Prevalence and Risk Factors for Superinfection with a Difficult-to-Treat Pathogen in Periprosthetic Joint Infections
Abstract
1. Introduction
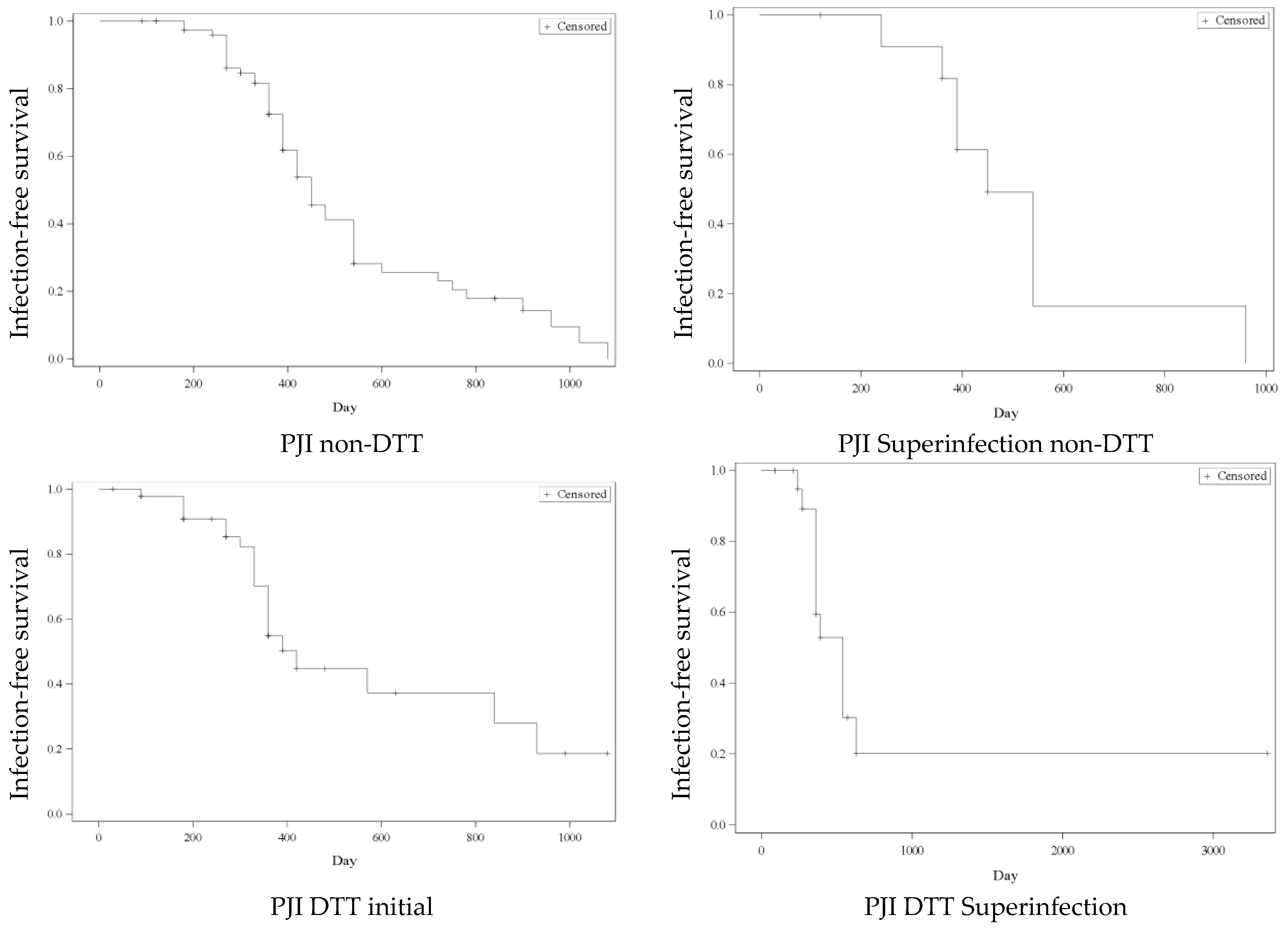
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Patient Parameters
4.3. Definition of Periprosthetic Joint Infection and DTT Pathogens
- -
- PJI caused by a non-DTT pathogen without superinfection.
- -
- PJI caused by a DTT pathogen without superinfection.
- -
- PJI initially caused by a non-DTT pathogen but with subsequent superinfection or pathogen switch to a DTT pathogen later in the course of the infection.
- -
- PJI with superinfection of any kind (DTT and non-DTT pathogens).
4.4. Treatment Regimen
4.5. Statistical Analysis
4.6. Ethics Approval
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neuprez, A.; Neuprez, A.H.; Kaux, J.F.; Kurth, W.; Daniel, C.; Thirion, T.; Huskin, J.P.; Gillet, P.; Bruyère, O.; Reginster, J.Y. Total joint replacement improves pain, functional quality of life, and health utilities in patients with late-stage knee and hip osteoarthritis for up to 5 years. Clin. Rheumatol. 2020, 39, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Egerci, O.F.; Yapar, A.; Dogruoz, F.; Selcuk, H.; Kose, O. Preventive strategies to reduce the rate of periprosthetic infections in total joint arthroplasty; a comprehensive review. Arch. Orthop. Trauma Surg. 2024, 144, 5131–5146. [Google Scholar] [CrossRef] [PubMed]
- Jämsen, E.; Stogiannidis, I.; Malmivaara, A.; Pajamäki, J.; Puolakka, T.; Konttinen, Y.T. Outcome of prosthesis exchange for infected knee arthroplasty: The effect of treatment approach. Acta Orthop. 2009, 80, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Yaghmour, K.M.; Chisari, E.; Khan, W.S. Single-Stage Revision Surgery in Infected Total Knee Arthroplasty: A PRISMA Systematic Review. J. Clin. Med. 2019, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Whitehouse, M.R.; Lenguerrand, E.; Blom, A.W.; Beswick, A.D. Re-Infection Outcomes Following One- And Two-Stage Surgical Revision of Infected Knee Prosthesis: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0151537. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, A.; Kolin, D.A.; Farley, K.X.; Wilson, J.M.; McLawhorn, A.S.; Cross, M.B.; Sculco, P.K. Projected Economic Burden of Periprosthetic Joint Infection of the Hip and Knee in the United States. J. Arthroplast. 2021, 36, 1484–1489.e3. [Google Scholar] [CrossRef] [PubMed]
- Kurd, M.F.; Ghanem, E.; Steinbrecher, J.; Parvizi, J. Two-stage Exchange Knee Arthroplasty: Does Resistance of the Infecting Organism Influence the Outcome? Clin. Orthop. Relat. Res. 2010, 468, 2060–2066. [Google Scholar] [CrossRef] [PubMed]
- Zmistowski, B.; Fedorka, C.J.; Sheehan, E.; Deirmengian, G.; Austin, M.S.; Parvizi, J. Prosthetic Joint Infection Caused by Gram-Negative Organisms. J. Arthroplast. 2011, 26, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Tornero, E.; Morata, L.; Martínez-Pastor, J.C.; Bori, G.; Mensa, J.; Soriano, A. Prosthetic joint infections due to methicillin-resistant and methicillin-susceptible staphylococci treated with open debridement and retention of the prosthesis. Rev. Esp. Quim. Quimioter. 2013, 26, 353–359. [Google Scholar]
- Kheir, M.M.; Tan, T.L.; Higuera, C.; George, J.; Della Valle, C.J.; Shen, M.; Parvizi, J. Periprosthetic Joint Infections Caused by Enterococci Have Poor Outcomes. J. Arthroplast. 2017, 32, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.H.; Lee, M.S.; Hsu, K.Y.; Chang, Y.H.; Shih, H.N.; Ueng, S.W. Gram-negative prosthetic joint infections: Risk factors and outcome of treatment. Clin. Infect. Dis. 2009, 49, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Kuo, F.C.; Goswami, K.; Shohat, N.; Blevins, K.; Rondon, A.J.; Parvizi, J. Two-Stage Exchange Arthroplasty Is a Favorable Treatment Option Upon Diagnosis of a Fungal Periprosthetic Joint Infection. J. Arthroplast. 2018, 33, 3555–3560. [Google Scholar] [CrossRef] [PubMed]
- Izakovicova, P.; Borens, O.; Trampuz, A. Periprosthetic joint infection: Current concepts and outlook. EFORT Open Rev. 2019, 4, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Mirza, Y.H.; Tansey, R.; Sukeik, M.; Shaath, M.; Haddad, F.S. Biofilm and the Role of Antibiotics in the Treatment of Periprosthetic Hip and Knee Joint Infections. Open Orthop. J. 2016, 10, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, M.D.; Hischebeth, G.T.R.; Randau, T.M.; Gathen, M.; Schildberg, F.A.; Fröschen, F.S.; Kohlhof, H.; Gravius, S. Difficult-to-treat pathogens significantly reduce infection resolution in periprosthetic joint infections. Diagn. Microbiol. Infect. Dis. 2020, 98, 115114. [Google Scholar] [CrossRef] [PubMed]
- Tuchscherr, L.; Heitmann, V.; Hussain, M.; Viemann, D.; Roth, J.; von Eiff, C.; Peters, G.; Becker, K.; Löffler, B. Staphylococcus aureus small-colony variants are adapted phenotypes for intracellular persistence. J. Infect. Dis. 2010, 202, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Vuong, C.; Kidder, J.B.; Jacobson, E.R.; Otto, M.; Proctor, R.A.; Somerville, G.A. Staphylococcus epidermidis polysaccharide intercellular adhesin production significantly increases during tricarboxylic acid cycle stress. J. Bacteriol. 2005, 187, 2967–2973. [Google Scholar] [CrossRef] [PubMed]
- Akgün, D.; Perka, C.; Trampuz, A.; Renz, N. Outcome of hip and knee periprosthetic joint infections caused by pathogens resistant to biofilm-active antibiotics: Results from a prospective cohort study. Arch. Orthop. Trauma Surg. 2018, 138, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Faschingbauer, M.; Bieger, R.; Kappe, T.; Weiner, C.; Freitag, T.; Reichel, H. Difficult to treat: Are there organism-dependent differences and overall risk factors in success rates for two-stage knee revision? Arch. Orthop. Trauma Surg. 2020, 140, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Hipfl, C.; Winkler, T.; Janz, V.; Perka, C.; Müller, M. Management of Chronically Infected Total Knee Arthroplasty With Severe Bone Loss Using Static Spacers With Intramedullary Rods. J. Arthroplast. 2019, 34, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Darwich, A.; Dally, F.J.; Abu Olba, K.; Mohs, E.; Gravius, S.; Hetjens, S.; Assaf, E.; Bdeir, M. Superinfection with Difficult-to-Treat Pathogens Significantly Reduces the Outcome of Periprosthetic Joint Infections. Antibiotics 2021, 10, 1145. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Lee, S.H.; Lin, Y.C.; Wang, Y.C.; Chang, C.J.; Hsieh, P.H. Increased periprosthetic hip and knee infection projected from 2014 to 2035 in Taiwan. J. Infect. Public Health 2020, 13, 1768–1773. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, P.; Maltenfort, M.G.; Chen, A.F.; Parvizi, J. Projected Increase in Periprosthetic Joint Infections Secondary to Rise in Diabetes and Obesity. J. Arthroplast. 2016, 31, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Bozic, K.J.; Lau, E.; Kurtz, S.; Ong, K.; Rubash, H.; Vail, T.P.; Berry, D.J. Patient-related risk factors for periprosthetic joint infection and postoperative mortality following total hip arthroplasty in Medicare patients. J. Bone Jt. Surg. Am. 2012, 94, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Poultsides, L.A.; Ma, Y.; Della Valle, A.G.; Chiu, Y.L.; Sculco, T.P.; Memtsoudis, S.G. In-hospital surgical site infections after primary hip and knee arthroplasty--incidence and risk factors. J. Arthroplast. 2013, 28, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplast. 2018, 33, 1309–1314.e2. [Google Scholar] [CrossRef] [PubMed]
- Tetreault, M.W.; Wetters, N.G.; Moric, M.; Gross, C.E.; Della Valle, C.J. Is synovial C-reactive protein a useful marker for periprosthetic joint infection? Clin. Orthop. Relat. Res. 2014, 472, 3997–4003. [Google Scholar] [CrossRef] [PubMed]
- Vanderstappen, C.; Verhoeven, N.; Stuyck, J.; Bellemans, J. Intra-articular versus serum C-reactive protein analysis in suspected periprosthetic knee joint infection. Acta Orthop. Belg. 2013, 79, 326–330. [Google Scholar] [PubMed]
- Grzelecki, D.; Kocon, M.; Mazur, R.; Grajek, A.; Kowalczewski, J. The diagnostic accuracy of blood C-reactive protein and erythrocyte sedimentation rate in periprosthetic joint infections—A 10-year analysis of 1510 revision hip and knee arthroplasties from a single orthopaedic center. J. Orthop. Surg. Res. 2025, 20, 276. [Google Scholar] [CrossRef] [PubMed]
- Unter Ecker, N.; Suero, E.M.; Gehrke, T.; Haasper, C.; Zahar, A.; Lausmann, C.; Hawi, N.; Citak, M. Serum C-reactive protein relationship in high- versus low-virulence pathogens in the diagnosis of periprosthetic joint infection. J. Med. Microbiol. 2019, 68, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, E.; Antoci, V., Jr.; Pulido, L.; Joshi, A.; Hozack, W.; Parvizi, J. The use of receiver operating characteristics analysis in determining erythrocyte sedimentation rate and C-reactive protein levels in diagnosing periprosthetic infection prior to revision total hip arthroplasty. Int. J. Infect. Dis. 2009, 13, e444–e449. [Google Scholar] [CrossRef] [PubMed]
- Grossi, O.; Asseray, N.; Bourigault, C.; Corvec, S.; Valette, M.; Navas, D.; Happi-Djeukou, L.; Touchais, S.; Bémer, P.; Boutoille, D.; et al. Gram-negative prosthetic joint infections managed according to a multidisciplinary standardized approach: Risk factors for failure and outcome with and without fluoroquinolones. J. Antimicrob. Chemother. 2016, 71, 2593–2597. [Google Scholar] [CrossRef] [PubMed]
- Baertl, S.; Lovasz, D.; Kees, M.G.; Walter, N.; Schindler, M.; Li, J.; Reinhard, J.; Alt, V.; Rupp, M. Periprosthetic Joint Infection and Concomitant Sepsis: Unveiling Clinical Manifestations, Risk Factors, and Patient Outcomes. J. Arthroplast. 2025, 40, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.J.; Chae, Y.J.; Jung, S.J.; Gong, H.S. Incidence and risk factors for periprosthetic joint infection: A common data model analysis. Jt. Dis. Relat. Surg. 2022, 33, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.; Chang, C.H.; Lin, Y.C.; Lee, S.H.; Hsieh, P.H.; Chang, Y. Different microbiological profiles between hip and knee prosthetic joint infections. J. Orthop. Surg. (Hong Kong) 2019, 27, 2309499019847768. [Google Scholar] [CrossRef] [PubMed]
- Enz, A.; Mueller, S.C.; Warnke, P.; Ellenrieder, M.; Mittelmeier, W.; Klinder, A. Periprosthetic Fungal Infections in Severe Endoprosthetic Infections of the Hip and Knee Joint-A Retrospective Analysis of a Certified Arthroplasty Centre of Excellence. J. Fungi 2021, 7, 404. [Google Scholar] [CrossRef] [PubMed]
- Tornero, E.; Senneville, E.; Euba, G.; Petersdorf, S.; Rodriguez-Pardo, D.; Lakatos, B.; Ferrari, M.C.; Pilares, M.; Bahamonde, A.; Trebse, R.; et al. Characteristics of prosthetic joint infections due to Enterococcus sp. and predictors of failure: A multi-national study. Clin. Microbiol. Infect. 2014, 20, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Baumgärtner, T.; Bdeir, M.; Dally, F.J.; Gravius, S.; Hai, A.A.E.; Assaf, E.; Hetjens, S.; Miethke, T.; Darwich, A. Rifampin-resistant periprosthetic joint infections are associated with worse functional outcome in both acute and chronic infection types. Diagn. Microbiol. Infect. Dis. 2024, 110, 116447. [Google Scholar] [CrossRef] [PubMed]
- Achermann, Y.; Eigenmann, K.; Ledergerber, B.; Derksen, L.; Rafeiner, P.; Clauss, M.; Nüesch, R.; Zellweger, C.; Vogt, M.; Zimmerli, W. Factors associated with rifampin resistance in staphylococcal periprosthetic joint infections (PJI): A matched case-control study. Infection 2013, 41, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Resende, V.A.C.; Neto, A.C.; Nunes, C.; Andrade, R.; Espregueira-Mendes, J.; Lopes, S. Higher age, female gender, osteoarthritis and blood transfusion protect against periprosthetic joint infection in total hip or knee arthroplasties: A systematic review and meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 8–43. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Ling, L.; Qi, L.; Liu, Z.; Zhang, W.; Yang, Z.; Wang, W.; Tu, C.; Li, Z. Patients’ risk factors for periprosthetic joint infection in primary total hip arthroplasty: A meta-analysis of 40 studies. BMC Musculoskelet. Disord. 2021, 22, 776. [Google Scholar] [CrossRef] [PubMed]
- Chrastil, J.; Anderson, M.B.; Stevens, V.; Anand, R.; Peters, C.L.; Pelt, C.E. Is Hemoglobin A1c or Perioperative Hyperglycemia Predictive of Periprosthetic Joint Infection or Death Following Primary Total Joint Arthroplasty? J. Arthroplast. 2015, 30, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Berríos-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017, 152, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Babkin, Y.; Raveh, D.; Lifschitz, M.; Itzchaki, M.; Wiener-Well, Y.; Kopuit, P.; Jerassy, Z.; Yinnon, A.M. Incidence and risk factors for surgical infection after total knee replacement. Scand. J. Infect. Dis. 2007, 39, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Alijanipour, P.; Heller, S.; Parvizi, J. Prevention of periprosthetic joint infection: What are the effective strategies? J. Knee Surg. 2014, 27, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R. Executive summary: Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, 1–10. [Google Scholar] [CrossRef] [PubMed]
- American Society of Anesthesiologists. ASA Physical Status Classification System. 2014. Available online: https://dam.assets.ohio.gov/image/upload/med.ohio.gov/portals/0/resources/asa%20physical%20status%20classification%20system.pdf (accessed on 20 May 2025).
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Lunz, A.; Lehner, B.; Voss, M.N.; Knappe, K.; Jaeger, S.; Innmann, M.M.; Renkawitz, T.; Omlor, G.W. Impact and Modification of the New PJI-TNM Classification for Periprosthetic Joint Infections. J. Clin. Med. 2023, 12, 1262. [Google Scholar] [CrossRef] [PubMed]
- Alt, V.; Rupp, M.; Langer, M.; Baumann, F.; Trampuz, A. Can the oncology classification system be used for prosthetic joint infection?: The PJI-TNM system. Bone Jt. Res. 2020, 9, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Zmistowski, B.; Berbari, E.F.; Bauer, T.W.; Springer, B.D.; Della Valle, C.J.; Garvin, K.L.; Mont, M.A.; Wongworawat, M.D.; Zalavras, C.G. New Definition for Periprosthetic Joint Infection: From the Workgroup of the Musculoskeletal Infection Society. Clin. Orthop. Relat. Res. 2011, 469, 2992–2994. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-joint infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Ochsner, P.E. Management of infection associated with prosthetic joints. Infection 2003, 31, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Sprowson, A.P.; Jensen, C.; Chambers, S.; Parsons, N.R.; Aradhyula, N.M.; Carluke, I.; Inman, D.; Reed, M.R. The use of high-dose dual-impregnated antibiotic-laden cement with hemiarthroplasty for the treatment of a fracture of the hip: The Fractured Hip Infection trial. Bone Jt. J. 2016, 98-B, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.K.; Rasouli, M.R.; Parvizi, J. Periprosthetic joint infection: Current concept. Indian. J. Orthop. 2013, 47, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Renz, N.; Trampuz, A. Management of Periprosthetic Joint Infection. Hip Pelvis 2018, 30, 138–146. [Google Scholar] [CrossRef] [PubMed]
| Age (Years) | Sex | Side | BMI (Kg/m2) | ASA Score | Anticoagulation | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD (Range) | N (%) | N (%) | Mean ± SD (Range) | Mean ± SD | N (%) | |||||
| Female | Male | Right | Left | Yes | No | |||||
| Total | n = 169 | 71.1 ± 13.1 (20–97) | 84 (49.7%) | 85 (50.3%) | 81 (47.9%) | 88 (52.1%) | 29.5 ± 6.5 (16.4–46.8) | 3 ± 0.6 | 67 (39.5%) | 102 (60.5%) |
| PJI non-DTT | n = 91 | 70.6 ± 13.9 (20–93) | 47 (51.7%) | 44 (48.3%) | 38 (41.8%) | 53 (58.2%) | 28.8 ± 6.5 (17.6–46.8) | 3 ± 0.6 | 31 (34.1%) | 60 (65.9%) |
| PJI DTT total | n = 78 | 71.5 ± 12 (35–97) | 37 (47.4%) | 41 (52.6%) | 41 (52.6%) | 37 (47.4%) | 30.3 ± 6.4 (16.4–45.1) | 3 ± 0.6 | 37 (47.4%) | 41 (52.6%) |
| PJI DTT initial | n = 54 | 71.8 ± 10.9 (48–89) | 28 (51.9%) | 26 (48.1%) | 26 (48.1%) | 28 (51.9%) | 30.7 ± 6.3 (16.4–45.1) | 3 ± 0.6 | 24 (44.4%) | 30 (55.6%) |
| PJI DTT super-infection | n = 24 | 71.1 ± 14.6 (35–97) | 9 (37.5%) | 15 (62.5%) | 15 (62.5%) | 9 (37.5%) | 29.5 ± 6.7 (18.5–42.3) | 3 ± 0.8 | 13 (54.2%) | 11 (45.8%) |
| PJI super-infection total | n = 40 | 69.1 ± 13.3 (35–97) | 15 (37.5%) | 25 (62.5%) | 20 (50%) | 20 (50%) | 30.4 ± 6.9 (18.5–44.1) | 3 ± 0.7 | 15 (37.5%) | 25 (62.5%) |
| p-value * | 0.6809 | 0.5898 | 0.2663 | 0.3324 | 0.7147 | 0.0743 | ||||
| Affected Joint | Type of Infection | CCI Age Adjusted | CRP (mg/L) at Admission | Antibiotic Treatment | Treatment Before Admission | Surgical Treatment | Number of Revisions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | Mean ± SD (Range) | Mean ± SD (range) | N (%) | N (%) | Mean ± SD (Range) | ||||||||||
| Hip | Knee | Shoulder | Acute | Chronic | 0–1 | 2–3 | 4–5 | Total Duration in Days | Antibiotic | Surgical | Prosthesis Retention | Prosthesis Exchange with Cement Spacer | Prosthesis Exchange Without Cement Spacer | ||||
| Total | n = 169 | 83 (49.1%) | 75 (44.4%) | 11 (6.5%) | 73 (43.2%) | 96 (56.8%) | 9 (5.3%) | 108 (63.9%) | 52 (30.8%) | 77.4 ± 83 (2–386) | 54.8 ± 36.7 (4–228) | 24 (14.2%) | 25 (14.8%) | 66 (40.7%) | 75 (46.3%) | 21 (13%) | 3.3 ± 3 (1–20) |
| PJI non-DTT | n = 91 | 36 (39.6%) | 47 (51.6%) | 8 (8.8%) | 33 (36.3%) | 58 (63.7%) | 6 (6.6%) | 60 (65.9%) | 25 (27.5%) | 105.6 ± 101.6 (2–390) | 46.3 ± 29.5 (4–217) | 13 (14.3%) | 11 (12.1%) | 44 (52.4%) | 33 (39.3%) | 7 (8.3%) | 2.3 ± 1.9 (1–14) |
| PJI DTT total | n = 78 | 47 (60.3%) | 28 (35.9%) | 3 (3.8%) | 37 (47.4%) | 41 (52.6%) | 3 (3.8%) | 48 (61.5%) | 27 (34.6%) | 64.4 ± 81.8 (2–381) | 64.2 ± 41.4 (4–228) | 11 (14.1%) | 14 (17.9%) | 22 (28.2%) | 42 (53.8%) | 14 (18%) | 4.4 ± 3.5 (1–20) |
| PJI DTT initial | n = 54 | 34 (63%) | 20 (37%) | 0 (0%) | 22 (40.8%) | 32 (59.2%) | 1 (1.9%) | 36 (66.7%) | 17 (31.5%) | 51.86 ± 63.01 (2–381) | 61.1 ± 39.7 (4–181) | 6 (11.1%) | 6 (11.1%) | 15 (28.8%) | 31 (57.4%) | 8 (14.8%) | 3.7 ± 3.3 (1–20) |
| PJI DTT Superinfection | n = 24 | 13 (54.2%) | 8 (33.3%) | 3 (12.5%) | 15 (62.5%) | 9 (37.5%) | 2 (8.3%) | 12 (50%) | 10 (41.7%) | 92.7 ± 109.7 (2–370) | 71.2 ± 45.2 (14–228) | 5 (20.8%) | 5 (20.8%) | 7 (30.2%) | 11 (45.8%) | 6 (24%) | 6 ± 3.6 (2–14) |
| PJI Superinfection total | n = 40 | 17 (42.5%) | 17 (42.5%) | 6 (15%) | 19 (47.5%) | 21 (52.5%) | 2 (5%) | 27 (67.5%) | 11 (27.5%) | 92.9 ± 93.8 (2–370) | 68.3 ± 45.6 (14–228) | 6 (15%) | 6 (15%) | 11 (27.5%) | 23 (57.5%) | 6 (15%) | 5.3 ± 3.4 (2–14) |
| p-value * | 0.0025 | 0.3155 | 0.9614 | 0.0055 | 0.0023 | 0.8534 | 0.0080 | <0.0001 | |||||||||
| Coagulase-Negative Staphylococci N (%) | Enterococci N (%) | Pseudomonas aeruginosa N (%) | Candida albicans N (%) | Polymicrobial * N (%) | ||
|---|---|---|---|---|---|---|
| PJI DTT total | n = 78 | 47 (60.3%) | 25 (32.1%) | 5 (6.4%) | 4 (5.1%) | 3 (3.9%) |
| PJI DTT initial | n = 54 | 34 (63%) | 12 (22.2%) | 3 (5.6%) | 3 (5.6%) | 2 (3.7%) |
| PJI DTT superinfection | n = 24 | 11 (45.8%) | 9 (37.5%) | 2 (8.3%) | 1 (4.2%) | 1 (4.2%) |
| Risk Factor | p-Value * | Odds Ratio | Confidence Interval | |
|---|---|---|---|---|
| PJI DTT total | CRP at admission (≥92.1 mg/L) | 0.001 | 6.981 | 1.367–35.63 |
| Hip joint | 0.0225 | 3.478 | 0.361–33.538 | |
| PJI DTT Superinfection | Number of revisions (≥3) | <0.0001 | 1.288 | 1.100–1.508 |
| Chronic type of infections | 0.0387 | 3.449 | 1.159–10.262 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwich, A.; Baumgärtner, T.; Hetjens, S.; Gravius, S.; Bdeir, M. Prevalence and Risk Factors for Superinfection with a Difficult-to-Treat Pathogen in Periprosthetic Joint Infections. Antibiotics 2025, 14, 752. https://doi.org/10.3390/antibiotics14080752
Darwich A, Baumgärtner T, Hetjens S, Gravius S, Bdeir M. Prevalence and Risk Factors for Superinfection with a Difficult-to-Treat Pathogen in Periprosthetic Joint Infections. Antibiotics. 2025; 14(8):752. https://doi.org/10.3390/antibiotics14080752
Chicago/Turabian StyleDarwich, Ali, Tobias Baumgärtner, Svetlana Hetjens, Sascha Gravius, and Mohamad Bdeir. 2025. "Prevalence and Risk Factors for Superinfection with a Difficult-to-Treat Pathogen in Periprosthetic Joint Infections" Antibiotics 14, no. 8: 752. https://doi.org/10.3390/antibiotics14080752
APA StyleDarwich, A., Baumgärtner, T., Hetjens, S., Gravius, S., & Bdeir, M. (2025). Prevalence and Risk Factors for Superinfection with a Difficult-to-Treat Pathogen in Periprosthetic Joint Infections. Antibiotics, 14(8), 752. https://doi.org/10.3390/antibiotics14080752





