Very Early Transition to Oral Antibiotics in Uncomplicated Enterobacterales Bloodstream Infections: Effectiveness and Impact on Carbon Footprint Saving
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics and Outcomes
2.2. Factors Associated with Very Early Oral Transition
2.3. Assessment of the Oral Transition with High Doses of Beta-Lactams and Cephalosporins
2.4. Carbon Footprint Analysis and Hospital Stay Costs
3. Discussion
4. Materials and Methods
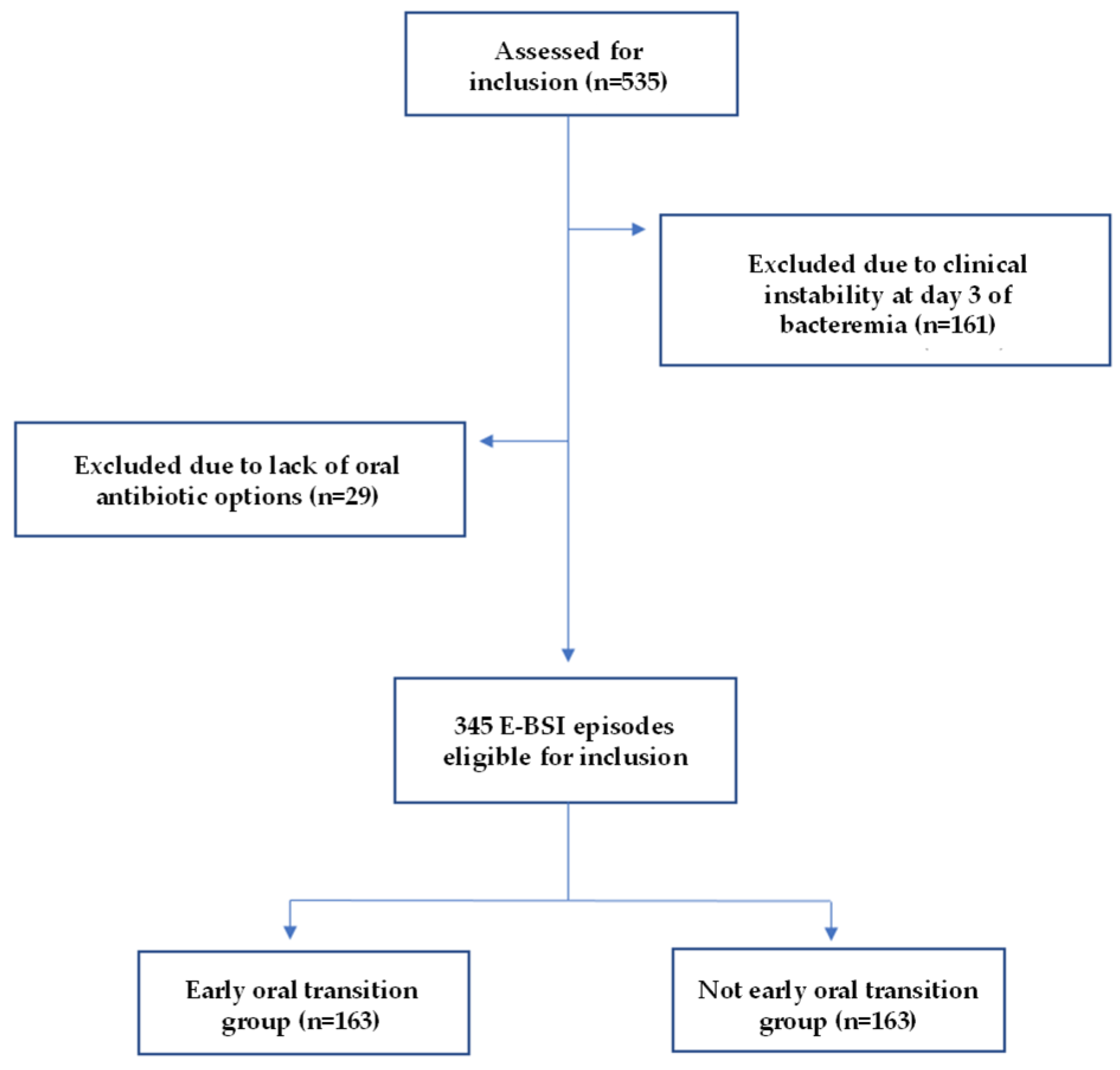
4.1. Study Design, Setting, and Patients
4.2. Variables and Definitions
4.3. Microbiology
4.4. Carbon Footprint and Economic Costs
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIC | Akaike Information Criterion |
| BLs | Beta-lactams |
| BSIs | Bloodstream infections |
| CCI | Charlson Comorbidity Index |
| E-BSIs | Enterobacterales bloodstream infections |
| EO group | Early oral group |
| ESBL | Extended-spectrum beta-lactamase |
| ESCMID | European Society of Clinical Microbiology and Infectious Diseases |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| IQR | Interquartile range |
| IV | Intravenous |
| KgCO2eq | Kilograms of carbon dioxide equivalents |
| MDR | Multidrug resistance |
| nEO group | Non-early oral group |
| QLs | Quinolones |
| RCTs | Randomized controlled trials |
| SEIMC | Spanish Society of Infectious Diseases and Clinical Microbiology |
| TS | Trimethoprim/sulfamethoxazole |
| UTI | Urinary tract infection |
References
- Ince, D.; Fiawoo, S.; Choudhury, R.; Cosgrove, S.E.; Dobrzynski, D.; Gold, H.; Lee, J.H.; Percival, K.M.; Shulder, S.; Sony, D.; et al. Epidemiology of Gram-Negative Bloodstream Infections in the United States: Results From a Cohort of 24 Hospitals. Open Forum Infect. Dis. 2023, 10, ofad265. [Google Scholar] [CrossRef] [PubMed]
- Wald-Dickler, N.; Holtom, P.D.; Phillips, M.C.; Centor, R.M.; Lee, R.A.; Baden, R.; Spellberg, B. Oral Is the New IV. Challenging Decades of Blood and Bone Infection Dogma: A Systematic Review. Am. J. Med. 2022, 135, 369–379.e1. [Google Scholar] [CrossRef] [PubMed]
- Amodio-Groton, M.; Madu, A.; Madu, C.N.; Briceland, L.L.; Seligman, M.; McMaster, P.; Miller, M.H. Sequential parenteral and oral ciprofloxacin regimen versus parenteral therapy for bacteremia: A pharmacoeconomic analysis. Ann. Pharmacother. 1996, 30, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Monmaturapoj, T.; Montakantikul, P.; Mootsikapun, P.; Tragulpiankit, P. A prospective, randomized, double dummy, placebo-controlled trial of oral cefditoren pivoxil 400mg once daily as switch therapy after intravenous ceftriaxone in the treatment of acute pyelonephritis. Int. J. Infect. Dis. 2012, 16, e843–e949. [Google Scholar] [CrossRef] [PubMed]
- Park, T.Y.; Choi, J.S.; Song, T.J.; Do, J.H.; Choi, S.H.; Oh, H.C. Early Oral Antibiotic Switch Compared with Conventional Intravenous Antibiotic Therapy for Acute Cholangitis with Bacteremia. Dig. Dis. Sci. 2014, 59, 2790–2796. [Google Scholar] [CrossRef] [PubMed]
- Omrani, A.S.; Abujarir, S.H.; Ben Abid, F.; Shaar, S.H.; Yilmaz, M.; Shaukat, A.; Alsamawi, M.S.; Elgara, M.S.; Alghazzawi, M.I.; Shunnar, K.M.; et al. Switch to oral antibiotics in Gram-negative bacteraemia: A randomized, open-label, clinical trial. Clin. Microbiol. Infect. 2024, 30, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Al-Hasan, M.; Rac, H. Transition from intravenous to oral antimicrobial therapy in patients with uncomplicated and complicated bloodstream infections. Clin Microbiol Infect. 2020, 26, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Engers, D.W.; Tamma, P.D.; Fiawoo, S.; Fong, K.; Jariwala, R.; Jenkins, T.C. Transition to Oral Antibiotic Therapy for Hospitalized Adults With Gram-Negative Bloodstream Infections. JAMA Netw. Open 2024, 7, e2349864. [Google Scholar] [CrossRef] [PubMed]
- Casado, A.; Gimeno, A.; Aguilar-Guisado, M.; García, M.; Rodríguez, J.F.; Rivas, P.A.; Bueno, C.; Lepe, J.A.; Cisneros, J.M.; Molina, J. Safety of early oral ambulatory treatment of adult patients with bloodstream infections discharged from the emergency department. Antimicrob. Agents Chemother. 2023, 67, e007802. [Google Scholar] [CrossRef] [PubMed]
- Tingsgård, S.; Israelsen, S.B.; Jørgensen, H.L.; Østergaard, C.; Benfield, T. Early Switch From Intravenous to Oral Antibiotics for Patients With Uncomplicated Gram-Negative Bacteremia. JAMA Netw. Open 2024, 7, e2352314. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Conley, A.T.; Cosgrove, S.E.; Harris, A.D.; Lautenbach, E.; Amoah, J.; Avdic, E.; Tolomeo, P.; Wise, J.; Subudhi, S.; et al. Association of 30-Day Mortality with Oral Step-Down vs Continued Intravenous Therapy in Patients Hospitalized with Enterobacteriaceae Bacteremia. JAMA Intern. Med. 2019, 179, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Pradubkham, T.; Suwanpimolkul, G.; Gross, A.E.; Nakaranurack, C. Intravenous to oral transition of antibiotics for gram-negative bloodstream infection at a University hospital in Thailand: Clinical outcomes and predictors of treatment failure. PLoS ONE 2022, 3, e0273369. [Google Scholar] [CrossRef] [PubMed]
- Yahav, D.; Franceschini, E.; Koppel, F.; Turjeman, A.; Babich, T.; Bitterman, R.; Neuberger, A.; Ghanem-Zoubi, N.; Santoro, A.; Eliakim-Raz, N.; et al. Seven Versus 14 Days of Antibiotic Therapy for Uncomplicated Gram-negative Bacteremia: A Noninferiority Randomized Controlled Trial. Clin. Infect. Dis. 2019, 69, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.; Montero-Mateos, E.; Praena-Segovia, J.; León-Jiménez, E.; Natera, C.; López-Cortés, L.E.; Valiente, L.; Rosso-Fernández, C.M.; Herrero, M.; Aller-García, A.I.; et al. Seven-versus 14-day course of antibiotics for the treatment of bloodstream infections by Enterobacterales: A randomized, controlled trial. Clin. Microbiol. Infect. 2022, 28, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Daneman, N.; Rishu, A.; Pinto, R.; Rogers, B.A.; Shehabi, Y.; Parke, R.; Cook, D.; Arabi, Y.; Muscedere, J.; Reynolds, S.; et al. Antibiotic Treatment for 7 versus 14 Days in Patients with Bloodstream Infections. N. Engl. J. Med. 2025, 392, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Punjabi, C.; Tien, V.; Meng, L.; Deresinski, S.; Holubar, M. Oral Fluoroquinolone or Trimethoprim-Sulfamethoxazole vs ß-Lactams as Step-Down Therapy for Enterobacteriaceae Bacteremia: Systematic Review and Meta-analysis. Open Forum Infect. Dis. 2019, 6, ofz364. [Google Scholar] [CrossRef] [PubMed]
- Mponponsuo, K.; Brown, K.A.; Fridman, D.J.; Johnstone, J.; Langford, B.J.; Lee, S.M.; MacFadden, D.R.; Patel, S.N.; Schwartz, K.L.; Daneman, N. Highly versus less bioavailable oral antibiotics in the treatment of gram-negative bloodstream infections: A propensity-matched cohort analysis. Clin. Microbiol. Infect. 2023, 29, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Mercuro, N.J.; Stogsdill, P.; Wungwattana, M. Retrospective analysis comparing oral stepdown therapy for enterobacteriaceae bloodstream infections: Fluoroquinolones versus β-lactams. Int. J. Antimicrob. Agents 2024, 51, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Bjork, L.; Hopkins, T.; Yang, L.; Teng, C. Comparative-Effectiveness of Oral Beta-Lactams and Fluoroquinolones for Stepdown Therapy in Patients with Enterobacterales Bloodstream Infections: A Retrospective Cohort Study. Int. J. Med. Sci. 2023, 20, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Alzaidi, S.; Veillette, J.J.; May, S.S.; Olson, J.; Jackson, K.; Waters, C.D.; Butler, A.M.; Hutton, M.A.; Buckel, W.R.; Webb, B.J. Oral β-Lactams, Fluoroquinolones, or Trimethoprim-Sulfamethoxazole for Definitive Treatment of Uncomplicated Escherichia coli or Klebsiella Species Bacteremia From a Urinary Tract Source. Open Forum Infect. Dis. 2023, 11, ofad657. [Google Scholar] [CrossRef] [PubMed]
- Nisly, S.A.; Mcclain, D.L.; Fillius, A.G.; Davis, K.A. Oral antibiotics for the treatment of Gram-negative bloodstream infections: A retrospective comparison of three antibiotic classes. J. Glob. Antimicrob. Resist. 2020, 20, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.R.; Tong, S.Y.C.; Davis, J.S.; Paterson, D.L.; Syed-Omar, S.F.; Peck, K.R.; Chung, D.R.; Cooke, G.S.; Libau, E.A.; Rahman, S.B.A.; et al. Early oral stepdown antibiotic therapy versus continuing intravenous therapy for uncomplicated Gram-negative bacteraemia (the INVEST trial): Study protocol for a multicentre, randomised controlled, open-label, phase III, non-inferiority trial. Trials 2022, 23, 572. [Google Scholar] [CrossRef] [PubMed]
- Boix-Palop, L.; Calbo, E. Early transition to oral therapy in gram-negative bloodstream infections: What is the next step ? Clin. Microbiol. Infect. 2024, 30, 428–430. [Google Scholar] [CrossRef] [PubMed]
- Hospenthal, D.R.; Waters, C.D.; Beekmann, S.E.; Polgreen, P.M. Practice Patterns of Infectious Diseases Physicians in Transitioning From Intravenous to Oral Therapy in Patients With Bacteremia. Open Forum Infect. Dis. 2019, 7, ofz386. [Google Scholar] [CrossRef] [PubMed]
- Rieger, K.; Bosso, J.; MacVane, S.; Temple, Z.; Wahlquist, A.; Bohm, N. Intravenous-only or Intravenous Transitioned to Oral Antimicrobials for Enterobacteriaceae-Associated Bacteremic Urinary Tract Infection. Pharmacotherapy 2017, 37, 1479–1483. [Google Scholar] [CrossRef] [PubMed]
- Thaden, J.T.; Tamma, P.D.; Doi, Y.; Daneman, N. Variability in Oral Antibiotic Step-Down Therapy in the Management of Gram-Negative Bloodstream Infections. Int. J. Antimicrob. Agents 2022, 58, 106451. [Google Scholar] [CrossRef] [PubMed]
- McMeekin, N.; Geue, C.; Briggs, A.; Rombach, I.; Li, H.K.; Bejon, P.; McNally, M.; Atkins, B.L.; Ferguson, J.; Scarborough, M.; et al. Cost-effectiveness of oral versus intravenous antibiotics ( OVIVA ) in patients with bone and joint infection: Evidence from a non-inferiority trial. Wellcome Open Res. 2019, 4, 108. [Google Scholar] [CrossRef] [PubMed]
- Yoong, J.; Yuen, K.H.; Molton, J.S.; Ding, Y.; Cher, B.P.; Chan, M.; Kalimuddin, S.; Oon, J.; Young, B.; Low, J.; et al. Cost minimization analysis of oral versus intravenous antibiotic treatment for Klebsiella pneumoniae liver abscess. Sci. Rep. 2023, 13, 9774. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.; Jayachandran, A. Clinical Impact of Oral Step-Down Therapy for Gram- Negative Bacteremia: A Retrospective Study. HCA Healthc. J. Med. 2023, 4, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef] [PubMed]
- Ethan, E.; Halm, M.D.; Alvin, S. Management of community-acquired pneumonia. N. Engl. J. Med. 2002, 347, 2039–2045. [Google Scholar]
- Heil, E.L.; Bork, J.T.; Abbo, L.M.; Barlam, T.F.; Cosgrove, S.E.; Davis, A.; Ha, D.R.; Jenkins, T.C.; Kaye, K.S.; Lewis, J.S., 2nd; et al. Optimizing the management of uncomplicated gram-negative bloodstream infections: Consensus guidance using a modified Delphi process. Open Forum Infect. Dis. 2021, 8, ofab434. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.D.; Kaye, K.S.; Stout, J.E.; McGarry, S.A.; Trivette, S.L.; Briggs, J.P.; Lamm, W.; Clark, C.; MacFarquhar, J.; Walton, A.L.; et al. Health care-associated bloodstream infections in adults: A reason to change the accepted definition of community-acquired infections. Ann. Intern. Med. 2002, 137, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef] [PubMed]
- Hilf, M.; Yu, V.L.; Sharp, J.; Zuravleff, J.J.; Korvick, J.A.; Muder, R.R. Antibiotic therapy for Pseudomonas aeruginosa bacteremia: Outcome correlations in a prospective study of 200 patients. Am. J. Med. 1989, 87, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Herreros, J.M.; Cobo-Reinoso, J.; Pujol-Rojo, M.; Rodríguez-Baño, J.; Salavert-Lletí, M. Guía para el diagnóstico y tratamiento del paciente con bacteriemia. Guías de la Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC). Enferm. Infecc. Microbiol. Clin. 2007, 25, 111–130. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2011, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Weisz, U.; Pichler, P.-P.; Jaccard, I.S.; Haas, W.; Matej, S.; Bachner, F.; Nowak, P.; Weisz, H. Carbon emission trends and sustainability options in Austrian health care. Resour. Conserv. Recycl. 2020, 160, 104862. [Google Scholar] [CrossRef]
- PAS 2050:2011; Specification for the Assessment of the Life Cycle Greenhouse Gas Emissions of Goods and Services. BSI: London, UK, 2011.
- ISO 14067:2018; Greenhouse Gases—Carbon Footprint of Products—Requirements and Guidelines for Quantification. ISO: Geneva, Switzerland, 2018.
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
| EO Group (n = 163) (%) | nEO Group (n = 182) (%) | p | |
|---|---|---|---|
| Sex (male) | 85 (52.2) | 93 (51.1) | 0.85 |
| Age a | 75 (64–84) | 77 (67–86) | 0.06 |
| Charlson index a (n = 344) | 4 (3–6) | 5 (4–6) | 0.002 |
| Diabetes Mellitus | 33 (20.3) | 49 (26.9) | 0.146 |
| Immunosuppression b | 14 (8.6) | 22 (12.1) | 0.29 |
| Corticosteroids | 6 (42.9) | 6 (27.3) | 0.33 |
| Biologic treatment | 3 (21.4) | 6 (27.3) | 1.00 |
| Neutropenia | 1 (7.1) | 1 (4.6) | 1.00 |
| Solid organ transplant | 2 (14.3) | 1 (4.6) | 0.55 |
| Hematopoietic cell transplant | 0 (0.0) | 3 (13.6) | 0.27 |
| Chemotherapy | 5 (35.7) | 10 (45.5) | 0.73 |
| AIDS | 0 (0.0) | 0 (0.0) | NA |
| Bacteremia characteristics | |||
| Classification according to acquisition-site (n = 339) | |||
| Community-acquired | 99 (61.5) | 97 (54.5) | 0.19 |
| Health care-associated infection | 45 (28.0) | 51 (28.7) | 0.89 |
| Nosocomial | 17 (10.6) | 30 (16.9) | 0.09 |
| Source of infection | |||
| Urinary-tract | 129 (79.1) | 118 (64.8) | 0.003 |
| Biliary-tract | 25 (15.3) | 39 (21.4) | 0.15 |
| Unknown | 8 (4.9) | 8 (4.4) | 0.82 |
| Others c | 1 (0.6) | 17 (9.3) | <0.001 |
| Initial Pitt-score a (n = 333) | 0 (0–0) | 0 (0–1) | 0.14 |
| Sepsis (n = 341) | 29 (17.9) | 43 (24.0) | 0.17 |
| Septic shock (n = 341) | 3 (1.9) | 11 (6.2) | 0.06 |
| ICU admission (n = 339) | 4 (2.5) | 10 (5.7) | 0.18 |
| Initial temperature a (n = 333) | 37.8 (37.2–38.5) | 37.8 (37·2–38.3) | 0·51 |
| Initial Leukocyte count a(n = 343) | 11.9 (8.9–16.3) | 11.5 (8.3–15.0) | 0.38 |
| Initial C-RP a (n = 337) | 86.3 (37.0–175.0) | 98.3 (38.2–174.9) | 0.53 |
| Initial PCT a (n = 259) | 0.9 (0.4–3.3) | 1.6 (0.6–6.7) | 0.04 |
| Microbiology | |||
| Days from blood culture extraction to clinician information a (n = 341) | 1 (1–2) | 1 (1–2) | 0.46 |
| Microorganism | |||
| E. coli | 121 (74.2) | 141 (77.5) | 0.53 |
| K.pneumoniae | 28 (17.2) | 32 (17.6) | 0.92 |
| Others d | 14 (8.6) | 9 (5.0) | 0.18 |
| Any resistance mechanism | 16 (9.8) | 35 (19.2) | 0.01 |
| ESBL | 15 (9.2) | 33 (18.1) | 0.02 |
| Others e | 1 (0.6) | 2 (1.1) | 1.00 |
| Multidrug resistance | 25 (15.3) | 31 (17.0) | 0.67 |
| Antibiotic treatment | |||
| Any empiric antibiotic | 158 (96.9) | 180 (98.9) | 0.26 |
| Appropriate empiric antibiotic (n = 328) | 139 (91.5) | 152 (86.4) | 0.15 |
| Days from bacteremia to first antibiotic administration a | 0 (0–1) | 0 (0–0) | 0.20 |
| Days of intravenous antibiotics a (n = 344) | 2 (1–3) | 6 (4–8) | <0.001 |
| Total length of antibiotic treatment a (n = 344) | 10 (7–14) | 10 (7–13) | 0.56 |
| Source control before oral treatment (n = 339) | |||
| Yes | 12 (7.5) | 38 (21.4) | <0.001 |
| No | 4 (2.5) | 5 (2.8) | 1.00 |
| Not indicated | 142 (88.2) | 128 (71.9) | <0.001 |
| Outcomes | |||
| Days to clinical stability from antibiotic initiation a | 1 (1–2) | 2 (1–3) | <0.001 |
| Phlebitis during admission (n = 334) | 3 (1.9) | 10 (5.8) | 0.09 |
| Length of hospital stay a (n = 333) | 3 (1–5) | 8 (5–14) | <0.001 |
| In-hospital mortality | 2 (1.2) | 7 (3.9) | 0.18 |
| 30-day mortality | 2 (1.2) | 5 (2.8) | 0.45 |
| Re-admission due to a new infection (n = 327) | 10 (6.3) | 26 (15.4) | 0.009 |
| Re-admission due to relapse of the E-BSI (n = 322) | 3 (1.9) | 7 (4.2) | 0.34 |
| Variable | Odds Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| Charlson index | 0.85 | 0.77–0.95 | 0.003 |
| Urinary tract infection | 2.02 | 1.18–3.48 | 0.01 |
| ESBL-producing isolate | 0.39 | 0.19–0.80 | 0.01 |
| Source control previous to the oral transition | 0.39 | 0.19–0.85 | 0.02 |
| Days to clinical stability | 0.51 | 0.39–0.66 | <0.001 |
| Carbon Footprint | |||
|---|---|---|---|
| Total KgCO2eq Per Group | Mean KgCO2eq Per Patient | Absolute Savings (KgCO2eq) a | |
| EO group (n = 163) | 44.279 | 0.272 | −38.794 |
| nEO group (n = 182) | 92.704 | 0.509 | −43.316 |
| Difference | −0.238 | ||
| Hospital Stay Cost | Antibiotics Cost | Intravenous Kit Cost | Total Savings (EUR) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median Hospital Stay (Days) | Cost Per Day of Hospital Stay (€) | Mean Total Cost Per Patient (EUR) | Absolut Savings (EUR) a | Total Days of Antibiotics Per Group | Total Cost of Antibiotics Per Group (EUR) | Mean Cost of Antibiotics Per Day Per Patient (EUR) | Absolute Savings (EUR) b | Total Days of Intravenous Kit Use Per Group | Total Cost of Intravenous Kit Per Group (EUR) | Mean Cost of Intravenous Kit Per Day Per Patient (EUR) | Absolute Savings (EUR) c | ||
| EO group (n = 163) | 4 | 266.22 | 1064.88 | −260,363.32 | 1899 | 5189.28 | 2.73 | −8736.46 | 319 | 1226.92 | 3.85 | −458.21 | −269,557.99 |
| nEO group (n = 182) | 10 | 266.22 | 2662.2 | −290,712.42 | 1473 | 10,801.80 | 7.33 | −6776.63 | 632 | 3338.56 | 5.28 | −907.80 | −298,396.85 |
| Difference | −1597.32 | −4.60 | −1.44 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateu, A.; Martínez-Urrea, A.; Gallego, C.; Gisbert, L.; Dietl, B.; Xercavins, M.; López-Sánchez, M.; Álvarez, S.; García Rodríguez, S.; Roselló, T.; et al. Very Early Transition to Oral Antibiotics in Uncomplicated Enterobacterales Bloodstream Infections: Effectiveness and Impact on Carbon Footprint Saving. Antibiotics 2025, 14, 751. https://doi.org/10.3390/antibiotics14080751
Mateu A, Martínez-Urrea A, Gallego C, Gisbert L, Dietl B, Xercavins M, López-Sánchez M, Álvarez S, García Rodríguez S, Roselló T, et al. Very Early Transition to Oral Antibiotics in Uncomplicated Enterobacterales Bloodstream Infections: Effectiveness and Impact on Carbon Footprint Saving. Antibiotics. 2025; 14(8):751. https://doi.org/10.3390/antibiotics14080751
Chicago/Turabian StyleMateu, Aina, Ana Martínez-Urrea, Clara Gallego, Laura Gisbert, Beatriz Dietl, Mariona Xercavins, Maria López-Sánchez, Silvia Álvarez, Sergi García Rodríguez, Toni Roselló, and et al. 2025. "Very Early Transition to Oral Antibiotics in Uncomplicated Enterobacterales Bloodstream Infections: Effectiveness and Impact on Carbon Footprint Saving" Antibiotics 14, no. 8: 751. https://doi.org/10.3390/antibiotics14080751
APA StyleMateu, A., Martínez-Urrea, A., Gallego, C., Gisbert, L., Dietl, B., Xercavins, M., López-Sánchez, M., Álvarez, S., García Rodríguez, S., Roselló, T., Pérez, J., Calbo, E., & Boix-Palop, L. (2025). Very Early Transition to Oral Antibiotics in Uncomplicated Enterobacterales Bloodstream Infections: Effectiveness and Impact on Carbon Footprint Saving. Antibiotics, 14(8), 751. https://doi.org/10.3390/antibiotics14080751







