criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef]
- Sarita, B.; Samadhan, D.; Hassan, M.Z.; Kovaleva, E.G. A comprehensive review of probiotics and human health-current prospective and applications. Front. Microbiol. 2025, 15, 1487641. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics mechanism of action on immune cells and beneficial effects on human health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]
- Marková, K.; Kreisinger, J.; Vinkler, M. Are there consistent effects of gut microbiota composition on performance, productivity and condition in poultry? Poult. Sci. 2024, 103, 103752. [Google Scholar] [CrossRef]
- Dinalli, V.P.; Costa, M.C.; Venâncio, E.J.; Filho, J.A.B.; Bessegatto, J.A.; Holkem, A.T.; Alfieri, A.A.A.; da Silva, C.A.; Oba, A. Impact of Chlorella vulgaris and probiotic supplementation on performance, immunity and intestinal microbiota of broiler chickens. PLoS ONE 2025, 20, e0313736. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Prajapati, K.; Bisani, K.; Prajapati, H.; Prajapati, S.; Agrawal, D.; Singh, S.; Saraf, M.; Goswami, D. Advances in probiotics research: Mechanisms of action, health benefits, and limitations in applications. Syst. Microbiol. Biomanufacturing 2024, 4, 386–406. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, J.; Zhong, H.; Li, N.; Xu, H.; Zhu, Q.; Liu, P. Effect of probiotics on the meat flavour and gut microbiota of chicken. Sci. Rep. 2017, 7, 6400. [Google Scholar] [CrossRef] [PubMed]
- Emami, N.K.; Calik, A.; White, M.B.; Kimminau, E.A.; Dalloul, R.A. Effect of Probiotics and Multi-Component Feed Additives on Microbiota, Gut Barrier and Immune Responses in Broiler Chickens During Subclinical Necrotic Enteritis. Front. Vet. Sci. 2020, 7, 572142. [Google Scholar] [CrossRef]
- Rychlik, I. Composition and Function of Chicken Gut Microbiota. Animals 2020, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Moore, R.J.; Stanley, D.; Chousalkar, K.K. The gut microbiota of laying hens and its manipulation with prebiotics and probiotics to enhance gut health and food safety. Appl. Environ. Microbiol. 2020, 86, e00600-20. [Google Scholar] [CrossRef]
- Bist, R.B.; Bist, K.; Poudel, S.; Subedi, D.; Yang, X.; Paneru, B.; Mani, S.; Wang, D.; Chai, L. Sustainable poultry farming practices: A critical review of current strategies and future prospects. Poult. Sci. 2024, 103, 104295. [Google Scholar] [CrossRef]
- Park, I.; Lee, Y.; Goo, D.; Zimmerman, N.P.; Smith, A.H.; Rehberger, T.; Lillehoj, H.S. The effects of dietary Bacillus subtilis supplementation, as a alternative to antibiotics, on growth performance, intestinal immunity, and epithelial barrier integrity in broiler chickens infected with Eimeria maxima. Poult. Sci. 2020, 99, 725–733. [Google Scholar] [CrossRef]
- Ritzi, M.M.; Abdelrahman, W.; Mohnl, M.; Dalloul, R.A. Effects of probiotics and application methods on performance and response of broiler chickens to an Eimeria challenge. Poult. Sci. 2014, 93, 2772–2778. [Google Scholar] [CrossRef]
- Jha, R.; Das, R.; Oak, S.; Mishra, P. Probiotics (Direct-Fed Microbials) in Poultry Nutrition and Their Effects on Nutrient Utilization, Growth and Laying Performance, and Gut Health: A Systematic Review. Animals 2020, 10, 1863. [Google Scholar] [CrossRef]
- Zhen, R.; Feng, J.; He, D.; Chen, Y.; Chen, T.; Cai, W.; Xiong, Y.; Qiu, Y.; Jiang, Z.; Wang, L.; et al. Effects of Niacin on Resistance to Enterotoxigenic Escherichia coli Infection in Weaned Piglets. Front. Nutr. 2022, 9, 865311. [Google Scholar] [CrossRef]
- Gondim, D.R.; Cecilia, J.A.; Rodrigues, T.N.B.; Vilarrasa-García, E.; Rodríguez-Castellón, E.; Azevedo, D.C.S.; Silva, I.J., Jr. Protein Adsorption onto Modified Porous Silica by Single and Binary Human Serum Protein Solutions. Int. J. Mol. Sci. 2021, 22, 9164. [Google Scholar] [CrossRef]
- Yangbo, J.; Weiqiang, H.; Qihang, Z.; Lin, L.; Jie, Z.; Yongfu, C. Inactivated Lactiplantibacillus plantarum Ps-8 enhances growth performance and intestinal health in broiler chickens via gut microbiota and serum metabolite modulation. Poult. Sci. 2025, 104, 105611. [Google Scholar] [CrossRef]
- Rodríguez-Sorrento, A.; Castillejos, L.; López-Colom, P.; Cifuentes-Orjuela, G.; Rodríguez-Palmero, M.; Moreno-Muñoz, J.A.; Martín-Orúe, S.M. Effects of Bifidobacterium longum subsp. infantis CECT 7210 and Lactobacillus rhamnosus HN001, combined or not with oligofructose-enriched inulin, on weaned pigs orally challenged with Salmonella typhimurium. Front. Microbiol. 2020, 11, 2012. [Google Scholar] [CrossRef] [PubMed]
- Tsuruda, C.T.; Souza, P.C.D.; Nishio, E.K.; Almeida, R.S.; Panagio, L.A.; Baptista, A.A.S.; Garcia, S.; Pinheiro, D.O.; Araújo, E.J.A.; Chue-Gonçalves, M.; et al. Lactobacillus rhamnosus V5 prevents Salmonella enterica serovar typhimurium invasion in cell culture and mice infection. Microbiol. Res. J. Int. 2020, 30, 44–57. [Google Scholar] [CrossRef]
- Borda-Molina, D.; Vital, M.; Sommerfeld, V.; Rodehutscord, M.; Camarinha-Silva, A. Insights Into Broilers′ Gut Microbiota Fed with Phosphorus, Calcium, and Phytase Supplemented Diets. Front. Microbiol. 2016, 7, 228625. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, H.; Zhang, L.; Su, Y.; Shi, D.; Xiao, H.; Tian, Y. High-Throughput Sequencing Technology to Reveal the Composition and Function of Cecal Microbiota in Dagu Chicken. BMC Microbiol. 2016, 16, 259. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The firmicutes/bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8836–8847. [Google Scholar] [CrossRef] [PubMed]
- Bemark, M.; Pitcher, M.J.; Dionisi, C.; Spencer, J. Gut-associated lymphoid tissue: A microbiota-driven hub of B cell immunity. Trends Immunol. 2024, 45, 211–223. [Google Scholar] [CrossRef]
- Broom, L.J.; Kogut, M.H. The role of the gut microbiome in shaping the immune system of chickens. Vet. Immunol. Immunopathol. 2018, 204, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Eicher, S.D.; Applegate, T.J. Development of intestinal mucin 2, IgA, and polymeric Ig receptor expressions in broiler chickens and Pekin ducks. Poult. Sci. 2015, 94, 172–180. [Google Scholar] [CrossRef]
- Gyawali, I.; Zhou, G.; Xu, G.; Li, G.; Wang, Y.; Zeng, Y.; Li, J.; Zhou, J.; Zhu, C.; Shu, G.; et al. Supplementation of microencapsulated probiotics modulates gut health and intestinal microbiota. Food Sci. Nutr. 2023, 11, 4547–4561. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Rajput, D.S.; Zeng, D.; Khalique, A.; Rajput, S.S.; Wang, H.; Zhao, Y.; Sun, N.; Ni, X. Pretreatment with probiotics ameliorates gut health and necrotic enteritis in broiler chickens, as a substitute to antibiotics. AMB Express 2020, 10, 220. [Google Scholar] [CrossRef]
- Ayalew, H.; Zhang, H.; Wang, J.; Wu, S.; Qiu, K.; Qi, G.; Tekeste, A.; Wassie, T.; Chanie, D. Potential feed additives as antibiotic alternatives in broiler production. Front. Vet. Sci. 2022, 9, 916473. [Google Scholar] [CrossRef]
- Korver, D.R. Intestinal Nutrition: Role of Vitamins and Biofactors and Gaps of Knowledge. Poult. Sci. 2022, 101, 101665. [Google Scholar] [CrossRef]
- Sachdeva, A.; Tomar, T.; Malik, T.; Bains, A.; Karnwal, A. Exploring probiotics as a sustainable alternative to antimicrobial growth promoters: Mechanisms and benefits in animal health. Front. Sustain. Food Syst. 2025, 8, 1523678. [Google Scholar] [CrossRef]
- Grozina, A.A.; Ilina, L.A.; Laptev, G.Y.; Yildirim, E.A.; Ponomareva, E.S.; Filippova, V.A.; Tyurina, D.G.; Fisinin, V.I.; Kochish, I.I.; Griffin, D.K.; et al. Probiotics as an Alternative to Antibiotics in Modulating the Intestinal Microbiota and Performance of Broiler Chickens. J. Appl. Microbiol. 2023, 134, lxad213. [Google Scholar] [CrossRef]
- Adli, D.N.; Sholikin, M.M.; Sitaresmi, P.I.; Ani Nurgiartiningsih, V.M.; Crooijmans, R.P. Dose-Dependent Effects of Probiotics on the Reproductive Performance, Egg Characteristics, and Seminal Traits of Broiler Breeders: A Model-Based Meta-Analysis. J. Anim. Physiol. Anim. Nutr. 2025, 1–19. [Google Scholar] [CrossRef]
- McFarland, L.V. Efficacy of single-strain probiotics versus multi-strain mixtures: Systematic review of strain and disease specificity. Dig. Dis. Sci. 2021, 66, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, G.; Qu, G.; Liu, B.; Zhang, X.; Li, G.; Jin, N.; Li, C.; Bai, J.; Zhao, C. Effects of dietary Lactobacillus rhamnosus GG supplementation on the production performance, egg quality, eggshell ultrastructure, and lipid metabolism of late-phase laying hens. BMC Vet. Res. 2023, 19, 150. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Chen, X.; Zheng, A.; Liu, G.; Ren, Y.; Chen, Z. Dietary supplementation of compound probiotics to improve performance, egg quality, biochemical parameters and intestinal morphology of laying hens. Front. Vet. Sci. 2024, 11, 1505151. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Wang, B.; Zhang, H.; Qiu, K.; Wu, S. The change of albumen quality during the laying cycle and its potential physiological and molecular basis of laying hens. Poult. Sci. 2024, 103, 104004. [Google Scholar] [CrossRef] [PubMed]
- Dangsawat, O.; Rattanawut, J.; Srisawat, T.; Sowanpreecha, R.; Tang Phuc Khang, L.; Srinual, O.; Hung, N.D.; Do-Hyung, K.; Husna, N.N.; Dwinanti, S.H.; et al. Bacillus aryabhattai CKNJh11 as a promising probiotic improves growth performance and egg quality in laying hens. Sci. Rep. 2025, 15, 13659. [Google Scholar] [CrossRef]
- Zou, Q.; Fan, X.; Xu, Y.; Wang, T.; Li, D. Effects of dietary supplementation probiotic complex on growth performance, blood parameters, fecal harmful gas, and fecal microbiota in AA+ male broilers. Front. Microbiol. 2022, 13, 1088179. [Google Scholar] [CrossRef]
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef]
- Lee, A.R.; Kim, S.H.; Kim, S.K. Effects of supplementation with a novel strain of Paenibacillus konkukensis on laying performance, tibial characteristics, and caecal microbiota in laying hens. Ital. J. Anim. Sc. 2025, 24, 827–841. [Google Scholar] [CrossRef]
- Muneeb, M.; Khan, E.U.; Ali, M.; Suleman, M.; Shaheen, M.S.; Zafar, M.S.; Ahmad, S. Effects of replacing antibiotics with probiotics and antimicrobial peptides on performance, gut health, carcass traits, meat quality, and welfare in broilers infected with Eimeria and Clostridium perfringens. Trop. Anim. Health Prod. 2025, 57, 184. [Google Scholar] [CrossRef]
- Zeng, X.; Li, Q.; Yang, C.; Yu, Y.; Fu, Z.; Wang, H.; Fan, X.; Yue, X.; Xu, Y. Effects of Clostridium butyricum- and Bacillus spp.-Based Potential Probiotics on the Growth Performance, Intestinal Morphology, Immune Responses, and Caecal Microbiota in Broilers. Antibiotics 2021, 10, 624. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Bourassa, D. Probiotics in Poultry: Unlocking Productivity Through Microbiome Modulation and Gut Health. Microorganisms 2025, 13, 257. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-A.; Bayo, J.; Cha, J.; Choi, Y.J.; Jung, M.Y.; Kim, D.-H.; Kim, Y.; Du, C. Investigating the probiotic characteristics of four microbial strains with potential application in feed industry. PLoS ONE 2019, 14, e0218922. [Google Scholar] [CrossRef]
- Kalus, K.; Opaliński, S.; Maurer, D.; Rice, S.; Koziel, J.A.; Korczyński, M.; Dobrzański, Z.; Kołacz, R.; Gutarowska, B. Odour reducing microbial-mineral additive for poultry manure treatment. Front. Environ. Sci. Eng. 2017, 11, 7. [Google Scholar] [CrossRef]
- Khalid, A.; Khalid, F.; Mahreen, N.; Hussain, S.M.; Shahzad, M.M.; Khan, S.; Wang, Z. Effect of Spore-Forming Probiotics on the Poultry Production: A Review. Food Sci. Anim. Resour. 2022, 42, 968–980. [Google Scholar] [CrossRef]
- Mi, J.; Chen, X.; Liao, X. Screening of single or combined administration of probiotics to reduce ammonia emissions from laying hens. Poult. Sci. 2019, 98, 3977–3988. [Google Scholar] [CrossRef] [PubMed]
- Misiukiewicz, A.; Gao, M.; Filipiak, W.; Cieslak, A.; Patra, A.K.; Szumacher-Strabel, M. Review: Methanogens and methane production in the digestive systems of non-ruminant farm animals. Animals 2021, 15, 100060. [Google Scholar] [CrossRef]
- Adamu, G.; Hassim, H.A.; Kumar, P.; Sazili, A.Q.; Mohd Zainudin, M.H. Controlling odour emissions in poultry production through dietary interventions: Prospects and challenges. World’s Poult. Sci. J. 2024, 80, 1101–1122. [Google Scholar] [CrossRef]
- Pewan, S.B.; Otto, J.R.; Huerlimann, R.; Budd, A.M.; Mwangi, F.W.; Edmunds, R.C.; Holman, B.W.B.; Henry, M.L.E.; Kinobe, R.T.; Adegboye, O.A.; et al. Genetics of Omega-3 Long-Chain Polyunsaturated Fatty Acid Metabolism and Meat Eating Quality in Tattykeel Australian White Lambs. Genes 2020, 11, 587. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations. Meat Quality 2014. Available online: http://www.fao.org/ag/againfo/themes/en/meat/quality_meat (accessed on 12 April 2025).
- Mohammed, A.A.; Zaki, R.S.; Negm, E.A.; Mahmoud, M.A.; Cheng, H.W. Effects of dietary supplementation of a probiotic (Bacillus subtilis) on bone mass and meat quality of broiler chickens. Poult. Sci. 2021, 100, 100906. [Google Scholar] [CrossRef]
- Das, A.K.; Nanda, P.; Madane, P.; Biswas, S.; Das, A.; Zhang, W.; Lorenzo, J.M. A comprehensive review on antioxidant dietary fibre enriched meat-based functional foods. Trends Food Sci. Technol. 2020, 99, 323–336. [Google Scholar] [CrossRef]
- Franco, L.; Boulianne, M.; Parent, E.; Barjesteh, N.; Costa, M.C. Colonization of the Gastrointestinal Tract of Chicks with Different Bacterial Microbiota Profiles. Animals 2023, 13, 2633. [Google Scholar] [CrossRef]
- Rubio, L.A. Possibilities of early life programming in broiler chickens via intestinal microbiota modulation. Poult. Sci. 2019, 98, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Al-Yemni, A.H.; Al-Abdullatif, A.A.; Attia, Y.A.; Hussein, E. Effects of different dietary levels of blue lupine (Lupinus angustifolius) seed meal with or without probiotics on the performance, carcass criteria, immune organs, and gut morphology of broiler chickens. Front. Vet. Sci. 2020, 7, 124. [Google Scholar] [CrossRef]
- Neveling, D.P.; van Emmenes, L.; Ahire, J.J.; Pieterse, E.; Smith, C.; Dicks, L.M.T. Effect of a Multi-Species Probiotic on the Colonisation of Salmonella in Broilers. Probiotics Antimicrob. Proteins 2020, 12, 896–905. [Google Scholar] [CrossRef]
- Shanmugasundaram, R.; Markazi, A.; Mortada, M.; Ng, T.T.; Applegate, T.J.; Bielke, L.R.; Syed, B.; Pender, C.M.; Curry, S.; Murugesan, G.R.; et al. Research Note: Effect of synbiotic supplementation on caecal Clostridium perfringens load in broiler chickens with different necrotic enteritis challenge models. Poult. Sci. 2020, 99, 2452–2458. [Google Scholar] [CrossRef]
- Lokapirnasari, W.P.; Pribadi, T.B.; Al Arif, A.; Soeharsono, S.; Hidanah, S.; Harijani, N.; Najwan, R.; Huda, K.; Wardhani, H.C.P.; Rahman, N.F.N.; et al. Potency of probiotics Bifidobacterium spp. and Lactobacillus casei to improve growth performance and business analysis in organic laying hens. Vet. World 2019, 12, 860–867. [Google Scholar] [CrossRef]
- Tsai, M.-Y.; Shih, B.-L.; Liaw, R.-B.; Chen, W.-T.; Lee, T.-Y.; Hung, H.-W.; Hung, K.-H.; Lin, Y.-F. Effect of Dietary Supplementation of Bacillus subtilis TLRI 211-1 on Laying Performance, Egg Quality and Blood Characteristics of Leghorn Layers. Anim. Biosci. 2023, 36, 609–618. [Google Scholar] [CrossRef]
- Saili, T.; Aka, R.; Auza, F.A.; Salido, W.L.; Sari, A.M.; Napirah, A. Production Performance of Local Village Chicken Fed by Agriculture By-Product Supplemented with Herbal Probiotics and Mud Clams Extract (Polymesoda erosa) in Kendari-South-East Sulawesi. J. Peternak. Indones. 2019, 21, 327. [Google Scholar] [CrossRef]
- Gao, H.; Xiao, J.; Zhang, Q.; Man, H.; Tang, X.; Wang, Z.; Fang, R.; Jiang, S. Effect of supplementation continuously in pullet and early lay period with Bacillus subtilis and yeast cell wall on the intestinal morphology, bone parameters, and egg quality of hens. Front. Vet. Sci. 2025, 12, 1584627. [Google Scholar] [CrossRef]
- Khogali, M.K.; Wen, K.; Jauregui, D.; Malik, H.E.; Liu, L.; Zhao, M.; Gong, D.; Geng, T. Probiotics-induced changes in intestinal structure and gut microbiota are associated with reduced rate of pimpled eggs in the late laying period of hens. J. Poult. Sci. 2022, 59, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, C.; Wang, H.; Tang, H.; Chen, Z.; Dou, X.; Cheng, J.; Li, Z.; Wang, Z.; Mei, Y.; et al. Probiotic potential of enterococcus lactis in improving egg production and quality in quails during late egg-laying period. Poult. Sci. 2025, 104, 104765. [Google Scholar] [CrossRef]
- Ezema, C.; Eze, D.C. Probiotic effect of yeast (Saccharomyces cerevisiae) on hen-day egg performance, serum and egg cholesterol levels in laying chicken. Pak. J. Nutr. 2015, 14, 44–46. [Google Scholar] [CrossRef][Green Version]
- Hossain, M.T.; Sardar, D.; Afsana, S.; Datta, M.; Habib, M.A. Comparative analysis between multi-strain probiotics and antibiotic as starter feed supplement of poultry on growth performance, serum metabolites and meat quality. Vet. Anim. Sci. 2024, 24, 100346. [Google Scholar] [CrossRef]
- Tang, X.; Liu, X.; Liu, H. Effects of Dietary Probiotic (Bacillus subtilis) Supplementation on Carcass Traits, Meat Quality, Amino Acid, and Fatty Acid Profile of Broiler Chickens. Front. Vet. Sci. 2021, 8, 767802. [Google Scholar] [CrossRef]
- Tajudeen, H.; Ha, S.H.; Hosseindoust, A.; Mun, J.Y.; Park, S.; Park, S.; Choi, P.S.; Hermes, R.G.; Taechavasonyoo, A.; Rodriguez, R.; et al. Effect of dietary inclusion of Bacillus-based probiotics on performance, egg quality, and the faecal microbiota of laying hens. Anim. Biosci. 2024, 37, 689–696. [Google Scholar] [CrossRef]
- Iakubchak, O.M.; Vivych, A.Y.; Hryb, J.V.; Danylenko, S.H.; Taran, T.V. Production and Meat Quality of Broiler Chickens with the Use of a Probiotic Complex of Bifidobacteria and Lactobacilli. Regul. Mech. Biosyst. 2024, 15, 477–482. [Google Scholar] [CrossRef]
- Ivanović, S.; Baltić, M.Ž.; Popov-Raljić, J.; Pisinov, B.; Maslić-Strižak, D.; Stojanović, Z.; Pavlović, I. The effect of different probiotics on broiler meat quality. Afr. J. Microbiol. Res. 2012, 6, 937–943. [Google Scholar] [CrossRef]
- Begdildayeva, N.; Karahan, A.G.; Kondybayev, A.; Kudaibergenova, A.; Nurgazina, A.; Akhmetsadykov, N.; Ospanova, A.; Akhmetsadykova, S.; Wang, D.Y. Probiotics effects on the growth performance and meat quality of broiler chickens. Anim. Prod. Sci. 2024, 64, AN23424. [Google Scholar] [CrossRef]
- Freitas, A.S.; Carvalho, L.M.; Soares, A.L.; Oliveira, M.E.S.; Madruga, M.S.; Neto, A.C.S.; Carvalho, R.H.; Ida, E.I.; Shimokomaki, M. Pale, soft and exudative (PSE) and dark, firm and dry (DFD) meat determination in broiler chicken raised under tropical climate management conditions. Int. J. Poult. Sci. 2017, 16, 81–87. [Google Scholar] [CrossRef]
- Sharipova, A.; Khaziev, D.; Kanareikina, S.; Kanareikin, V.; Rebezov, M.; Kazanina, M.; Andreeva, A.; Okuskhanova, E.; Yessimbekov, Z.; Bykova, O. The effects of a probiotic dietary supplementation on the amino acid and mineral composition of broilers meat. Annu. Res. Rev. Biol. 2017, 21, 1–7. [Google Scholar] [CrossRef]
- Jadhav, V.V.; Han, J.; Fasina, Y.; Harrison, S.H. Connecting gut microbiomes and short chain fatty acids with the serotonergic system and behavior in Gallus gallus and other avian species. Front. Physiol. 2022, 13, 1035538. [Google Scholar] [CrossRef]
- Bernabucci, U. Climate change: Impact on livestock and how can we adapt. Anim. Front. 2019, 9, 3–5. [Google Scholar] [CrossRef]
- Chen, S.; Yong, Y.; Ju, X. Effect of heat stress on growth and production performance of livestock and poultry: Mechanism to prevention. J. Therm. Biol. 2021, 99, 103019. [Google Scholar] [CrossRef] [PubMed]
- Oni, A.I.; Abiona, J.A.; Fafiolu, A.O.; Oke, O.E. Early-age thermal manipulation and supplemental antioxidants on physiological, biochemical and productive performance of broiler chickens in hot-tropical environments. Stress 2024, 27, 2319803. [Google Scholar] [CrossRef] [PubMed]
- Kikusato, M.; Xue, G.; Pastor, A.; Niewold, T.A.; Toyomizu, M. Effects of plant-derived isoquinoline alkaloids on growth performance and intestinal function of broiler chickens under heat stress. Poult. Sci. 2021, 100, 957–963. [Google Scholar] [CrossRef]
- Aydin, S.S.; Hatipoglu, D. Probiotic strategies for mitigating heat stress effects on broiler chicken performance. Int. J. Biometeorol. 2024, 68, 2153–2171. [Google Scholar] [CrossRef]
- Verma, A.; Inslicht, S.S.; Bhargava, A. Gut-Brain Axis: Role of Microbiome, Metabolomics, Hormones, and Stress in Mental Health Disorders. Cells 2024, 13, 1436. [Google Scholar] [CrossRef]
- Hu, J.; Chen, H.; Cheng, H.W. Effect of direct-fed microbials, Bacillus subtilis, on production performance, serotonin concentrations and behavioral parameters in a selected dominant strain of white leghorn hens. Int. J. Poult. Sci 2018, 17, 106–115. [Google Scholar] [CrossRef][Green Version]
| Probiotic Strains | Health/Performance Effects | Reference |
|---|---|---|
| Lactobacillus reuteri | Early supplementation improves microbial diversity, increases beneficial microbes, reduces pathogen levels, and lowers disease susceptibility (up to 6 weeks of age). | [148,160] |
| Bacillus subtilis, Clostridium butyricum, Enterococcus faecalis | Supplementation increases the thymus and bursa of Fabricius relative to body weight (by 29.3%), indicating enhanced humoral and cellular immune responses and improved overall immunity. | [100,161] |
| Multi-strain probiotics (unspecified) | Enhance immune responses, increase lysozyme activity, and boost IgA, IgG, IgM, and T-lymphocyte activity in intestinal mucosa. | [99,162] |
| Bifidobacterium spp., Lactobacillus casei (0.5%) | Improve intestinal mucosa, inhibit harmful bacteria from spreading, and significantly increase egg production. | [163] |
| Bacillus subtilis | Increased egg production, improvements in egg weight, shell thickness, and Haugh unit, increased egg freshness and quality | [164] |
| Bacillus subtilis, Enterococcus faecium | Enhance feed intake and conversion efficiency; improve growth and egg production. | [165] |
| Bacillus subtilis, yeast cell wall components | Improve egg weight, shell thickness, yolk pigmentation, albumen height, and Haugh unit. | [166] |
| Clostridium butyricum, B. subtilis | Improve the villus height to crypt depth ratio; better nutrient absorption. | [167] |
| Enterococcus lactis | Enhances nutrient absorption, growth, egg production, feed conversion, and calcium/phosphorus metabolism (notably during late laying). | [168,169] |
| Lactobacillus rhamnosus | Improves egg-laying performance, feed-to-egg ratio, and eggshell strength. | [170] |
| Pediococcus acidilactici | Maintains laying rate and feed efficiency when the metabolizable energy in the diet is reduced. | [140] |
| Saccharomyces cerevisiae | An increase in hen-day egg performance and a reduction in circulating cholesterol, mitigate oxidative stress. | [171] |
| Multi-strain preparation probiotics | Improved body weight gain, feed conversion ratio, reduced cholesterol, increased HDL content, lowered LDL, lower total triglycerides, meat yield, Increased meat protein in broilers | [172] |
| Bacillus-based probiotics | Decreased abdominal fat and improved fatty acid composition with increased unsaturated fatty acids in broilers. | [173] |
| Bacillus-based probiotics | Improved hen-day egg production and average egg weight, especially at higher dosages. | [174] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pewan, S.B.; Kabantiyok, D.; Emennaa, P.E.; Dawurung, J.S.; Dawurung, C.J.; Duwil, R.K.; Olorundare, O.O.; Ngukat, H.Y.; Umaru, M.G.; Ugwuoke, G.M.; et al. Advancing Nigerian Indigenous Poultry Health and Production, Use of Probiotics as Viable Alternatives to Antibiotics: A Review. Antibiotics 2025, 14, 846. https://doi.org/10.3390/antibiotics14080846
Pewan SB, Kabantiyok D, Emennaa PE, Dawurung JS, Dawurung CJ, Duwil RK, Olorundare OO, Ngukat HY, Umaru MG, Ugwuoke GM, et al. Advancing Nigerian Indigenous Poultry Health and Production, Use of Probiotics as Viable Alternatives to Antibiotics: A Review. Antibiotics. 2025; 14(8):846. https://doi.org/10.3390/antibiotics14080846
Chicago/Turabian StylePewan, Shedrach Benjamin, Dennis Kabantiyok, Paulinus Ekene Emennaa, Joshua Shehu Dawurung, Christiana J. Dawurung, Reuben Kefas Duwil, Olufunke Olufunmilola Olorundare, Hassan Yader Ngukat, Moses Gani Umaru, Garba Mathias Ugwuoke, and et al. 2025. "Advancing Nigerian Indigenous Poultry Health and Production, Use of Probiotics as Viable Alternatives to Antibiotics: A Review" Antibiotics 14, no. 8: 846. https://doi.org/10.3390/antibiotics14080846
APA StylePewan, S. B., Kabantiyok, D., Emennaa, P. E., Dawurung, J. S., Dawurung, C. J., Duwil, R. K., Olorundare, O. O., Ngukat, H. Y., Umaru, M. G., Ugwuoke, G. M., & Ezema, C. (2025). Advancing Nigerian Indigenous Poultry Health and Production, Use of Probiotics as Viable Alternatives to Antibiotics: A Review. Antibiotics, 14(8), 846. https://doi.org/10.3390/antibiotics14080846







