Trends in Positive Urine Culture Rates and Antimicrobial Resistance in Non-Hospitalized Children from Western Romania: A Retrospective Observational Study
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
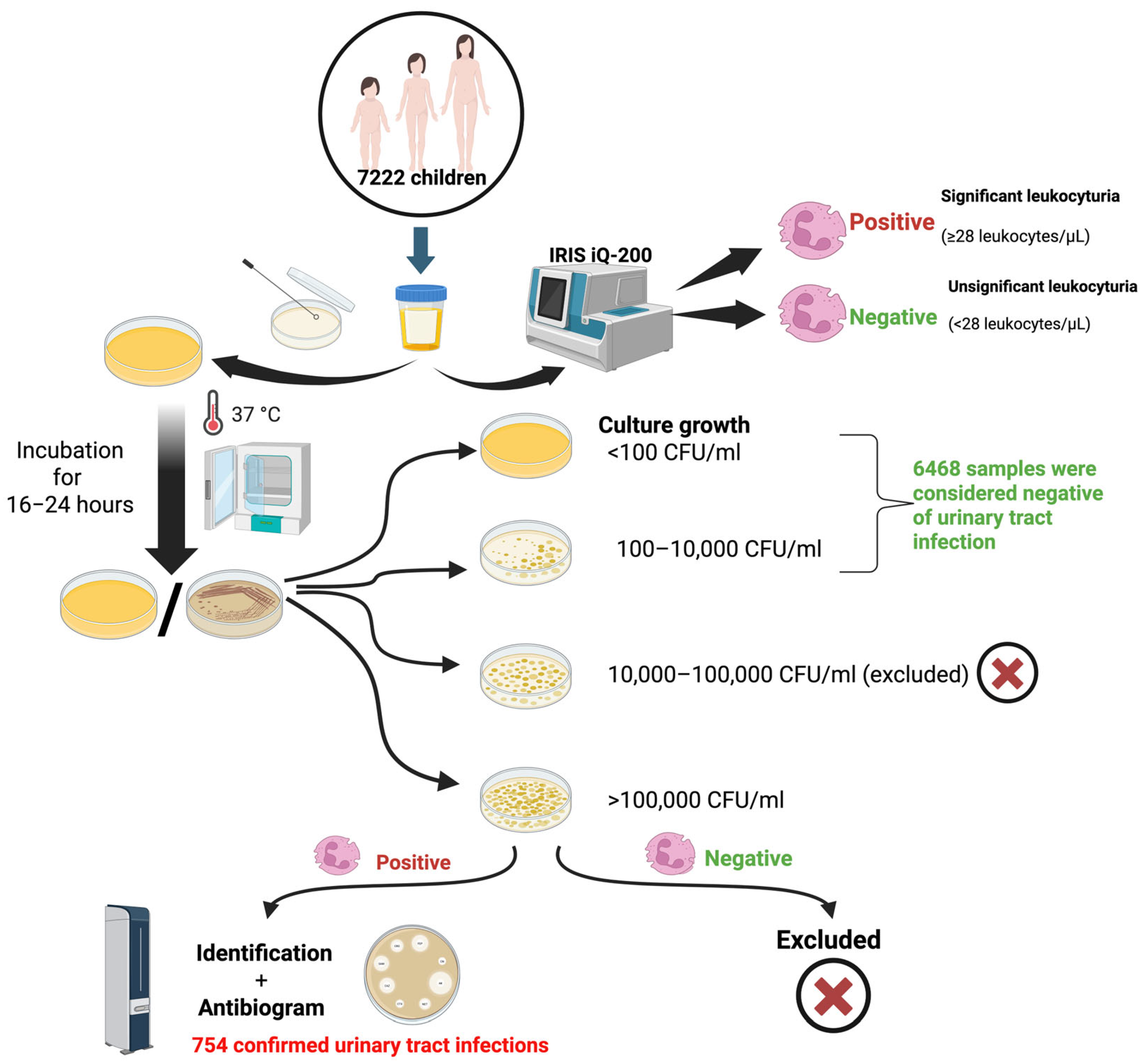
4.1. Study Design
4.2. Sample Collection
4.3. Culture
4.4. Interpretation of Results and Diagnosis Procedure
4.5. Assays
4.6. Data Collection and Statistical Analyses
4.7. Ethical Approval
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFU | Colony-forming unit; |
| UTI | Urinary tract infection; |
| Spp. | Several species; |
| ESBL | Extended-spectrum beta-lactamase. |
References
- Abraham, S.N.; Miao, Y. The Nature of Immune Responses to Urinary Tract Infections. Nat. Rev. Immunol. 2015, 15, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Al Lawati, H.; Blair, B.M.; Larnard, J. Urinary Tract Infections: Core Curriculum 2024. Am. J. Kidney Dis. 2024, 83, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Midiri, A.; Gerace, E.; Marra, M.; Zummo, S.; Biondo, C. Urinary Tract Infections: The Current Scenario and Future Prospects. Pathogens 2023, 12, 623. [Google Scholar] [CrossRef]
- Barola, S.; Grossman, O.K.; Abdelhalim, A. Urinary Tract Infections in Children. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK599548/ (accessed on 12 April 2025).
- Kaufman, J.; Temple-Smith, M.; Sanci, L. Urinary Tract Infections in Children: An Overview of Diagnosis and Management. BMJ Paediatr. Open 2019, 3, e000487. [Google Scholar] [CrossRef]
- Leung, A.K.C.; Wong, A.H.C.; Leung, A.A.M.; Hon, K.L. Urinary Tract Infection in Children. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 2–18. [Google Scholar] [CrossRef]
- Okarska-Napierała, M.; Wasilewska, A.; Kuchar, E. Urinary Tract Infection in Children: Diagnosis, Treatment, Imaging—Comparison of Current Guidelines. J. Pediatr. Urol. 2017, 13, 567–573. [Google Scholar] [CrossRef]
- Jamshidbeigi, T.; Adibi, A.; Hashemipour, S.M.A.; Sarokhani, D.; Dehkordi, A.H.; Fakhri, M.; Alaienezhad, S. A Systematic Review and Meta-Analysis of Prevalence of Urinary Tract Infection in Childhood. J. Ren. Inj. Prev. 2023, 12, e32160. [Google Scholar] [CrossRef]
- Flokas, M.E.; Detsis, M.; Alevizakos, M.; Mylonakis, E. Prevalence of ESBL-Producing Enterobacteriaceae in Paediatric Urinary Tract Infections: A Systematic Review and Meta-Analysis. J. Infect. 2016, 73, 547–557. [Google Scholar] [CrossRef] [PubMed]
- E Silva, A.C.S.; Oliveira, E.A.; Mak, R.H. Urinary Tract Infection in Pediatrics: An Overview. J. Pediatr. 2020, 96 (Suppl. S1), 65–79. [Google Scholar] [CrossRef]
- Suh, W.; Kim, B.N.; Kang, H.M.; Yang, E.A.; Rhim, J.-W.; Lee, K.-Y. Febrile Urinary Tract Infection in Children: Changes in Epidemiology, Etiology, and Antibiotic Resistance Patterns over a Decade. Clin. Exp. Pediatr. 2021, 64, 293–300. [Google Scholar] [CrossRef]
- Alfuraiji, N.; Al-Hamami, A.; Ibrahim, M.; Rajab, H.K.; Hussain, B.W. Uropathogenic Escherichia coli Virulence Characteristics and Antimicrobial Resistance amongst Pediatric Urinary Tract Infections. J. Med. Life 2022, 15, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Duicu, C.; Cozea, I.; Delean, D.; Aldea, A.A.; Aldea, C. Antibiotic Resistance Patterns of Urinary Tract Pathogens in Children from Central Romania. Exp. Ther. Med. 2021, 22, 748. [Google Scholar] [CrossRef] [PubMed]
- Miron, V.D.; Filimon, C.; Cabel, T.; Mihăescu, R.I.; Bar, G.; Leu, D.; Craiu, M. Urinary Tract Infections in Children: Clinical and Antimicrobial Resistance Data from Bucharest Area, Romania. Germs 2021, 11, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Rusu, A.; Petca, A.; Mares, C.; Petca, R.-C.; Popescu, R.I.; Negoita, S.; Danau, R.A.; Chibelean, C.B.; Jinga, V. Urinary Tract Infections in a Romanian Population: Antimicrobial Resistance of Uropathogens—A Multiregional Study. Farmacia 2023, 71, 19. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 13.0 [Internet]. 2023. Available online: http://www.eucast.org (accessed on 10 April 2025).
- O’Brien, K.; Edwards, A.; Hood, K.; Butler, C.C. Prevalence of Urinary Tract Infection in Acutely Unwell Children in General Practice: A Prospective Study with Systematic Urine Sampling. Br. J. Gen. Pract. 2013, 63, e156–e164. [Google Scholar] [CrossRef]
- Daniel, M.; Szymanik-Grzelak, H.; Sierdziński, J.; Podsiadły, E.; Kowalewska-Młot, M.; Pańczyk-Tomaszewska, M. Epidemiology and Risk Factors of UTIs in Children—A Single-Center Observation. J. Pers. Med. 2023, 13, 138. [Google Scholar] [CrossRef]
- Alrasheedy, M.; Abousada, H.J.; Abdulhaq, M.M.; Alsayed, R.A.; Alghamdi, K.A.; Alghamdi, F.D.; Al Muaibid, A.F.; Ajjaj, R.G.; Almohammadi, S.S.; Almohammadi, S.S.; et al. Prevalence of Urinary Tract Infection in Children in the Kingdom of Saudi Arabia. Arch. Ital. Urol. Androl. 2021, 93, 206–210. [Google Scholar] [CrossRef]
- Freeman, M.C.; Garn, J.V.; Sclar, G.D.; Boisson, S.; Medlicott, K.; Alexander, K.T.; Penakalapati, G.; Anderson, D.; Mahtani, A.G.; Grimes, J.E.T.; et al. The Impact of Sanitation on Infectious Disease and Nutritional Status: A Systematic Review and Meta-Analysis. Int. J. Hyg. Environ. Health 2017, 220, 928–949. [Google Scholar] [CrossRef]
- Hellström, A.L.; Hanson, E.; Hansson, S.; Hjälmås, K.; Jodal, U. Micturition Habits and Incontinence in 7-Year-Old Swedish School Entrants. Eur. J. Pediatr. 1990, 149, 434–437. [Google Scholar] [CrossRef]
- Schum, T.R.; Kolb, T.M.; McAuliffe, T.L.; Simms, M.D.; Underhill, R.L.; Lewis, M. Sequential Acquisition of Toilet-Training Skills: A Descriptive Study of Gender and Age Differences in Normal Children. Pediatrics 2002, 109, E48. [Google Scholar] [CrossRef]
- Shaikh, N.; Morone, N.E.; Bost, J.E.; Farrell, M.H. Prevalence of Urinary Tract Infection in Childhood: A Meta-Analysis. Pediatr. Infect. Dis. J. 2008, 27, 302–308. [Google Scholar] [CrossRef]
- Boon, H.A.; Struyf, T.; Crèvecoeur, J.; Delvaux, N.; Van Pottelbergh, G.; Vaes, B.; Van den Bruel, A.; Verbakel, J.Y. Incidence Rates and Trends of Childhood Urinary Tract Infections and Antibiotic Prescribing: Registry-Based Study in General Practices (2000 to 2020). BMC Prim. Care 2022, 23, 177. [Google Scholar] [CrossRef]
- Aziz, M.; Davis, G.S.; Park, D.E.; Idris, A.H.; Sariya, S.; Wang, Y.; Zerbonne, S.; Nordstrom, L.; Weaver, B.; Statham, S.; et al. Pediatric Urinary Tract Infections Caused by Poultry-Associated Escherichia coli. Microbiol. Spectr. 2024, 12, e0341523. [Google Scholar] [CrossRef]
- Bergeron, C.R.; Prussing, C.; Boerlin, P.; Daignault, D.; Dutil, L.; Reid-Smith, R.J.; Zhanel, G.G.; Manges, A.R. Chicken as Reservoir for Extraintestinal Pathogenic Escherichia coli in Humans, Canada. Emerg. Infect. Dis. 2012, 18, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Brătfelan, D.O.; Tabaran, A.; Colobatiu, L.; Mihaiu, R.; Mihaiu, M. Prevalence and Antimicrobial Resistance of Escherichia coli Isolates from Chicken Meat in Romania. Animals 2023, 13, 3488. [Google Scholar] [CrossRef] [PubMed]
- Connolly, G.; Clark, C.M.; Campbell, R.E.; Byers, A.W.; Reed, J.B.; Campbell, W.W. Poultry Consumption and Human Health: How Much Is Really Known? A Systematically Searched Scoping Review and Research Perspective. Adv. Nutr. 2022, 13, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Cogan, T.A.; Bloomfield, S.F.; Humphrey, T.J. The Effectiveness of Hygiene Procedures for Prevention of Cross-Contamination from Chicken Carcases in the Domestic Kitchen. Lett. Appl. Microbiol. 1999, 29, 354–358. [Google Scholar] [CrossRef]
- Linton, A.H.; Howe, K.; Bennett, P.M.; Richmond, M.H.; Whiteside, E.J. The Colonization of the Human Gut by Antibiotic Resistant Escherichia coli from Chickens. J. Appl. Bacteriol. 1977, 43, 465–469. [Google Scholar] [CrossRef]
- Isac, R.; Doros, G.; Stolojanu, C.-A.; Steflea, R.M.; Stroescu, R.F.; Olariu, I.-C.; Micsescu-Olah, A.-M.; Gafencu, M. General Characteristics and Current State of Antibiotic Resistance in Pediatric Urinary Tract Infection-A Single Center Experience. Antibiotics 2024, 13, 684. [Google Scholar] [CrossRef]
- Hickling, D.R.; Sun, T.-T.; Wu, X.-R. Anatomy and Physiology of the Urinary Tract: Relation to Host Defense and Microbial Infection. Microbiol. Spectr. 2015, 3, 1–17. [Google Scholar] [CrossRef]
- Yamamoto, S.; Tsukamoto, T.; Terai, A.; Kurazono, H.; Takeda, Y.; Yoshida, O. Genetic Evidence Supporting the Fecal-Perineal-Urethral Hypothesis in Cystitis Caused by Escherichia coli. J. Urol. 1997, 157, 1127–1129. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef]
- Stumpf, R.M.; Wilson, B.A.; Rivera, A.; Yildirim, S.; Yeoman, C.J.; Polk, J.D.; White, B.A.; Leigh, S.R. The Primate Vaginal Microbiome: Comparative Context and Implications for Human Health and Disease. Am. J. Phys. Anthropol. 2013, 152 (Suppl. S57), 119–134. [Google Scholar] [CrossRef] [PubMed]
- Hickey, R.J.; Zhou, X.; Settles, M.L.; Erb, J.; Malone, K.; Hansmann, M.A.; Shew, M.L.; Van Der Pol, B.; Fortenberry, J.D.; Forney, L.J. Vaginal Microbiota of Adolescent Girls Prior to the Onset of Menarche Resemble Those of Reproductive-Age Women. mBio 2015, 6, e00097-15. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-F.; Chen, W.-L.; Huang, I.-F.; Chen, J.-R.; Chiou, Y.-H.; Chen, Y.-S.; Lee, S.S.-J.; Hung, W.-Y.; Hung, C.-H.; Wang, J.-L. Urinary Tract Infection in Infants Caused by Extended-Spectrum Beta-Lactamase-Producing Escherichia coli: Comparison between Urban and Rural Hospitals. Pediatr. Nephrol. 2016, 31, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Puro, N.A.; Feyereisen, S. Telehealth Availability in US Hospitals in the Face of the COVID-19 Pandemic. J. Rural Health 2020, 36, 577–583. [Google Scholar] [CrossRef]
- Shaver, J. The State of Telehealth Before and After the COVID-19 Pandemic. Prim. Care Clin. Off. Pract. 2022, 49, 517–530. [Google Scholar] [CrossRef]
- Cai, T.; Tascini, C.; Novelli, A.; Anceschi, U.; Bonkat, G.; Wagenlehner, F.; Bjerklund Johansen, T.E. The Management of Urinary Tract Infections during the COVID-19 Pandemic: What Do We Need to Know? Uro 2022, 2, 55–64. [Google Scholar] [CrossRef]
- Harju, E.; Speierer, A.; Jungo, K.T.; Levati, S.; Baggio, S.; Tancredi, S.; Noor, N.; Rodondi, P.-Y.; Cullati, S.; Imboden, M.; et al. Changes in Healthcare Utilization During the COVID-19 Pandemic and Potential Causes—A Cohort Study From Switzerland. Int. J. Public Health 2023, 68, 1606010. [Google Scholar] [CrossRef]
- Liang, D.; Wang, M.E.; Dahlen, A.; Liao, Y.; Saunders, A.C.; Coon, E.R.; Schroeder, A.R. Incidence of Pediatric Urinary Tract Infections Before and During the COVID-19 Pandemic. JAMA Netw. Open 2024, 7, e2350061. [Google Scholar] [CrossRef]
- Werbel, K.; Jankowska, D.; Wasilewska, A.; Taranta-Janusz, K. Clinical and Epidemiological Analysis of Children’s Urinary Tract Infections in Accordance with Antibiotic Resistance Patterns of Pathogens. J. Clin. Med. 2021, 10, 5260. [Google Scholar] [CrossRef]
- Alsubaie, M.A.; Alsuheili, A.Z.; Aljehani, M.N.; Alothman, A.A.; Alzahrani, A.S.; Mohammedfadel, H.A.; Alnajjar, A.A. Pediatric Community Acquired Urinary Tract Infections Due to Extended-Spectrum Beta-Lactamase versus Non-Extended-Spectrum Beta-Lactamase Producing Bacteria. Pediatr. Int. 2023, 65, e15620. [Google Scholar] [CrossRef] [PubMed]
- Bryce, A.; Hay, A.D.; Lane, I.F.; Thornton, H.V.; Wootton, M.; Costelloe, C. Global Prevalence of Antibiotic Resistance in Paediatric Urinary Tract Infections Caused by Escherichia coli and Association with Routine Use of Antibiotics in Primary Care: Systematic Review and Meta-Analysis. BMJ 2016, 352, i939. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Kanwar, N.; Morgan, G.M.; Mendes, R.E.; Lee, B.R.; Banerjee, D.; Selvarangan, R. Antimicrobial Susceptibility Trends in E. coli Causing Pediatric Urinary Tract Infections in the United States. Pathogens 2024, 13, 1068. [Google Scholar] [CrossRef] [PubMed]
- Perween, N.; Rai, S.; Nandwani, S.; Kumar, S.K. Retrospective Analysis of Urinary Tract Infection in the Pediatric Population at a Tertiary Care Centre. Cureus 2022, 14, e24796. [Google Scholar] [CrossRef]
- Whelan, S.O.; Kyne, S.; Dore, A.; Glynn, M.; Higgins, F.; Hanahoe, B.; Moriarty, F.; Moylett, E.; Cormican, M. Paediatric Escherichia coli Urinary Tract Infection: Susceptibility Trends and Clinical Management-a Retrospective Analysis of a 10-Year Period. Ir. J. Med. Sci. 2024, 193, 1891–1900. [Google Scholar] [CrossRef]
- Godaly, G.; Ambite, I.; Svanborg, C. Innate Immunity and Genetic Determinants of Urinary Tract Infection Susceptibility. Curr. Opin. Infect. Dis. 2015, 28, 88–96. [Google Scholar] [CrossRef]
- Lu, J.; Liu, X.; Wei, Y.; Yu, C.; Zhao, J.; Wang, L.; Hu, Y.; Wei, G.; Wu, S. Clinical and Microbial Etiology Characteristics in Pediatric Urinary Tract Infection. Front. Pediatr. 2022, 10, 844797. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Z.; Zheng, L.; Gong, Z.; Li, Y.; Jin, Y.; Huang, Y.; Chi, M. Urinary Tract Infections Caused by Uropathogenic Escherichia coli: Mechanisms of Infection and Treatment Options. Int. J. Mol. Sci. 2023, 24, 10537. [Google Scholar] [CrossRef]
- Kılıç, F.E.; Küçükkelepçe, O. Evaluating Antibiotic Resistance in Pediatric UTIs: Five-Year Data from a Tertiary Hospital in Turkey. Medicina 2025, 61, 402. [Google Scholar] [CrossRef]
- Rodríguez-Lozano, J.; de Malet, A.; Cano, M.E.; de la Rubia, L.; Wallmann, R.; Martínez-Martínez, L.; Calvo, J. Antimicrobial Susceptibility of Microorganisms That Cause Urinary Tract Infections in Pediatric Patients. Enfermedades Infecc. Y Microbiol. Clin. 2018, 36, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Pierantoni, L.; Andreozzi, L.; Ambretti, S.; Dondi, A.; Biagi, C.; Baccelli, F.; Lanari, M. Three-Year Trend in Escherichia coli Antimicrobial Resistance among Children’s Urine Cultures in an Italian Metropolitan Area. Children 2021, 8, 597. [Google Scholar] [CrossRef] [PubMed]
- Altamimi, I.; Almazyed, A.; Alshammary, S.; Altamimi, A.; Alhumimidi, A.; Alnutaifi, R.; Malhis, M.; Altamimi, A. Bacterial Pathogens and Antimicrobial Susceptibility Patterns of Urinary Tract Infections in Children during COVID-19 2019–2020: A Large Tertiary Care Center in Saudi Arabia. Children 2023, 10, 971. [Google Scholar] [CrossRef]
- Shkalim Zemer, V.; Ashkenazi, S.; Levinsky, Y.; Richenberg, Y.; Jacobson, E.; Nathanson, S.; Shochat, T.; Kushnir, S.; Cohen, M.; Cohen, A.H. Pathogens Causing Pediatric Community Acquired Urinary Tract Infections and Their Increasing Antimicrobial Resistance: A Nationwide Study. Pathogens 2024, 13, 201. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Oliveira, Í.; Pereira, M.; Mendes, P.; Virtuoso, M.J.; Pereira, A. Local Antimicrobial Resistance Trends in Pediatric Urinary Tract Infection: The Importance of Local Surveillance of a Global Problem. Cureus 2024, 16, e54700. [Google Scholar] [CrossRef]
- Esposito, S.; Maglietta, G.; Di Costanzo, M.; Ceccoli, M.; Vergine, G.; La Scola, C.; Malaventura, C.; Falcioni, A.; Iacono, A.; Crisafi, A.; et al. Retrospective 8-Year Study on the Antibiotic Resistance of Uropathogens in Children Hospitalised for Urinary Tract Infection in the Emilia-Romagna Region, Italy. Antibiotics 2021, 10, 1207. [Google Scholar] [CrossRef]
- Logan, L.K.; Braykov, N.P.; Weinstein, R.A.; Laxminarayan, R.; CDC Epicenters Prevention Program. Extended-Spectrum β-Lactamase-Producing and Third-Generation Cephalosporin-Resistant Enterobacteriaceae in Children: Trends in the United States, 1999–2011. J. Pediatr. Infect. Dis. Soc. 2014, 3, 320–328. [Google Scholar] [CrossRef]
- Romanian Ministry of Health. Order No. 1101 of September 30, 2016 for the Approval the Norms for the Surveillance, Prevention and Limitation of Healthcare-Associated Infections. Available online: https://Legislatie.Just.Ro/Public/DetaliiDocument/182388 (accessed on 26 May 2025). (In Romanian).
- Government of Romania. Decision No. 879 of November 9, 2018 for the Establishment of the National Committee for Limiting Antimicrobial Resistance. Available online: https://Legislatie.Just.Ro/Public/DetaliiDocument/207007 (accessed on 16 May 2025). (In Romanian).
- Parliament of Romania. Law No. 3 of January 8, 2021 for the Prevention, Diagnosis, and Treatment of Healthcare-Associated Infections. Available online: https://Legislatie.Just.Ro/Public/DetaliiDocument/235999 (accessed on 16 May 2025). (In Romanian).
- Romanian Ministry of Health. Order No. 487 of March 23, 2020 for the Approval of the Treatment Protocol for SARS-CoV-2 Infection. Available online: https://Legislatie.Just.Ro/Public/DetaliiDocument/224341 (accessed on 16 May 2025). (In Romanian).
- Peñalva, G.; Fernández-Urrusuno, R.; Turmo, J.M.; Hernández-Soto, R.; Pajares, I.; Carrión, L.; Vázquez-Cruz, I.; Botello, B.; García-Robredo, B.; Cámara-Mestres, M.; et al. Long-Term Impact of an Educational Antimicrobial Stewardship Programme in Primary Care on Infections Caused by Extended-Spectrum β-Lactamase-Producing Escherichia coli in the Community: An Interrupted Time-Series Analysis. Lancet Infect. Dis. 2020, 20, 199–207. [Google Scholar] [CrossRef]
- Lee, J.; Pai, H.; Kim, Y.K.; Kim, N.H.; Eun, B.W.; Kang, H.J.; Park, K.H.; Choi, E.H.; Shin, H.Y.; Kim, E.C.; et al. Control of Extended-Spectrum Beta-Lactamase-Producing Escherichia coli and Klebsiella Pneumoniae in a Children’s Hospital by Changing Antimicrobial Agent Usage Policy. J. Antimicrob. Chemother. 2007, 60, 629–637. [Google Scholar] [CrossRef]
- Lan, C.-K.; Hsueh, P.-R.; Wong, W.-W.; Fung, C.-P.; Lau, Y.-T.; Yeung, J.Y.K.; Young, G.T.; Su, C.-C. Association of Antibiotic Utilization Measures and Reduced Incidence of Infections with Extended-Spectrum Beta-Lactamase-Producing Organisms. J. Microbiol. Immunol. Infect. 2003, 36, 182–186. [Google Scholar]
- Thamlikitkul, V.; Tangkoskul, T.; Seenama, C. Fecal Carriage Rate of Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae as a Proxy Composite Indicator of Antimicrobial Resistance in a Community in Thailand. Open Forum Infect. Dis. 2019, 6, ofz425. [Google Scholar] [CrossRef]
- Subbiah, M.; Top, E.M.; Shah, D.H.; Call, D.R. Selection Pressure Required for Long-Term Persistence of blaCMY-2-Positive IncA/C Plasmids. Appl. Environ. Microbiol. 2011, 77, 4486–4493. [Google Scholar] [CrossRef] [PubMed]
- Cottell, J.L.; Webber, M.A.; Piddock, L.J.V. Persistence of Transferable Extended-Spectrum-β-Lactamase Resistance in the Absence of Antibiotic Pressure. Antimicrob. Agents Chemother. 2012, 56, 4703–4706. [Google Scholar] [CrossRef] [PubMed]
- Fasugba, O.; Mitchell, B.G.; Mnatzaganian, G.; Das, A.; Collignon, P.; Gardner, A. Five-Year Antimicrobial Resistance Patterns of Urinary Escherichia coli at an Australian Tertiary Hospital: Time Series Analyses of Prevalence Data. PLoS ONE 2016, 11, e0164306. [Google Scholar] [CrossRef] [PubMed]
- Cullen, I.M.; Manecksha, R.P.; McCullagh, E.; Ahmad, S.; O’Kelly, F.; Flynn, R.J.; McDermott, T.; Murphy, P.; Grainger, R.; Fennell, J.P.; et al. The Changing Pattern of Antimicrobial Resistance within 42,033 Escherichia coli Isolates from Nosocomial, Community and Urology Patient-Specific Urinary Tract Infections, Dublin, 1999–2009. BJU Int. 2012, 109, 1198–1206. [Google Scholar] [CrossRef]
- Hastak, P.; Fourment, M.; Darling, A.E.; Gottlieb, T.; Cheong, E.; Merlino, J.; Myers, G.S.A.; Djordjevic, S.P.; Roy Chowdhury, P. Escherichia coli ST8196 Is a Novel, Locally Evolved, and Extensively Drug Resistant Pathogenic Lineage within the ST131 Clonal Complex. Emerg. Microbes Infect. 2020, 9, 1780–1792. [Google Scholar] [CrossRef]
- Abuhandan, M.; Güzel, B.; Oymak, Y.; Çiftçi, H. Antibiotic Sensitivity and Resistance in Children with Urinary Tract Infection in Sanliurfa. Turk. J. Urol. 2013, 39, 106–110. [Google Scholar] [CrossRef]
- Wu, C.-T.; Lee, H.-Y.; Chen, C.-L.; Tuan, P.-L.; Chiu, C.-H. High Prevalence and Antimicrobial Resistance of Urinary Tract Infection Isolates in Febrile Young Children without Localizing Signs in Taiwan. J. Microbiol. Immunol. Infect. 2016, 49, 243–248. [Google Scholar] [CrossRef]
- Samanci, S.; Pınarbaşı, A.S. Microbial Etiology and Antibiotic Resistance in Urinary Tract Infections in Children; View from an Area Where Antibiotics Are Overused. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 7680–7687. [Google Scholar] [CrossRef]
- Wang, J.-T.; Chen, P.-C.; Chang, S.-C.; Shiau, Y.-R.; Wang, H.-Y.; Lai, J.-F.; Huang, I.-W.; Tan, M.-C.; Lauderdale, T.-L.Y. TSAR Hospitals Antimicrobial Susceptibilities of Proteus mirabilis: A Longitudinal Nationwide Study from the Taiwan Surveillance of Antimicrobial Resistance (TSAR) Program. BMC Infect. Dis. 2014, 14, 486. [Google Scholar] [CrossRef]
- Edlin, R.S.; Shapiro, D.J.; Hersh, A.L.; Copp, H.L. Antibiotic Resistance Patterns of Outpatient Pediatric Urinary Tract Infections. J. Urol. 2013, 190, 222–227. [Google Scholar] [CrossRef]
- Stock, I. Natural Antibiotic Susceptibility of Proteus spp., with Special Reference to P. mirabilis and P. penneri Strains. J. Chemother. 2003, 15, 12–26. [Google Scholar] [CrossRef]
- Al Benwan, K.; Jamal, W. Etiology and Antibiotic Susceptibility Patterns of Urinary Tract Infections in Children in a General Hospital in Kuwait: A 5-Year Retrospective Study. Med. Princ. Pract. 2022, 31, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Bagnasco, F.; Lorenzini Ceradelli, F.; Mesini, A.; Saffioti, C.; Ricci, E.; Russo, C.; Mariani, M.; Ugolotti, E.; Caci, E.; Contu, D.; et al. Etiology and Oral Antibiotic Susceptibility Patterns of the First Urinary Tract Infection Episode in Infants Under 6 Months of Age: A 17-Year, Retrospective, Single-Center Study in Italy. Microorganisms 2025, 13, 607. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-S.; Kim, D.R.; Baek, J.Y.; Shin, A.; Kim, K.-R.; Park, H.; Son, S.; Cho, H.; Kim, Y.-J. Susceptibility to Fosfomycin and Nitrofurantoin of ESBL-Positive Escherichia coli and Klebsiella pneumoniae Isolated From Urine of Pediatric Patients. J. Korean Med. Sci. 2023, 38, e361. [Google Scholar] [CrossRef] [PubMed]
- Bitsori, M.; Maraki, S.; Raissaki, M.; Bakantaki, A.; Galanakis, E. Community-Acquired Enterococcal Urinary Tract Infections. Pediatr. Nephrol. 2005, 20, 1583–1586. [Google Scholar] [CrossRef]
- Roberts, K.B.; Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management. Urinary Tract Infection: Clinical Practice Guideline for the Diagnosis and Management of the Initial UTI in Febrile Infants and Children 2 to 24 Months. Pediatrics 2011, 128, 595–610. [Google Scholar] [CrossRef]
- Guan, L.; Beig, M.; Wang, L.; Navidifar, T.; Moradi, S.; Motallebi Tabaei, F.; Teymouri, Z.; Abedi Moghadam, M.; Sedighi, M. Global Status of Antimicrobial Resistance in Clinical Enterococcus faecalis Isolates: Systematic Review and Meta-Analysis. Ann. Clin. Microbiol. Antimicrob. 2024, 23, 80. [Google Scholar] [CrossRef]
- Sattari-Maraji, A.; Jabalameli, F.; Node Farahani, N.; Beigverdi, R.; Emaneini, M. Antimicrobial Resistance Pattern, Virulence Determinants and Molecular Analysis of Enterococcus faecium Isolated from Children Infections in Iran. BMC Microbiol. 2019, 19, 156. [Google Scholar] [CrossRef]
- Karah, N.; Rafei, R.; Elamin, W.; Ghazy, A.; Abbara, A.; Hamze, M.; Uhlin, B.E. Guideline for Urine Culture and Biochemical Identification of Bacterial Urinary Pathogens in Low-Resource Settings. Diagnostics 2020, 10, 832. [Google Scholar] [CrossRef]
- Kaufman, J.; Temple-Smith, M.; Sanci, L. Urine Sample Collection from Young Pre-Continent Children: Common Methods and the New Quick-Wee Technique. Br. J. Gen. Pract. 2019, 70, 42–43. [Google Scholar] [CrossRef]
| Variable (n) | Samples Investigated for UTI | Univariate Logistic Regression | |||
|---|---|---|---|---|---|
| Age Groups (Years) | Positive (%) | Negative (%) | cOR | 95% CI | p Value |
| 1–5 (3784) | 336 (8.88) | 3448 (91.12) | Ref. | ||
| 6–12 (2302) | 276 (11.99) | 2026 (88.01) | 1.4 | 1.18–1.65 | <0.001 |
| 13–18 (1136) | 142 (12.5) | 994 (87.5) | 1.47 | 1.19–1.81 | <0.001 |
| Gender | |||||
| Male (2548) | 143 (5.63) | 2395 (94.37) | Ref. | ||
| Female (4684) | 611 (13.04) | 4073 (86.96) | 2.51 | 2.08–3.03 | <0.001 |
| Area of residence | |||||
| Rural (1667) | 181 (10.86) | 1486 (89.14) | Ref. | ||
| Urban (5555) | 573 (10.32) | 4982 (89.68) | 0.94 | 0.79–1.13 | 0.53 |
| Total (7222) | 754 (10.44) | 6468 (89.56) | |||
| Samples Investigated for UTI | Univariate Logistic Regression | ||||
|---|---|---|---|---|---|
| Year (n) | Positive (%) | Negative (%) | cOR | 95% CI | p Value |
| 2016 (991) | 123 (12.41) | 868 (87.59) | Ref. | ||
| 2017 (908) | 92 (10.13) | 816 (89.87) | 0.8 | 0.6–1.06 | 0.12 |
| 2018 (1121) | 111 (9.9) | 1010 (90.1) | 0.78 | 0.59–1.02 | 0.07 |
| 2019 (1212) | 124 (10.23) | 1088 (89.77) | 0.8 | 0.62–1.10 | 0.11 |
| 2020 (963) | 102 (10.59) | 861 (89.41) | 0.84 | 0.63–1.10 | 0.21 |
| 2021 (1146) | 115 (10.03) | 1031 (89.97) | 0.79 | 0.60–1.03 | 0.08 |
| 2022 (881) | 87 (9.88) | 794 (90.12) | 0.77 | 0.58–1.03 | 0.08 |
| Total (7222) | 754 (10.44) | 6468 (89.56) | |||
| Variable (n) | Samples Investigated for UTI | Multivariate Logistic Regression | |||
|---|---|---|---|---|---|
| Age Groups (Years) (Ref.: 1–5) | Positive (%) | Negative (%) | aOR | 95% CI | p Value |
| 6–12 (2302) | 276 (11.99) | 2026 (88.01) | 1.35 | 1.14–1.60 | <0.001 |
| 13–18 (1136) | 142 (12.5) | 994 (87.5) | 1.34 | 1.08–1.65 | 0.007 |
| Gender (Ref.: Males) | |||||
| Females (4684) | 611 (13.04) | 4073 (86.96) | 2.45 | 2.03–2.97 | <0.001 |
| Area of Residence (Ref.: Rural) | |||||
| Urban (5555) | 573 (10.32) | 4982 (89.68) | 0.98 | 0.82–1.17 | 0.81 |
| Year (Ref.: 2016) | |||||
| 2017 (908) | 92 (10.13) | 816 (89.87) | 0.82 | 0.62–1.10 | 0.19 |
| 2018 (1121) | 111 (9.9) | 1010 (90.1) | 0.81 | 0.61–1.06 | 0.13 |
| 2019 (1212) | 124 (10.23) | 1088 (89.77) | 0.84 | 0.64–1.09 | 0.19 |
| 2020 (963) | 102 (10.59) | 861 (89.41) | 0.9 | 0.68–1.20 | 0.48 |
| 2021 (1146) | 115 (10.03) | 1031 (89.97) | 0.84 | 0.64–1.10 | 0.2 |
| 2022 (881) | 87 (9.88) | 794 (90.12) | 0.81 | 0.60–1.08 | 0.16 |
| Pathogen | Number of Positive Urine Samples 754 (100%) |
|---|---|
| E. coli | 501 (66.45%) |
| Proteus mirabilis | 95 (12.6%) |
| Klebsiella pneumoniae | 40 (5.31%) |
| Enterococcus faecalis | 35 (4.64%) |
| Pseudomonas aeruginosa | 19 (2.52%) |
| Staphylococcus saprophyticus | 9 (1.19%) |
| Streptococcus agalactiae | 9 (1.19%) |
| Klebsiella oxytoca | 6 (0.8%) |
| Coagulase negative Staphylococcus | 5 (0.66%) |
| Enterococcus spp. | 4 (0.53%) |
| Morganella morganii | 4 (0.53%) |
| Enterobacter cloacae | 3 (0.4%) |
| Others * | 24 (3.2%) |
| E. coli | Antibiotic Resistance | ||
|---|---|---|---|
| Antibacterials (n) | Susceptible n (%) | Intermediate n (%) | Resistant n (%) |
| Ampicillin (501) | 185 (36.93) | 4 (0.8) | 312 (62.28) |
| Amoxicillin + clavulanic acid (501) | 376 (75.05) | 56 (11.18) | 69 (13.77) |
| Piperacillin + tazobactam (499) | 434 (86.97) | 26 (5.21) | 39 (7.82) |
| Cefotaxime (478) | 421 (88.08) | 17 (3.56) | 40 (8.37) |
| Ceftazidime (501) | 437 (87.23) | 32 (6.39) | 32 (6.39) |
| Cefepime (451) | 403 (89.36) | 21 (4.66) | 27 (5.99) |
| Ertapenem (451) | 451 (100) | 0 | 0 |
| Imipenem (501) | 501 (100) | 0 | 0 |
| Meropenem (501) | 501 (100) | 0 | 0 |
| Amikacin (451) | 391 (86.7) | 59 (13.08) | 1 (0.22) |
| Gentamicin (501) | 454 (90.62) | 1 (0.2) | 46 (9.18) |
| Ciprofloxacin (501) | 428 (85.43) | 8 (1.6) | 65 (12.97) |
| Norfloxacin (334) | 185 (55.39) | 0 (0) | 149 (44.61) |
| Fosfomycin (350) | 349 (99.71) | 0 (0) | 1 (0.29) |
| Nitrofurantoin (501) | 481 (96.01) | 17 (3.39) | 3 (0.6) |
| Trimethoprim + sulfamethoxazole (501) | 318 (63.47) | 0 | 183 (36.53) |
| E. coli | Antibiotic Resistance | Multinominal Statistical Analysis | |||||
|---|---|---|---|---|---|---|---|
| Antibacterials (n) | Year | Susceptible | Intermediate | Resistant | RRR | 95% CI | p Value |
| (n = 100%) | n (%) | n (%) | n (%) | ||||
| Ampicillin (501) | 2016 (77) | 23 (29.87) | 0 | 54 (70.13) | |||
| 2017 (60) | 21 (35) | 1 (1.67) | 38 (63.33) | ||||
| 2018 (83) | 24 (28.92) | 0 | 59 (71.08) | 1.02 * | 0.61–1.71 * | 0.93 * | |
| 2019 (79) | 32 (40.51) | 1 (1.27) | 46 (58.23) | 0.9 ** | 0.82–0.99 ** | 0.025 ** | |
| 2020 (72) | 29 (40.28) | 1 (1.39) | 42 (58.33) | ||||
| 2021 (73) | 32 (43.84) | 1 (1.37) | 40 (54.79) | ||||
| 2022 (57) | 24 (42.11) | 0 | 33 (57.89) | ||||
| Amoxicillin + clavulanic acid (501) | 2016 (77) | 54 (70.13) | 10 (12.99) | 13 (16.88) | |||
| 2017 (60) | 46 (76.67) | 10 (16.67) | 4 (6.67) | ||||
| 2018 (83) | 60 (72.29) | 13 (15.66) | 10 (12.05) | 0.8 * | 0.68–0.93 * | 0.003 * | |
| 2019 (79) | 58 (73.42) | 10 (12.66) | 11 (13.92) | 1.03 ** | 0.9–1.17 ** | 0.7 ** | |
| 2020 (72) | 52 (72.22) | 10 (13.89) | 10 (13.89) | ||||
| 2021 (73) | 60 (82.19) | 3 (4.11) | 10 (13.7) | ||||
| 2022 (57) | 46 (80.7) | 0 | 11 (19.3) | ||||
| Piperacillin + tazobactam (499) | 2016 (77) | 64 (83.12) | 9 (11.69) | 4 (5.19) | |||
| 2017 (60) | 52 (86.67) | 6 (10) | 2 (3.33) | ||||
| 2018 (83) | 71 (85.54) | 4 (4.82) | 8 (9.64) | 0.69 * | 0.55–0.88 * | 0.002 * | |
| 2019 (78) | 68 (87.18) | 1 (1.28) | 9 (11.54) | 1.07 ** | 0.9–1.27 ** | 0.43 ** | |
| 2020 (72) | 64 (88.89) | 3 (4.17) | 5 (6.94) | ||||
| 2021 (72) | 63 (87.5) | 3 (4.17) | 6 (8.33) | ||||
| 2022 (57) | 52 (91.23) | 0 | 5 (8.77) | ||||
| Cefotaxim (478) | 2016 (77) | 60 (77.92) | 9 (11.69) | 8 (10.39) | |||
| 2017 (60) | 53 (88.33) | 4 (6.67) | 3 (5) | ||||
| 2018 (83) | 71 (85.54) | 4 (4.82) | 8 (9.64) | 0.4 * | 0.26–0.64 * | <0.001 * | |
| 2019 (79) | 73 (92.41) | 0 | 6 (7.59) | 0.92 ** | 0.77–1.1 ** | 0.35 ** | |
| 2020 (72) | 61 (84.72) | 0 | 11 (15.28) | ||||
| 2021 (73) | 71 (97.26) | 0 | 2 (2.74) | ||||
| 2022 (34) | 32 (94.12) | 0 | 2 (5.88) | ||||
| Ceftazidim (501) | 2016 (77) | 60 (77.92) | 13 (16.88) | 4 (5.19) | |||
| 2017 (60) | 53 (88.33) | 5 (8.33) | 2 (3.33) | ||||
| 2018 (83) | 70 (84.34) | 6 (7.23) | 7 (8.43) | 0.74 * | 0.6–0.9 * | 0.003 * | |
| 2019 (79) | 73 (92.41) | 0 | 6 (7.59) | 0.94 ** | 0.78–1.14 ** | 0.55 ** | |
| 2020 (72) | 61 (84.72) | 0 | 11 (15.28) | ||||
| 2021 (73) | 68 (93.15) | 4 (5.48) | 1 (1.37) | ||||
| 2022 (57) | 52 (91.23) | 4 (7.02) | 1 (1.75) | ||||
| Cefepim (451) | 2016 (44) | 36 (81.82) | 8 (18.18) | 0 | |||
| 2017 (45) | 39 (86.67) | 4 (8.89) | 2 (4.44) | ||||
| 2018 (82) | 72 (87.8) | 4 (4.88) | 6 (7.32) | 0.67 * | 0.51–0.87 * | 0.003 * | |
| 2019 (79) | 73 (92.41) | 0 | 6 (7.59) | 0.99 ** | 0.8–1.23 ** | 0.95 ** | |
| 2020 (72) | 61 (84.72) | 0 | 11 (15.28) | ||||
| 2021 (73) | 71 (97.26) | 1 (1.37) | 1 (1.37) | ||||
| 2022 (56) | 51 (91.07) | 4 (7.14) | 1 (1.79) | ||||
| Amikacin (451) | 2016 (44) | 23 (52.27) | 21 (47.73) | 0 | |||
| 2017 (45) | 28 (62.22) | 17 (37.78) | 0 | ||||
| 2018 (82) | 72 (87.8) | 10 (12.2) | 0 | ||||
| 2019 (79) | 77 (97.47) | 2 (2.53) | 0 | 0.49 ** | 0.4–0.59 ** | <0.001 ** | |
| 2020 (72) | 68 (94.44) | 4 (5.56%) | 0 | ||||
| 2021 (73) | 70 (95.89) | 3 (4.11%) | 0 | ||||
| 2022 (56) | 53 (94.64) | 2 (3.57%) | 1 (1.79) | ||||
| Ciprofloxacin (501) | 2016 (77) | 61 (79.22) | 0 | 16 (20.78) | |||
| 2017 (60) | 52 (86.67) | 0 | 8 (13.33) | ||||
| 2018 (83) | 76 (91.57) | 0 | 7 (8.43) | 2.31 * | 1.25–4.25 * | 0.008 * | |
| 2019 (79) | 68 (86.08) | 0 | 11 (13.92) | 0.9 ** | 0.79–1.04 ** | 0.15 ** | |
| 2020 (72) | 60 (83.33) | 2 (2.78) | 10 (13.89) | ||||
| 2021 (73) | 63 (86.3) | 3 (4.11) | 7 (9.59) | ||||
| 2022 (57) | 48 (84.21) | 3 (5.26) | 6 (10.53) | ||||
| Norfloxacin *** (334) | 2016 (77) | 59 (76.62) | 0 | 18 (23.38) | |||
| 2017 (60) | 44 (73.33) | 0 | 16 (26.67) | ||||
| 2018 (83) | 71 (85.54) | 0 | 12 (14.46) | 2.29 ** | 1.94–2.71 ** | <0.001 ** | |
| 2019 (12) | 11 (91.67) | 0 | 1 (8.33) | ||||
| 2020 (0) | 0 | 0 | 0 | ||||
| 2021 (45) | 0 | 0 | 45 (100) | ||||
| 2022 (57) | 0 | 0 | 57 (100) | ||||
| Trimethoprim + sulfamethoxazole (501) | 2016 (77) | 39 (50.64) | 0 | 38 (49.36) | |||
| 2017 (60) | 33 (55) | 0 | 27 (45) | ||||
| 2018 (83) | 60 (72.28) | 0 | 23 (27.72) | 0.9 ** | 0.82–0.99 ** | 0.027 ** | |
| 2019 (79) | 52 (65.82) | 0 | 27 (34.18) | ||||
| 2020 (72) | 47 (65.28) | 0 | 25 (34.72) | ||||
| 2021 (73) | 47 (64.38) | 0 | 26 (35.62) | ||||
| 2022 (57) | 40 (70.18) | 0 | 17 (29.82) | ||||
| Proteus mirabilis | Antibiotic Resistance | ||
|---|---|---|---|
| Antibacterials (n) | Susceptible | Intermediate | Resistant |
| n (%) | n (%) | n (%) | |
| Ampicillin (93) | 35 (37.64) | 4 (4.3) | 54 (58.06) |
| Amoxicillin + clavulanic acid (78) | 61 (78.21) | 7 (8.97) | 10 (12.82) |
| Piperacillin + tazobactam (95) | 83 (87.36) | 6 (6.32) | 6 (6.32) |
| Cefotaxime (87) | 77 (88.5) | 7 (8.05) | 3 (3.45) |
| Ceftazidime (95) | 82 (86.32) | 5 (5.26) | 8 (8.42) |
| Cefepime (85) | 73 (85.88) | 7 (8.24) | 5 (5.88) |
| Ertapenem (81) | 78 (96.3) | 3 (3.7) | 0 |
| Imipenem (95) | 22 (23.16) | 46 (48.42) | 27 (28.42) |
| Meropenem (95) | 89 (93.68) | 3 (3.16) | 3 (3.16) |
| Amikacin (85) | 76 (89.41) | 9 (10.59) | 0 |
| Gentamicin (95) | 80 (84.21) | 1 (1.05) | 14 (14.74) |
| Ciprofloxacin (95) | 81 (85.27) | 1 (1.05) | 13 (13.68) |
| Norfloxacin (67) | 42 (62.69) | 0 | 25 (37.31) |
| Fosfomycin (42) | 35 (83.33) | 0 | 7 (16.67) |
| Nitrofurantoin (76) | 0 | 0 | 76 (100) |
| Trimethoprim + sulfamethoxazole (95) | 43 (45.26) | 0 | 52 (54.74) |
| Klebsiella pneumoniae | Antibiotic Resistance | ||
|---|---|---|---|
| Antibacterials (n) | Susceptible | Intermediate | Resistant |
| n (%) | n (%) | n (%) | |
| Ampicillin (40) | 0 | 0 | 40 (100) |
| Amoxicillin + clavulanic acid (40) | 25 (62.5) | 6 (15) | 9 (22.5) |
| Piperacillin + tazobactam (40) | 25 (62.5) | 5 (12.5) | 10 (25) |
| Cefotaxime (39) | 29 (74.36) | 3 (7.69) | 7 (17.95) |
| Ceftazidime (40) | 29 (72.5) | 3 (7.5) | 8 (20) |
| Cefepime (39) | 29 (74.36) | 4 (10.26) | 6 (15.38) |
| Ertapenem (37) | 37 (100) | 0 | 0 |
| Imipenem (40) | 38 (95) | 2 (5) | 0 |
| Meropenem (40) | 38 (95) | 2 (5) | 0 |
| Amikacin (39) | 37 (94.87) | 2 (5.13) | 0 |
| Gentamicin (40) | 40 (100) | 0 | 0 |
| Ciprofloxacin (40) | 23 (57.5) | 1 (2.5) | 16 (40) |
| Norfloxacin (23) | 9 (39.13) | 1 (4.35) | 13 (56.52) |
| Fosfomycin (20) | 9 (45) | 0 | 11 (55) |
| Nitrofurantoin (35) | 9 (25.71) | 14 (40) | 12 (34.29) |
| Trimethoprim + sulfamethoxazole (40) | 25 (62.5) | 0 | 15 (37.5) |
| Enterococcus faecalis | Antibiotic Resistance | ||
|---|---|---|---|
| Antibacterials (n) | Susceptible | Intermediate | Resistant |
| n (%) | n (%) | n (%) | |
| Ampicillin | 35 (100) | 0 | 0 |
| Imipenem | 10 (58.82) | 7 (41.18) | 0 |
| High-Level Gentamicin | 28 (80) | 0 | 7 (20) |
| High-Level Streptomycin | 22 (62.86) | 0 | 13 (37.14) |
| Ciprofloxacin | 30 (85.71) | 3 (8.57) | 2 (5.71) |
| Linezolid | 32 (94.12) | 2 (5.88) | 0 |
| Teicoplanin | 33 (94.29) | 0 | 2 (5.71) |
| Vancomycin | 33 (94.29) | 0 | 2 (5.71) |
| Tigecycline | 35 (100) | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marc, C.C.; Mot, M.D.; Licker, M.; Muntean, D.; Marti, D.T.; Ardelean, A.A.; Ciceu, A.; Sprintar, S.A.; Oatis, D.A.; Mihu, A.G.; et al. Trends in Positive Urine Culture Rates and Antimicrobial Resistance in Non-Hospitalized Children from Western Romania: A Retrospective Observational Study. Antibiotics 2025, 14, 723. https://doi.org/10.3390/antibiotics14070723
Marc CC, Mot MD, Licker M, Muntean D, Marti DT, Ardelean AA, Ciceu A, Sprintar SA, Oatis DA, Mihu AG, et al. Trends in Positive Urine Culture Rates and Antimicrobial Resistance in Non-Hospitalized Children from Western Romania: A Retrospective Observational Study. Antibiotics. 2025; 14(7):723. https://doi.org/10.3390/antibiotics14070723
Chicago/Turabian StyleMarc, Constantin Catalin, Maria Daniela Mot, Monica Licker, Delia Muntean, Daniela Teodora Marti, Ana Alexandra Ardelean, Alina Ciceu, Sergiu Adrian Sprintar, Daniela Adriana Oatis, Alin Gabriel Mihu, and et al. 2025. "Trends in Positive Urine Culture Rates and Antimicrobial Resistance in Non-Hospitalized Children from Western Romania: A Retrospective Observational Study" Antibiotics 14, no. 7: 723. https://doi.org/10.3390/antibiotics14070723
APA StyleMarc, C. C., Mot, M. D., Licker, M., Muntean, D., Marti, D. T., Ardelean, A. A., Ciceu, A., Sprintar, S. A., Oatis, D. A., Mihu, A. G., & Olariu, T. R. (2025). Trends in Positive Urine Culture Rates and Antimicrobial Resistance in Non-Hospitalized Children from Western Romania: A Retrospective Observational Study. Antibiotics, 14(7), 723. https://doi.org/10.3390/antibiotics14070723








