Preliminary Evaluation of the Synergistic Antibacterial Effects of Selected Commercial Essential Oil Compounds Against Methicillin-Resistant Staphylococcus aureus ATCC 43300
Abstract
1. Introduction
2. Results
2.1. Evaluation of the MIC and MBC Values for EOCs Against MRSA Strain
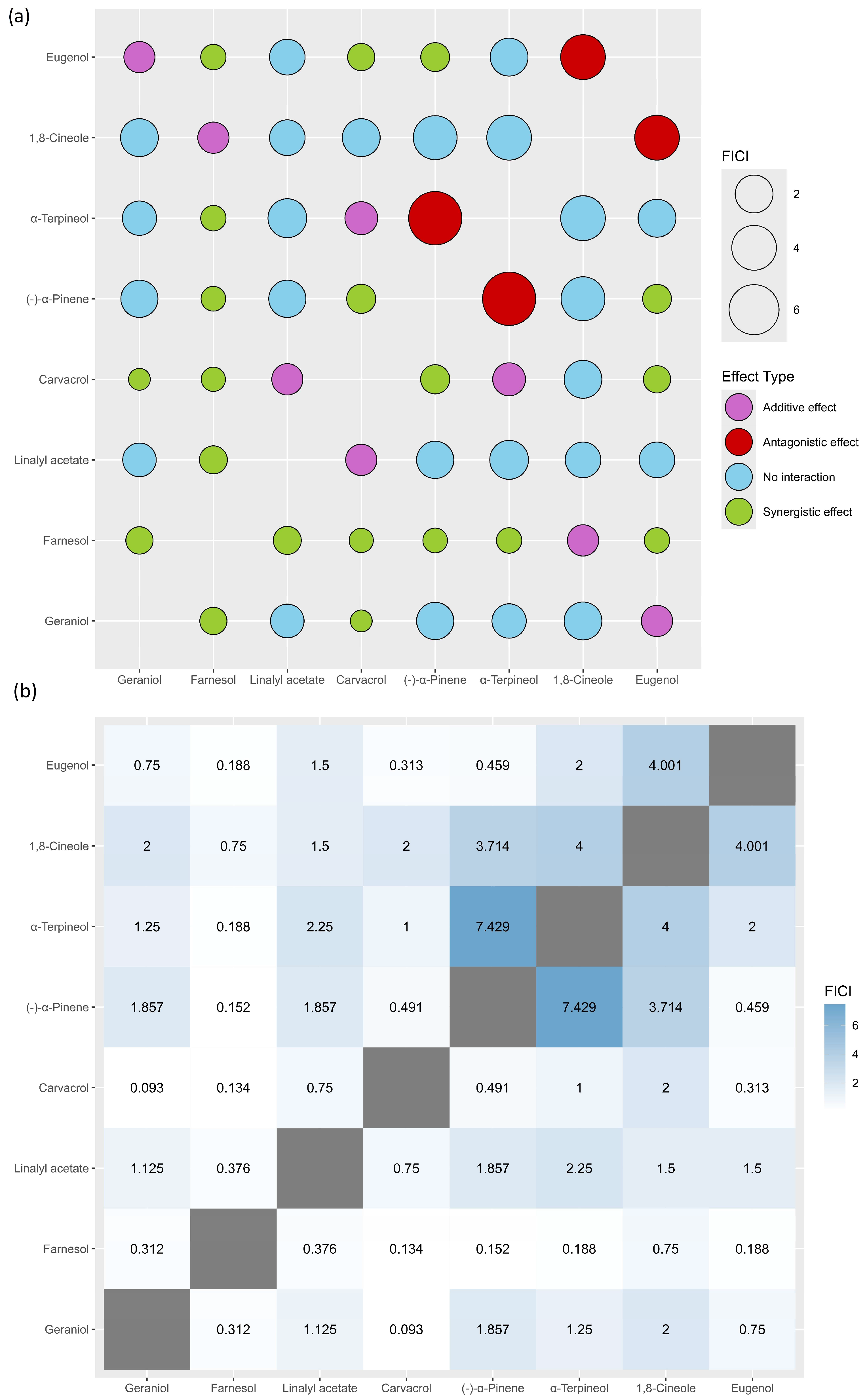
2.2. Evaluation of the Antibacterial Activity of EOCs in Combination Against MRSA Strain
3. Discussion
4. Materials and Methods
4.1. Reference Strain and Growth Conditions
4.2. Chemicals and Preparation of Dilution
4.3. Evaluation of MIC, MBC, MBC/MIC Ratio, and the Activity of Chemicals Against Reference Strain
4.4. The Checkerboard Assay
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, X.; Zhao, S.; Deng, Y.; Xu, W.; Wang, Z.; Wang, W.; Lv, R.; Liu, D. Antibacterial Activity and Mechanisms of α-Terpineol Against Foodborne Pathogenic Bacteria. Appl. Microbiol. Biotechnol. 2023, 107, 6641–6653. [Google Scholar] [CrossRef]
- Huang, J.; Yang, L.; Zou, Y.; Luo, S.; Wang, X.; Liang, Y.; Du, Y.; Feng, R.; Wei, Q. Antibacterial Activity and Mechanism of Three Isomeric Terpineols of Cinnamomum longepaniculatum Leaf Oil. Folia Microbiol. 2021, 66, 59–67. [Google Scholar] [CrossRef]
- Galan, D.M.; Ezeudu, N.E.; Garcia, J.; Geronimo, C.A.; Berry, N.M.; Malcolm, B.J. Eucalyptol (1,8-Cineole): An Underutilized Ally in Respiratory Disorders? J. Essent. Oil Res. 2020, 32, 103–110. [Google Scholar] [CrossRef]
- Khaleel, C.; Tabanca, N.; Buchbauer, G. α-Terpineol, a Natural Monoterpene: A Review of Its Biological Properties. Open Chem. 2018, 16, 349–361. [Google Scholar] [CrossRef]
- Sharafan, M.; Jafernik, K.; Ekiert, H.; Kubica, P.; Kocjan, R.; Blicharska, E.; Szopa, A. Illicium verum (Star Anise) and Trans-Anethole as Valuable Raw Materials for Medicinal and Cosmetic Applications. Molecules 2022, 27, 650. [Google Scholar] [CrossRef]
- Oliveira, K.C.; Franciscato, L.M.S.S.; Mendes, S.S.; Barizon, F.M.A.; Gonçalves, D.D.; Barbosa, L.N.; Faria, M.G.I.; Valle, J.S.; Casalvara, R.F.A.; Gonçalves, J.E.; et al. Essential Oil from the Leaves, Fruits and Twigs of Schinus terebinthifolius: Chemical Composition, Antioxidant and Antibacterial Potential. Molecules 2024, 29, 469. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Ziaee, M.; Palla, F. Essential Oils Extracted from Different Species of the Lamiaceae Plant Family as Prospective Bioagents Against Several Detrimental Pests. Molecules 2020, 25, 1556. [Google Scholar] [CrossRef]
- de Amorim, M.S.; Verdan, M.H.; Oliveira, C.S.; Santos, A.D. Essential Oils of Neotropical Myrtaceae Species from 2011 until 2023: An Update. Chem. Biodivers. 2025, 22, e202401503. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Saleh, M.A. Effect of Exposure to Light Emitted Diode (LED) Lights on Essential Oil Composition of Sweet Mint Plants. J. Environ. Sci. Health Part A 2019, 54, 435–440. [Google Scholar] [CrossRef]
- Karlberg, A.; Magnusson, K.; Nilsson, U. Air Oxidation of d-limonene (the Citrus Solvent) Creates Potent Allergens. Contact Dermat. 1992, 26, 332–340. [Google Scholar] [CrossRef]
- Folle, C.; Díaz-Garrido, N.; Mallandrich, M.; Suñer-Carbó, J.; Sánchez-López, E.; Halbaut, L.; Marqués, A.M.; Espina, M.; Badia, J.; Baldoma, L.; et al. Hydrogel of Thyme-Oil-PLGA Nanoparticles Designed for Skin Inflammation Treatment. Gels 2024, 10, 149. [Google Scholar] [CrossRef]
- Folle, C.; Marqués, A.M.; Mallandrich, M.; Suñer-Carbó, J.; Halbaut, L.; Sánchez-López, E.; López-Machado, A.L.; Díaz-Garrido, N.; Badia, J.; Baldoma, L.; et al. Colloidal Hydrogel Systems of Thymol-Loaded PLGA Nanoparticles Designed for Acne Treatment. Colloids Surf. B Biointerfaces 2024, 234, 113678. [Google Scholar] [CrossRef] [PubMed]
- Said, S.S.; Aloufy, A.K.; El-Halfawy, O.M.; Boraei, N.A.; El-Khordagui, L.K. Antimicrobial PLGA Ultrafine Fibers: Interaction with Wound Bacteria. Eur. J. Pharm. Biopharm. 2011, 79, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, I.; Piotrowska, M. Anastrozole as Aromatase Inhibitor—New Approaches to Breast Cancer Treatment in Postmenopausal Women. Nowotw. J. Oncol. 2019, 69, 26–35. [Google Scholar] [CrossRef]
- Sharma, M.; Grewal, K.; Jandrotia, R.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Essential Oils as Anticancer Agents: Potential Role in Malignancies, Drug Delivery Mechanisms, and Immune System Enhancement. Biomed. Pharmacother. 2022, 146, 112514. [Google Scholar] [CrossRef]
- Hadidi, M.; Pouramin, S.; Adinepour, F.; Haghani, S.; Jafari, S.M. Chitosan Nanoparticles Loaded with Clove Essential Oil: Characterization, Antioxidant and Antibacterial Activities. Carbohydr. Polym. 2020, 236, 116075. [Google Scholar] [CrossRef]
- Nur Fatin Nazurah, R.; Noranizan, M.A.; Nor-Khaizura, M.A.R.; Nur Hanani, Z.A. Chitosan Nanoparticles Incorporate with Curry Leaf Essential Oil: Physicochemical Characterization and In Vitro Release Properties. Int. J. Biol. Macromol. 2024, 273, 132972. [Google Scholar] [CrossRef]
- Talebi, S.M.; Naser, A.; Ghorbanpour, M. Chemical Composition and Antimicrobial Activity of the Essential Oils in Different Populations of Coriandrum Sativum L. (Coriander) from Iran and Iraq. Food Sci. Nutr. 2024, 12, 3872–3882. [Google Scholar] [CrossRef]
- Park, M.K.; Cha, J.Y.; Kang, M.; Jang, H.W.; Choi, Y. The Effects of Different Extraction Methods on Essential Oils from Orange and Tangor: From the Peel to the Essential Oil. Food Sci. Nutr. 2024, 12, 804–814. [Google Scholar] [CrossRef]
- Scully, J.; Mustafa, A.S.; Hanif, A.; Tunio, J.H.; Tunio, S.N.J. Immune Responses to Methicillin-Resistant Staphylococcus aureus Infections and Advances in the Development of Vaccines and Immunotherapies. Vaccines 2024, 12, 1106. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Aqib, A.I.; Muzammil, I.; Majeed, N.; Bhutta, Z.A.; Kulyar, M.F.-A.; Fatima, M.; Zaheer, C.-N.F.; Muneer, A.; Murtaza, M.; et al. MRSA Compendium of Epidemiology, Transmission, Pathophysiology, Treatment, and Prevention Within One Health Framework. Front. Microbiol. 2023, 13, 1067284. [Google Scholar] [CrossRef] [PubMed]
- Nandhini, P.; Kumar, P.; Mickymaray, S.; Alothaim, A.S.; Somasundaram, J.; Rajan, M. Recent Developments in Methicillin-Resistant Staphylococcus aureus (MRSA) Treatment: A Review. Antibiotics 2022, 11, 606. [Google Scholar] [CrossRef] [PubMed]
- Nazli, A.; Tao, W.; You, H.; He, X.; He, Y. Treatment of MRSA Infection: Where Are We? Curr. Med. Chem. 2024, 31, 4425–4460. [Google Scholar] [CrossRef]
- Bassetti, M.; Righi, E. Safety Profiles of Old and New Antimicrobials for the Treatment of MRSA Infections. Expert Opin. Drug Saf. 2016, 15, 467–481. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial Activity of Eugenol and Essential Oils Containing Eugenol: A Mechanistic Viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef]
- Fuentes, C.; Fuentes, A.; Barat, J.M.; Ruiz, M.J. Relevant Essential Oil Components: A Minireview on Increasing Applications and Potential Toxicity. Toxicol. Mech. Methods 2021, 31, 559–565. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Mnichowska-Polanowska, M.; Pruss, A.; Masiuk, H.; Dzięcioł, M.; Giedrys-Kalemba, S.; Sienkiewicz, M. The Effect of Fennel Essential Oil in Combination with Antibiotics on Staphylococcus aureus Strains Isolated from Carriers. Burns 2017, 43, 1544–1551. [Google Scholar] [CrossRef]
- Simbu, S.; Orchard, A.; van Vuuren, S. Essential Oil Compounds in Combination with Conventional Antibiotics for Dermatology. Molecules 2024, 29, 1225. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Łopusiewicz, Ł.; Pruss, A.; Kostek, M.; Sienkiewicz, M.; Bonikowski, R.; Wojciechowska-Koszko, I.; Dołęgowska, B. Antibacterial Activity of Selected Essential Oil Compounds Alone and in Combination with β-Lactam Antibiotics Against MRSA Strains. Int. J. Mol. Sci. 2020, 21, 7106. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Łopusiewicz, Ł.; Kostek, M.; Drozłowska, E.; Pruss, A.; Wojciuk, B.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Dołęgowska, B. The Antibacterial Activity of Lavender Essential Oil Alone and in Combination with Octenidine Dihydrochloride Against MRSA Strains. Molecules 2020, 25, 95. [Google Scholar] [CrossRef]
- Neagu, R.; Popovici, V.; Ionescu, L.-E.; Ordeanu, V.; Biță, A.; Popescu, D.M.; Ozon, E.A.; Gîrd, C.E. Phytochemical Screening and Antibacterial Activity of Commercially Available Essential Oils Combinations with Conventional Antibiotics Against Gram-Positive and Gram-Negative Bacteria. Antibiotics 2024, 13, 478. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.Y.; Hwang, S.T.; Sethi, G.; Fan, L.; Arfuso, F.; Ahn, K.S. Potential Anti-Inflammatory and Anti-Cancer Properties of Farnesol. Molecules 2018, 23, 2827. [Google Scholar] [CrossRef] [PubMed]
- Sell, C.S. Terpenoids. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; ISBN 978-0-471-23896-6. [Google Scholar]
- Delmondes, G.D.; Santiago Lemos, I.C.; Dias, D.D.; Cunha, G.L.; Araújo, I.M.; Barbosa, R.; Coutinho, H.D.; Felipe, C.F.; Barbosa-Filho, J.M.; Lima, N.T.; et al. Pharmacological Applications of Farnesol (C15H26O): A Patent Review. Expert Opin. Ther. Pat. 2020, 30, 227–234. [Google Scholar] [CrossRef]
- Kaneko, M.; Togashi, N.; Hamashima, H.; Hirohara, M.; Inoue, Y. Effect of Farnesol on Mevalonate Pathway of Staphylococcus aureus. J. Antibiot. 2011, 64, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Jabra-Rizk, M.A.; Meiller, T.F.; James, C.E.; Shirtliff, M.E. Effect of Farnesol on Staphylococcus aureus Biofilm Formation and Antimicrobial Susceptibility. Antimicrob. Agents Chemother. 2006, 50, 1463–1469. [Google Scholar] [CrossRef]
- Kuroda, M.; Nagasaki, S.; Ohta, T. Sesquiterpene Farnesol Inhibits Recycling of the C55 Lipid Carrier of the Murein Monomer Precursor Contributing to Increased Susceptibility to β-Lactams in Methicillin-Resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2007, 59, 425–432. [Google Scholar] [CrossRef]
- Vincenzi, M.D.; Stammati, A.; Vincenzi, A.D.; Silano, M. Constituents of Aromatic Plants: Carvacrol. Fitoterapia 2004, 75, 801–804. [Google Scholar] [CrossRef]
- de Lira, M.H.P.; Júnior, F.P.d.A.; Moraes, G.F.Q.; Macena, G.d.S.; Pereira, F.d.O.; Lima, I.O. Antimicrobial Activity of Geraniol: An Integrative Review. J. Essent. Oil Res. 2020, 32, 187–197. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and Human Health: A Comprehensive Review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- Asadi, S.; Nayeri-Fasaei, B.; Zahraei-Salehi, T.; Yahya-Rayat, R.; Shams, N.; Sharifi, A. Antibacterial and Anti-Biofilm Properties of Carvacrol Alone and in Combination with Cefixime Against Escherichia Coli. BMC Microbiol. 2023, 23, 55. [Google Scholar] [CrossRef]
- Magi, G.; Marini, E.; Facinelli, B. Antimicrobial Activity of Essential Oils and Carvacrol, and Synergy of Carvacrol and Erythromycin, Against Clinical, Erythromycin-Resistant Group A Streptococci. Front. Microbiol. 2015, 6, 165. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Młodzińska, P.; Kilanowicz, A.; Dudzińska, E.; Kwiatkowski, P. Interaction of Selected Commercial Antiseptics with Natural Products Against Methicillin-Resistant Staphylococcus aureus Strain. Appl. Sci. 2024, 14, 2060. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant Activity of Eugenol: A Structure–Activity Relationship Study. J. Med. Food 2011, 14, 975–985. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Pruss, A.; Wojciuk, B.; Dołęgowska, B.; Wajs-Bonikowska, A.; Sienkiewicz, M.; Mężyńska, M.; Łopusiewicz, Ł. The Influence of Essential Oil Compounds on Antibacterial Activity of Mupirocin-Susceptible and Induced Low-Level Mupirocin-Resistant MRSA Strains. Molecules 2019, 24, 3105. [Google Scholar] [CrossRef]
- de Araújo, A.C.J.; Freitas, P.R.; dos Santos Barbosa, C.R.; Muniz, D.F.; de Almeida, R.S.; Alencar de Menezes, I.R.; Ribeiro-Filho, J.; Tintino, S.R.; Coutinho, H.D.M. In Vitro and In Silico Inhibition of Staphylococcus aureus Efflux Pump NorA by α-Pinene and Limonene. Curr. Microbiol. 2021, 78, 3388–3393. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.M.; Lima, E.D.O.; De Souza, E.L.; Diniz, M.D.F.F.M.; Trajano, V.N.; De Medeiros, I.A. Inhibitory Effect of Beta-Pinene, Alpha-Pinene and Eugenol on the Growth of Potential Infectious Endocarditis Causing Gram-Positive Bacteria. Rev. Bras. Ciências Farm. 2007, 43, 121–126. [Google Scholar] [CrossRef]
- Borges, J.C.; de Almeida Campos, L.A.; Kretzschmar, E.A.M.; Cavalcanti, I.M.F. Incorporation of Essential Oils in Polymeric Films for Biomedical Applications. Int. J. Biol. Macromol. 2024, 269, 132108. [Google Scholar] [CrossRef] [PubMed]
- Il’ina, I.V.; Volcho, K.P.; Salakhutdinov, N.F. Acid-Catalyzed Transformations of Pinane Terpenoids. New Prospects. Russ. J. Org. Chem. 2008, 44, 1–23. [Google Scholar] [CrossRef]
- Miladinović, D.L.; Ilić, B.S.; Kocić, B.D.; Ćirić, V.M.; Nikolić, D.M. Antibacterial Investigation of Thyme Essential Oil and Its Main Constituents in Combination with Tetracycline. J. Med. Food 2015, 18, 935–937. [Google Scholar] [CrossRef]
- Gu, K.; Ouyang, P.; Hong, Y.; Dai, Y.; Tang, T.; He, C.; Shu, G.; Liang, X.; Tang, H.; Zhu, L.; et al. Geraniol Inhibits Biofilm Formation of Methicillin-Resistant Staphylococcus aureus and Increase the Therapeutic Effect of Vancomycin In Vivo. Front. Microbiol. 2022, 13, 960728. [Google Scholar] [CrossRef] [PubMed]
- Merghni, A.; Belmamoun, A.R.; Urcan, A.C.; Bobiş, O.; Lassoued, M.A. 1,8-Cineol (Eucalyptol) Disrupts Membrane Integrity and Induces Oxidative Stress in Methicillin-Resistant Staphylococcus aureus. Antioxidants 2023, 12, 1388. [Google Scholar] [CrossRef] [PubMed]
- Sebei, K.; Sakouhi, F.; Herchi, W.; Khouja, M.L.; Boukhchina, S. Chemical Composition and Antibacterial Activities of Seven Eucalyptus Species Essential Oils Leaves. Biol. Res. 2015, 48, 7. [Google Scholar] [CrossRef] [PubMed]
- Johansen, B.; Duval, R.E.; Sergere, J.-C. First Evidence of a Combination of Terpinen-4-Ol and α-Terpineol as a Promising Tool Against ESKAPE Pathogens. Molecules 2022, 27, 7472. [Google Scholar] [CrossRef]
- Chadha, J.; Ravi; Singh, J.; Harjai, K. α-Terpineol Synergizes with Gentamicin to Rescue Caenorhabditis elegans from Pseudomonas aeruginosa Infection by Attenuating Quorum Sensing-Regulated Virulence. Life Sci. 2023, 313, 121267. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Mnichowska-Polanowska, M.; Pruss, A.; Dzięcioł, M.; Masiuk, H. Activity of Essential Oils against Staphylococcus aureus Strains Isolated from Skin Lesions in the Course of Staphylococcal Skin Infections. Herba Pol. 2017, 63, 43–52. [Google Scholar] [CrossRef]
- Mogana, R.; Adhikari, A.; Tzar, M.N.; Ramliza, R.; Wiart, C. Antibacterial Activities of the Extracts, Fractions and Isolated Compounds from Canarium patentinervium Miq. against Bacterial Clinical Isolates. BMC Complement. Med. Ther. 2020, 20, 55. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Pruss, A.; Grygorcewicz, B.; Wojciuk, B.; Dołęgowska, B.; Giedrys-Kalemba, S.; Kochan, E.; Sienkiewicz, M. Preliminary Study on the Antibacterial Activity of Essential Oils Alone and in Combination with Gentamicin Against Extended-Spectrum β-Lactamase-Producing and New Delhi Metallo-β-Lactamase-1-Producing Klebsiella pneumoniae Isolates. Microb. Drug Resist. 2018, 24, 1368–1375. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NJ, USA, 2016. [Google Scholar]
- Wickham, H. Reshaping Data with the Reshape Package. J. Stat. Softw. 2007, 21, 1–20. [Google Scholar] [CrossRef]
- Wickham, H.; Henry, L.; Pedersen, T.; Luciani, T.; Decorde, M.; Lise, V. Svglite: An "SVG" Graphics Device; The R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
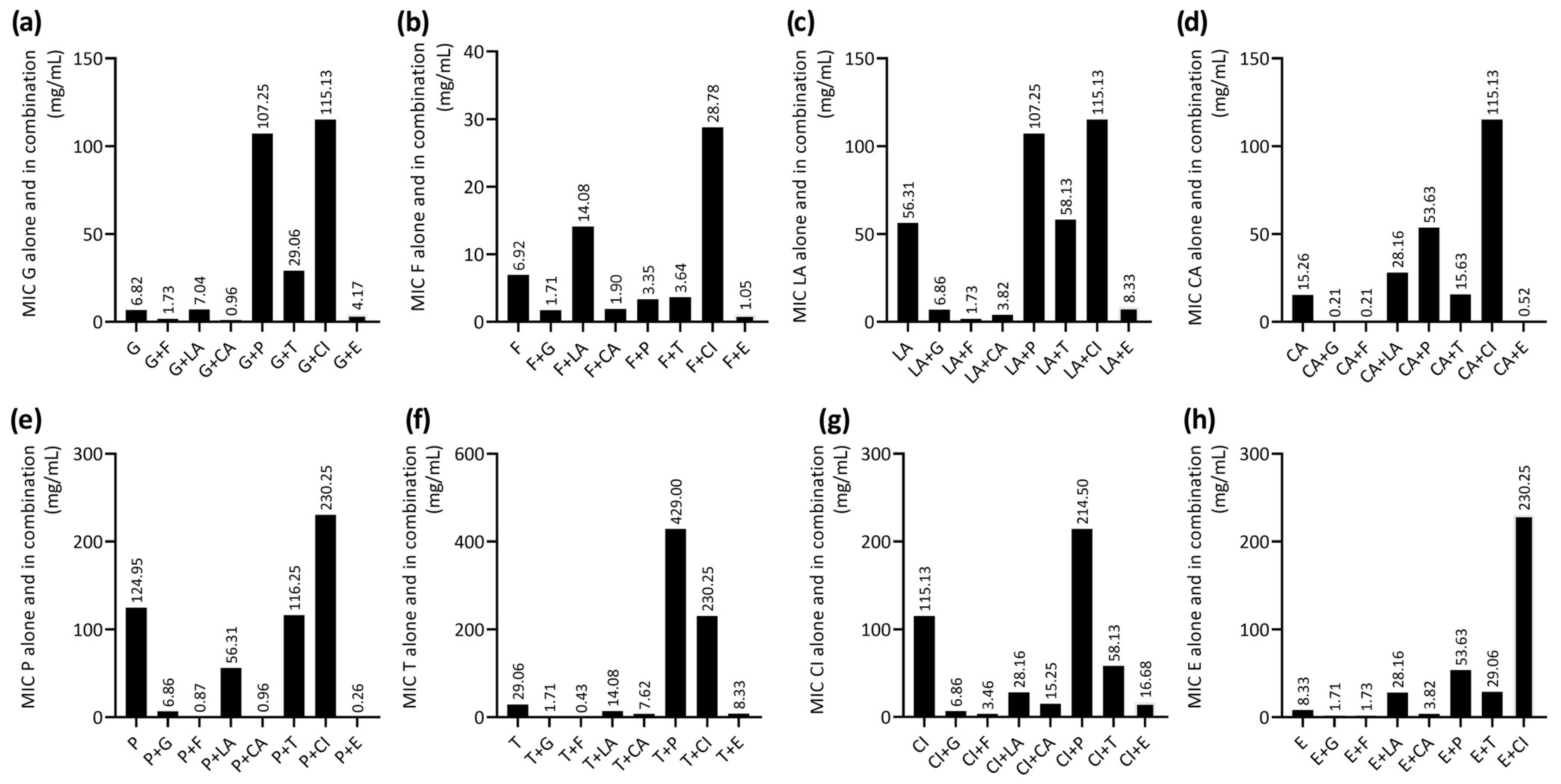
| EOC | MIC (mg/mL) | MBC (mg/mL) | MBC/MIC Ratio | Activity |
|---|---|---|---|---|
| Geraniol | 6.82 ± 0.00 | 6.82 ± 0.00 | 1 | Bactericidal |
| Farnesol | 6.92 ± 0.00 | 18.46 ± 0.00 | 3 | Bactericidal |
| Linalyl acetate | 56.31 ± 0.00 | 225.25 ± 0.00 | 4 | Bactericidal |
| Carvacrol | 15.26 ± 0.00 | 30.5 ± 0.00 | 2 | Bactericidal |
| (-)-α-Pinene | 124.95 ± 0.00 | 124.95 ± 0.00 | 1 | Bactericidal |
| α-Terpineol | 29.06 ± 0.00 | 29.06 ± 0.00 | 1 | Bactericidal |
| 1,8-Cineole | 115.13 ± 0.00 | 115.13 ± 0.00 | 1 | Bactericidal |
| Eugenol | 8.33 ± 0.00 | 8.33 ± 0.00 | 1 | Bactericidal |
| Vancomycin hydrochloride (control) | 0.0005 ± 0.0000 | 0.0005 ± 0.0000 | 1 | Bactericidal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartman, K.; Świerczyńska, M.; Wieczorek, A.; Baszuk, P.; Wojciechowska-Koszko, I.; Garbacz, K.; Sienkiewicz, M.; Kwiatkowski, P. Preliminary Evaluation of the Synergistic Antibacterial Effects of Selected Commercial Essential Oil Compounds Against Methicillin-Resistant Staphylococcus aureus ATCC 43300. Antibiotics 2025, 14, 733. https://doi.org/10.3390/antibiotics14070733
Hartman K, Świerczyńska M, Wieczorek A, Baszuk P, Wojciechowska-Koszko I, Garbacz K, Sienkiewicz M, Kwiatkowski P. Preliminary Evaluation of the Synergistic Antibacterial Effects of Selected Commercial Essential Oil Compounds Against Methicillin-Resistant Staphylococcus aureus ATCC 43300. Antibiotics. 2025; 14(7):733. https://doi.org/10.3390/antibiotics14070733
Chicago/Turabian StyleHartman, Kacper, Maja Świerczyńska, Amelia Wieczorek, Piotr Baszuk, Iwona Wojciechowska-Koszko, Katarzyna Garbacz, Monika Sienkiewicz, and Paweł Kwiatkowski. 2025. "Preliminary Evaluation of the Synergistic Antibacterial Effects of Selected Commercial Essential Oil Compounds Against Methicillin-Resistant Staphylococcus aureus ATCC 43300" Antibiotics 14, no. 7: 733. https://doi.org/10.3390/antibiotics14070733
APA StyleHartman, K., Świerczyńska, M., Wieczorek, A., Baszuk, P., Wojciechowska-Koszko, I., Garbacz, K., Sienkiewicz, M., & Kwiatkowski, P. (2025). Preliminary Evaluation of the Synergistic Antibacterial Effects of Selected Commercial Essential Oil Compounds Against Methicillin-Resistant Staphylococcus aureus ATCC 43300. Antibiotics, 14(7), 733. https://doi.org/10.3390/antibiotics14070733







