Synthetic Human Lactoferrin Peptide hLF(1-11) Shows Antifungal Activity and Synergism with Fluconazole and Anidulafungin Towards Candida albicans and Various Non-Albicans Candida Species, Including Candidozyma auris
Abstract
1. Introduction
2. Results
2.1. Minimum Inhibitory Concentration (MIC) Values
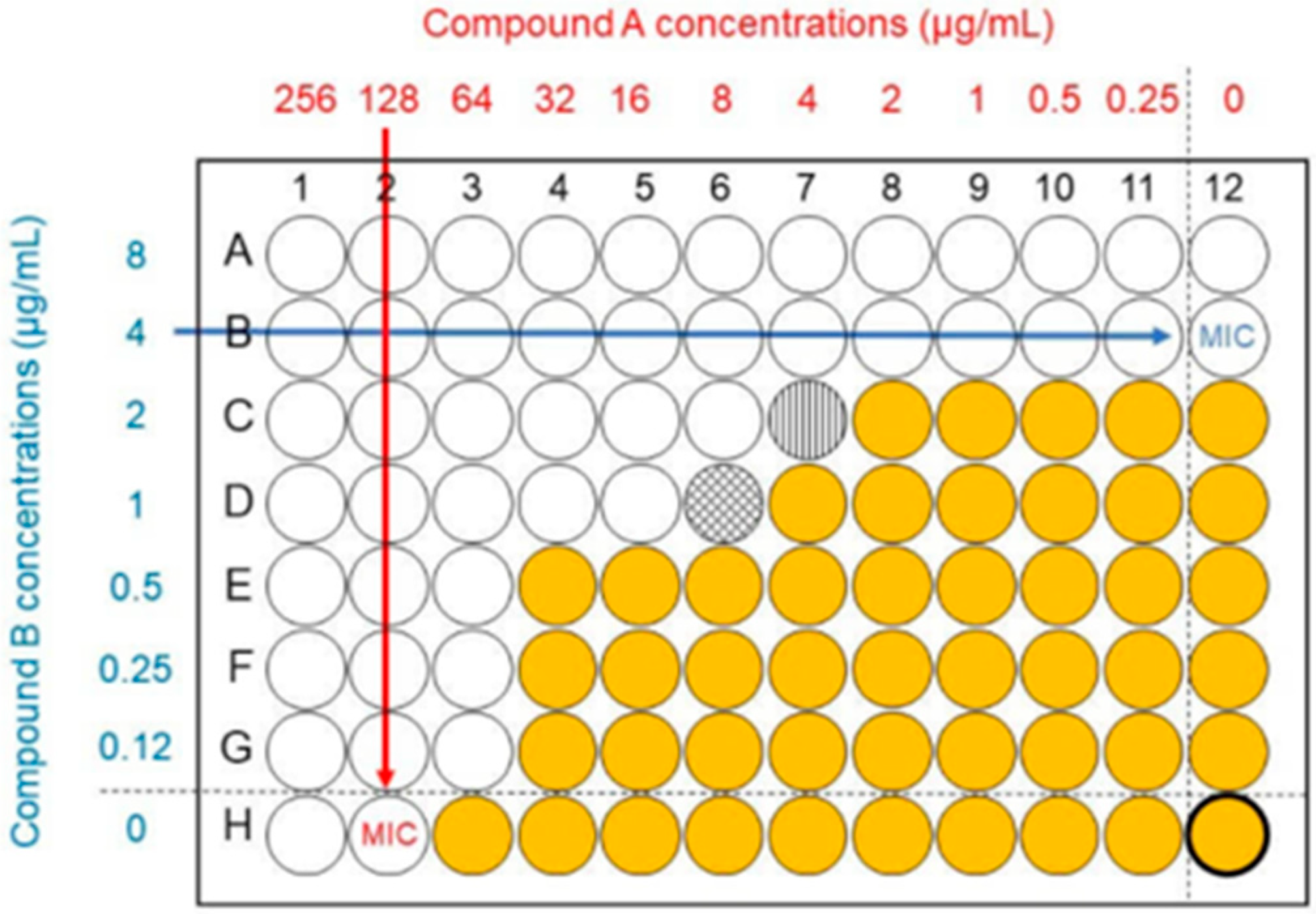
2.2. Synergy Studies
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Minimum Inhibitory Concentration (MIC) Values
4.3. Candida Strains, Cell Viability Testing
4.4. Antimicrobial Peptides
4.5. EUCAST Broth Microdilution Method
4.6. Minimal Inhibitory Growth Assays
4.7. Synergy Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ellis, M. Invasive fungal infections: Evolving challenges for diagnosis and therapeutics. Mol. Immunol. 2002, 38, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, A.A.; Lackner, M.; Lass-Flörl, C.; Boekhout, T. The changing spectrum of Saccharomycotina yeasts causing candidemia: Phylogeny mirrors antifungal susceptibility patterns for azole drugs and amphothericin B. FEMS Yeast Res. 2019, 19, foz037. [Google Scholar] [CrossRef]
- Masson, P.L.; Heremans, J.F.; Prignot, J.J.; Wauters, G. Immunohistochemical localization and bacteriostatic properties of an iron-binding protein from bronchial mucus. Thorax 1966, 21, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Lupetti, A.; Nibbering, P.H.; Campa, M.; Del Tacca, M.; Danesi, R. Molecular targeted treatments for fungal infections: The role of drug combinations. Trends Mol. Med. 2003, 9, 269–276. [Google Scholar] [CrossRef]
- Lum, K.Y.; Tay, S.T.; Le, C.F.; Lee, V.S.; Sabri, N.H.; Velayuthan, R.D.; Hassan, H.; Sekaran, S.D. Activity of Novel Synthetic Peptides against Candida albicans. Sci. Rep. 2015, 5, 9657. [Google Scholar] [CrossRef]
- Corrêa-Moreira, D.; Baptista, B.O.; Giosa, D.; Oliveira, M.M.E. Editorial: Emerging fungal pathogens: Perspectives. Front. Fungal Biol. 2024, 5, 1369062. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Andes, D.R.; Diekema, D.J.; Horn, D.L.; Reboli, A.C.; Rotstein, C.; Franks, B.; Azie, N.E. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2496 patients: Data from the Prospective Antifungal Therapy (PATH) registry 2004–2008. PLoS ONE 2014, 9, e101510. [Google Scholar] [CrossRef]
- Morales-López, S.E.; Parra-Giraldo, C.M.; Ceballos-Garzón, A.; Martínez, H.P.; Rodríguez, G.J.; Álvarez-Moreno, C.A.; Rodríguez, J.Y. Invasive Infections with Multidrug-Resistant Yeast Candida auris, Colombia. Emerg. Infect. Dis. 2017, 23, 162–164. [Google Scholar] [CrossRef]
- Pérez-Hansen, A.; Lass-Flörl, C.; Lackner, M. Antifungal susceptibility profiles of rare ascomycetous yeasts. J. Antimicrob. Chemother. 2019, 74, 2649–2656. [Google Scholar] [CrossRef]
- Stavrou, A.A.; Pérez-Hansen, A.; Lackner, M.; Lass-Flörl, C.; Boekhout, T. Elevated minimum inhibitory concentrations to antifungal drugs prevail in 14 rare species of candidemia-causing Saccharomycotina yeasts. Med. Mycol. 2020, 58, 987–995. [Google Scholar] [CrossRef]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Lass-Flörl, C.; Kanj, S.S.; Govender, N.P.; Thompson, G.R., 3rd; Ostrosky-Zeichner, L.; Govrins, M.A. Invasive candidiasis. Nat. Rev. Dis. Primers 2024, 10, 20. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Patterson, T.F. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J. Infect. Dis. 2017, 216, S445–S451. [Google Scholar] [CrossRef] [PubMed]
- Peyton, L.R.; Gallagher, S.; Hashemzadeh, M. Triazole antifungals: A review. Drugs Today 2015, 51, 705–718. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Rice, L.B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef]
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Ademe, M.; Girma, F. Candida auris: From Multidrug Resistance to Pan-Resistant Strains. Infect. Drug. Resist. 2020, 13, 1287–1294. [Google Scholar] [CrossRef]
- Lee, W.G.; Shin, J.H.; Uh, Y.; Kang, M.G.; Kim, S.H.; Park, K.H.; Jang, H.C. First three reported cases of nosocomial fungemia caused by Candida auris. J. Clin. Microbiol. 2011, 49, 3139–3142. [Google Scholar] [CrossRef]
- Adams, E.; Quinn, M.; Tsay, S.; Poirot, E.; Chaturvedi, S.; Southwick, K.; Greenko, J.; Fernandez, R.; Kallen, A.; Vallabhaneni, S.; et al. Candida auris in Healthcare Facilities, New York, USA, 2013–2017. Emerg. Infect. Dis. 2018, 24, 1816–1824. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef]
- Lee, J.S.; Im, D.S.; An, Y.-S.; Hong, J.M.; Gwag, B.J.; Joo, I.S. Chronic cerebral hypoperfusion in a mouse model of Alzheimer’s disease: An additional contributing factor of cognitive impairment. Neurosci. Lett. 2011, 489, 84–88. [Google Scholar] [CrossRef]
- Forsberg, K.; Woodworth, K.; Walters, M.; Berkow, E.L.; Jackson, B.; Chiller, T.; Vallabhaneni, S. Candida auris: The recent emergence of a multidrug-resistant fungal pathogen. Med. Mycol. 2019, 57, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sanglard, D. Emerging Threats in Antifungal-Resistant Fungal Pathogens. Front. Med. 2016, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S.; Shor, E.; Zhao, Y. Update on Antifungal Drug Resistance. Curr. Clin. Microbiol. Rep. 2015, 2, 84–95. [Google Scholar] [CrossRef]
- Chowdhary, A.; Sharma, C.; Duggal, S.; Agarwal, K.; Prakash, A.; Singh, P.K.; Jain, S.; Kathuria, S.; Randhawa, H.S.; Hagen, F.; et al. New clonal strain of Candida auris, Delhi, India. Emerg. Infect. Dis. 2013, 19, 1670–1673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Choi, K.; Wang, X.; Xi, L.; Lu, S. The Contribution of Human Antimicrobial Peptides to Fungi. Int. J. Mol. Sci. 2025, 26, 2494. [Google Scholar] [CrossRef]
- Brouwer, C.; Theelen, B.; van der Linden, Y.; Sarink, N.; Rahman, M.; Alwasel, S.; Cafarchia, C.; Welling, M.M.; Boekhout, T. Combinatory Use of hLF(1-11), a Synthetic Peptide Derived from Human Lactoferrin, and Fluconazole/Amphotericin B against Malassezia furfur Reveals a Synergistic/Additive Antifungal Effect. Antibiotics 2024, 13, 790. [Google Scholar] [CrossRef]
- Brouwer, C.; Welling, M.M.; Alwasel, S.; Boekhout, T. Potential health benefits of lactoferrin and derived peptides—How to qualify as a medical device? Crit. Rev. Microbiol. 2025, 1–25. [Google Scholar] [CrossRef]
- Mba, I.E.; Nweze, E.I. Antimicrobial Peptides Therapy: An Emerging Alternative for Treating Drug-Resistant Bacteria. Yale J. Biol. Med. 2022, 95, 445–463. [Google Scholar]
- Cezara, B.; Corina, C. Antimicrobial peptides: Opportunities and challenges in overcoming resistance. Microbiol. Res. 2024, 286, 127822. [Google Scholar] [CrossRef]
- Ammons, M.C.; Copié, V. Mini-review: Lactoferrin: A bioinspired, anti-biofilm therapeutic. Biofouling 2013, 29, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.L.; Heremans, J.F.; Ferin, J. Presence of an Iron-binding protein (lactoferrin) in the genital tract of the human female. I. Its immunohistochemical localization in the endometrium. Fertil. Steril. 1968, 19, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, C.; Boekhout, T.; Alwasel, S.; Rahman, M.; Janga, R.; Welling, M.M. Screening sensibility and antifungal activity after topical application of a synthetic lactoferrin-derived antimicrobial peptide. Am. J. Transl. Res. 2024, 16, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, C.; Roscini, L.; Cardinali, G.; Corte, L.; Pierantoni, D.C.; Robert, V.; Rahman, M.; Welling, M.M. Structure-activity relationship study of synthetic variants derived from the highly potent human antimicrobial peptide hLF (1-11). Cohesive J. Microbiol. Infect. Dis. 2018, 1, 1–19. [Google Scholar] [CrossRef]
- Lupetti, A.; Brouwer, C.; Bogaards, S.J.P.; Welling, M.M.; de Heer, E.; Campa, M.; van Dissel, J.T.; Friesen, R.H.E.; Nibbering, P.H. Human lactoferrin-derived peptide’s antifungal activities against disseminated Candida albicans infection. J. Infect. Dis. 2007, 196, 1416–1424. [Google Scholar] [CrossRef]
- Kurtzman, C.; Fell, J.; Boekhout, T. Definition, classification and nomenclature of the yeasts. In The Yeasts: A Taxonomic Study; Elsevier: Amsterdam, The Netherlands, 2011; pp. 3–9. [Google Scholar]
- Li, W.; Liu, B.; Lin, Y.; Xue, P.; Lu, Y.; Song, S.; Li, Y.; Szeto, I.M.; Ren, F.; Guo, H. The application of lactoferrin in infant formula: The past, present and future. Crit. Rev. Food Sci. Nutr. 2024, 64, 5748–5767. [Google Scholar] [CrossRef]
- Takashima, M.; Sugita, T. Taxonomy of Pathogenic Yeasts Candida, Cryptococcus, Malassezia, and Trichosporon. Med. Mycol. J. 2022, 63, 119–132. [Google Scholar] [CrossRef]
- Khunnamwong, P.; Lertwattanasakul, N.; Jindamorakot, S.; Limtong, S.; Lachance, M.A. Description of Diutina gen. nov., Diutina siamensis, f.a. sp. nov., and reassignment of Candida catenulata, Candida mesorugosa, Candida neorugosa, Candida pseudorugosa, Candida ranongensis, Candida rugosa and Candida scorzettiae to the genus Diutina. Int. J. Syst. Evol. Microbiol. 2015, 65, 4701–4709. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Guo, L.C.; Hu, S.; Wei, Y.H.; Hui, F.L.; Liu, X.Z.; Bai, F.Y. Pichia kurtzmaniana f.a. sp. nov., with the transfer of eight Candida species to Pichia. Int. J. Syst. Evol. Microbiol. 2024, 74, 006306. [Google Scholar] [CrossRef]
- Warshaw, E.M.; Fett, D.D.; Bloomfield, H.E.; Grill, J.P.; Nelson, D.B.; Quintero, V.; Carver, S.M.; Zielke, G.R.; Lederle, F.A. Pulse versus continuous terbinafine for onychomycosis: A randomized, double-blind, controlled trial. J. Am. Acad. Dermatol. 2005, 53, 578–584. [Google Scholar] [CrossRef]
- Thappeta, K.R.V.; Vikhe, Y.S.; Yong, A.M.H.; Chan-Park, M.B.; Kline, K.A. Combined Efficacy of an Antimicrobial Cationic Peptide Polymer with Conventional Antibiotics to Combat Multidrug-Resistant Pathogens. ACS Infect. Dis. 2020, 6, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, C.P.; Rahman, M.; Welling, M.M. Discovery and development of a synthetic peptide derived from lactoferrin for clinical use. Peptides 2011, 32, 1953–1963. [Google Scholar] [CrossRef] [PubMed]
- Lupetti, A.; Pauwels, E.K.J.; Nibbering, P.H.; Weling, M.M. Tc-99m-antimicrobial peptides: Promising candidates for infection imaging. Q. J. Nucl. Med. 2003, 47, 238–245. [Google Scholar]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- van der Velden, W.; van Iersel, T.M.P.; Blijlevens, N.M.A.; Donnelly, J.P. Safety and tolerability of the antimicrobial peptide human lactoferrin 1-11 (hLF1-11). BMC Med. 2009, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Beardsley, J.; Kim, H.Y.; Dao, A.; Kidd, S.; Alastruey-Izquierdo, A.; Sorrell, T.C.; Tacconelli, E.; Chakrabarti, A.; Harrison, T.S.; Bongomin, F.; et al. Candida glabrata (Nakaseomyces glabrata): A systematic review of clinical and microbiological data from 2011 to 2021 to inform the World Health Organization Fungal Priority Pathogens List. Med. Mycol. 2024, 62, myae041. [Google Scholar] [CrossRef]
- Fisher, M.C.; Denning, D.W. The WHO fungal priority pathogens list as a game-changer. Nat. Rev. Microbiol. 2023, 21, 211–212. [Google Scholar] [CrossRef]
- Rybak, J.M.; Doorley, L.A.; Nishimoto, A.T.; Barker, K.S.; Palmer, G.E.; Rogers, P.D. Abrogation of Triazole Resistance upon Deletion of CDR1 in a Clinical Isolate of Candida auris. Antimicrob. Agents Chemother. 2019, 63, e00057-19. [Google Scholar] [CrossRef]
- Chowdhary, A.; Prakash, A.; Sharma, C.; Kordalewska, M.; Kumar, A.; Sarma, S.; Tarai, B.; Singh, A.; Upadhyaya, G.; Upadhyay, S.; et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–17) in India: Role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J. Antimicrob. Chemother. 2018, 73, 891–899. [Google Scholar] [CrossRef]
- Sherry, L.; Rajendran, R.; Lappin, D.F.; Borghi, E.; Perdoni, F.; Falleni, M.; Tosi, D.; Smith, K.; Williams, C.; Jones, B.; et al. Biofilms formed by Candida albicans bloodstream isolates display phenotypic and transcriptional heterogeneity that are associated with resistance and pathogenicity. BMC Microbiol. 2014, 14, 182. [Google Scholar] [CrossRef]
- Sherry, L.; Ramage, G.; Kean, R.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R. Biofilm-Forming Capability of Highly Virulent, Multidrug-Resistant Candida auris. Emerg. Infect. Dis. 2017, 23, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Czajka, K.M.; Venkataraman, K.; Brabant-Kirwan, D.; Santi, S.A.; Verschoor, C.; Appanna, V.D.; Singh, R.; Saunders, D.P.; Tharmalingam, S. Molecular Mechanisms Associated with Antifungal Resistance in Pathogenic Candida Species. Cells 2023, 12, 2655. [Google Scholar] [CrossRef]
- Windsor, R.E.; Insall, J.N.; Urs, W.K.; Miller, D.V.; Brause, B.D. Two-stage reimplantation for the salvage of total knee arthroplasty complicated by infection. Further follow-up and refinement of indications. JBJS 1990, 72, 272–278. [Google Scholar] [CrossRef]
- Bluard-Deconinck, J.M.; Williams, J.; Evans, R.W.; van Snick, J.; Osinski, P.A.; Masson, P.L. Iron-binding fragments from the N-terminal and C-terminal regions of human lactoferrin. Biochem. J. 1978, 171, 321–327. [Google Scholar] [CrossRef]
- Nibbering, P.H.; Ravensbergen, E.; Welling, M.M.; van Berkel, L.A.; van Berkel, P.H.C.; Pauwels, E.K.J.; Nuijens, J.H. Human lactoferrin and peptides derived from its N terminus are highly effective against infections with antibiotic-resistant bacteria. Infect. Immun. 2001, 69, 1469–1476. [Google Scholar] [CrossRef]
- Lupetti, A.; Paulusma-Annema, A.; Welling, M.M.; Dogterom-Ballering, H.; Brouwer, C.P.J.M.; Senesi, S.; Dissel, J.T.v.; Nibbering, P.H. Synergistic Activity of the N-Terminal Peptide of Human Lactoferrin and Fluconazole against Candida Species. Antimicrob. Agents Chemother. 2003, 47, 262–267. [Google Scholar] [CrossRef]
- van der Does, A.M.; Bogaards, S.J.P.; Ravensbergen, B.; Beekhuizen, H.; van Dissel, J.T.; Nibbering, P.H. Antimicrobial peptide hLF1-11 directs granulocyte-macrophage colony-stimulating factor-driven monocyte differentiation toward macrophages with enhanced recognition and clearance of pathogens. Antimicrob. Agents Chemother. 2010, 54, 811–816. [Google Scholar] [CrossRef] [PubMed]
- van der Does, A.M.; Joosten, S.a.; Vroomans, E.; Bogaards, S.J.P.; van Meijgaarden, K.E.; Ottenhoff, T.H.M.; van Dissel, J.T.; Nibbering, P.H. The antimicrobial peptide hLF1-11 drives monocyte-dendritic cell differentiation toward dendritic cells that promote antifungal responses and enhance Th17 polarization. J. Innate Immun. 2012, 4, 284–292. [Google Scholar] [CrossRef]
- Trilles, L.; Fernández-Torres, B.; Dos Santos Lazéra, M.; Wanke, B.; de Oliveira Schubach, A.; de Almeida Paes, R.; Inza, I.; Guarro, J. In vitro antifungal susceptibilities of Sporothrix schenckii in two growth phases. Antimicrob. Agents Chemother. 2005, 49, 3952–3954. [Google Scholar] [CrossRef]
- Beggs, W.H. Growth phase in relation to ketoconazole and miconazole susceptibilities of Candida albicans. Antimicrob. Agents Chemother. 1984, 25, 316–318. [Google Scholar] [CrossRef]
- Meletiadis, J.; Meis, J.F.; Mouton, J.W.; Verweij, P.E. Analysis of growth characteristics of filamentous fungi in different nutrient media. J. Clin. Microbiol. 2001, 39, 478–484. [Google Scholar] [CrossRef]
- Cudic, M.; Condie, B.A.; Weiner, D.J.; Lysenko, E.S.; Xiang, Z.Q.; Insug, O.; Bulet, P.; Otvos, L., Jr. Development of novel antibacterial peptides that kill resistant isolates. Peptides 2002, 23, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Cudic, M.; Lockatell, C.V.; Johnson, D.E.; Otvos, L., Jr. In vitro and in vivo activity of an antibacterial peptide analog against uropathogens. Peptides 2003, 24, 807–820. [Google Scholar] [CrossRef]
- To, W.K.; Fothergill, A.W.; Rinaldi, M.G. Comparative evaluation of macrodilution and alamar colorimetric microdilution broth methods for antifungal susceptibility testing of yeast isolates. J. Clin. Microbiol. 1995, 33, 2660–2664. [Google Scholar] [CrossRef] [PubMed]
- Svensäter, G.; Björnsson, O.; Hamilton, I.R. Effect of carbon starvation and proteolytic activity on stationary-phase acid tolerance of Streptococcus mutans. Microbiology 2001, 147, 2971–2979. [Google Scholar] [CrossRef]
- Radetsky, M.; Wheeler, R.C.; Roe, M.H.; Todd, J.K. Microtiter broth dilution method for yeast susceptibility testing with validation by clinical outcome. J. Clin. Microbiol. 1986, 24, 600–606. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Rinaldi, M.G.; Galgiani, J.N.; Bartlett, M.S.; Body, B.A.; Espinel-Ingroff, A.; Fromtling, R.A.; Hall, G.S.; Hughes, C.E.; Odds, F.C. Collaborative investigation of variables in susceptibility testing of yeasts. Antimicrob. Agents Chemother. 1990, 34, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.J.; Espinel-Ingroff, A.; Moore, L.S.; Webb, C.D. Antifungal susceptibility testing of yeasts: A brief overview. Clin. Infect. Dis. 1993, 17 (Suppl. S2), S494–S500. [Google Scholar] [CrossRef]
- Rodríguez-Tudela, J.L.; Berenguer, J.; Martínez-Suárez, J.V.; Sanchez, R. Comparison of a spectrophotometric microdilution method with RPMI-2% glucose with the National Committee for Clinical Laboratory Standards reference macrodilution method M27-P for in vitro susceptibility testing of amphotericin B, flucytosine, and fluconazole against Candida albicans. Antimicrob. Agents Chemother. 1996, 40, 1998–2003. [Google Scholar] [CrossRef]
- Kean, R.; Delaney, C.; Rajendran, R.; Sherry, L.; Metcalfe, R.; Thomas, R.; McLean, W.; Williams, C.; Ramage, G. Gaining Insights from Candida Biofilm Heterogeneity: One Size Does Not Fit All. J. Fungi 2018, 4, 12. [Google Scholar] [CrossRef]
- Piedrahita, C.T.; Cadnum, J.L.; Jencson, A.L.; Shaikh, A.A.; Ghannoum, M.A.; Donskey, C.J. Environmental Surfaces in Healthcare Facilities are a Potential Source for Transmission of Candida auris and Other Candida Species. Infect Control Hosp. Epidemiol. 2017, 38, 1107–1109. [Google Scholar] [CrossRef]
- Schelenz, S.; Hagen, F.; Rhodes, J.L.; Abdolrasouli, A.; Chowdhary, A.; Hall, A.; Ryan, L.; Shackleton, J.; Trimlett, R.; Meis, J.F.; et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob. Resist. Infect. Control 2016, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Mercer, D.K.; Torres, M.D.T.; Duay, S.S.; Lovie, E.; Simpson, L.; von Köckritz-Blickwede, M.; de la Fuente-Nunez, C.; O’Neil, D.A.; Angeles-Boza, A.M. Antimicrobial Susceptibility Testing of Antimicrobial Peptides to Better Predict Efficacy. Front. Cell Infect. Microbiol. 2020, 10, 326. [Google Scholar] [CrossRef]
- Vaidyanathan, G.; Zalutsky, M.R. Applications of 211At and 223Ra in targeted alpha-particle radiotherapy. Curr. Radiopharm. 2011, 4, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Buda De Cesare, G.; Cristy, S.A.; Garsin, D.A.; Lorenz, M.C. Antimicrobial Peptides: A New Frontier in Antifungal Therapy. mBio 2020, 11, e02123-20. [Google Scholar] [CrossRef]
- McLeod, G.I.; Spector, M.P. Starvation- and Stationary-phase-induced resistance to the antimicrobial peptide polymyxin B in Salmonella typhimurium is RpoS (sigma(S)) independent and occurs through both phoP-dependent and -independent pathways. J. Bacteriol. 1996, 178, 3683–3688. [Google Scholar] [CrossRef] [PubMed]
- Vctor, L. Antibiotics in Laboratory Medicine; Lippincott Williams & Wilkins: Philadelphia, PN, USA, 2005. [Google Scholar]
- Woods, R.J.; Read, A.F. Combination antimicrobial therapy to manage resistance. Evol. Med. Public Health 2023, 11, 185–186. [Google Scholar] [CrossRef]
- Thayna, A.M.S.; Erica, O.M.; Gabriel, B.T.; Felipe, F.M.; Sergio Henrique, S.; André, O.C.; Valdirene, M.G. Synergistic action of synthetic peptides and amphotericin B causes disruption of the plasma membrane and cell wall in Candida albicans. Biosci. Rep. 2024, 44, BSR20232075. [Google Scholar] [CrossRef]
| Name | Old Name | Strain | Country of Origin | Source | MIC Values | |
|---|---|---|---|---|---|---|
| Mean | St. Dev | |||||
| Candida albicans | AA (LUMC) | Netherlands | Blood | 22.92 | 5.10 | |
| Candida albicans | ATCC 90028 | United States | Blood | 18.75 | 6.85 | |
| Candida albicans | ATCC 10231 | unknown | Bronchomycosis | 23.21 | 4.73 | |
| Candida albicans | CBS 562 | Uruguay | Skin | 20.46 | 6.31 | |
| Candida albicans | CMC 1968 | Italy | Human | 23.21 | 4.73 | |
| Candida albicans | Y01-19 | unknown | unknown | 16.67 | 6.46 | |
| Candida dubliniensis | CBS 7987 | Iceland | unknown | 22.92 | 5.10 | |
| Candida parapsilosis | ATCC 22019 | United States | Case of sprue | 23.44 | 8.99 | |
| Candida parapsilosis | CBS 604 | Puerto Rico | unknown | 20.83 | 6.46 | |
| Candida parapsilosis | CMC 2039 | Italy | Human | 18.75 | 6.85 | |
| Candida parapsilosis | LUMC | Netherlands | Human | 18.75 | 6.85 | |
| Candida tropicalis | CBS 1920 | unknown | unknown | 21.88 | 7.66 | |
| Candida tropicalis | CBS 94 | unknown | unknown | 45.83 | 10.21 | |
| Candida tropicalis | CMC 2041 | Italy | Human | 41.67 | 12.91 | |
| Candida tropicalis | LUMC | Netherlands | Human | 23.21 | 4.73 | |
| Candidozyma auris | Candida auris | 2MG-1491 | unknown | unknown | 21.25 | 6.04 |
| Candidozyma auris | Candida auris | CBS 10913 | Japan | Ear | 21.25 | 5.88 |
| Candidozyma auris | Candida auris | CBS 12372 | Korea | Blood | 19.38 | 6.38 |
| Candidozyma auris | Candida auris | CBS 12373 | Korea | Blood | 21.88 | 5.55 |
| Candidozyma auris | Candida auris | CBS 12805 | India | Blood | 20.00 | 6.46 |
| Candidozyma auris | Candida auris | CBS 12806 | India | Blood | 21.25 | 6.04 |
| Candidozyma auris | Candida auris | CBS 12807 | India | Blood | 20.00 | 6.46 |
| Candidozyma auris | Candida auris | CBS 12874 | India | Blood | 20.00 | 6.46 |
| Candidozyma auris | Candida auris | CBS 12875 | India | Blood | 20.00 | 6.46 |
| Candidozyma auris | Candida auris | CBS 12883 | India | Blood | 20.00 | 6.46 |
| Candidozyma auris | Candida auris | CBS 12884 | India | Blood | 21.25 | 6.04 |
| Candidozyma auris | Candida auris | CBS 12885 | India | Human | 22.50 | 5.27 |
| Candidozyma auris | Candida auris | CBS 14144 | Kuwait | Blood | 18.75 | 6.59 |
| Candidozyma auris | Candida auris | CBS 1491 | unknown | unknown | 20.00 | 6.85 |
| Candidozyma auris | Candida auris | CBS 14916 | Oman | Blood | 18.75 | 6.59 |
| Candidozyma auris | Candida auris | CBS 14918 | Oman | Blood | 20.00 | 6.46 |
| Candidozyma auris | Candida auris | CBS 1492 | unknown | unknown | 20.00 | 6.85 |
| Candidozyma auris | Candida auris | CBS 15108 | Oman | unknown | 20.00 | 6.46 |
| Candidozyma auris | Candida auris | CBS 15109 | Oman | Human | 20.00 | 6.46 |
| Candidozyma auris | Candida auris | CBS 15279 | Belgium | Kuwaiti patient | 18.18 | 6.53 |
| Candidozyma duobushaemuli | Candida pseudohaemulonii | CBS 10004 | Thailand | unknown | 19.79 | 8.31 |
| Candidozyma duobushaemuli | Candida pseudohaemulonii | CBS 12371 | Korea | unknown | 20.83 | 6.46 |
| Candidozyma duobushaemuli | Candida pseudohaemulonii | CBS 7798 | United States | unknown | 22.50 | 5.59 |
| Candidozyma duobushaemuli | Candida pseudohaemulonii | CBS 7800 | United States | unknown | 20.00 | 6.85 |
| Candidozyma duobusheamuli | Candida duobushaemulonii | CBS 7798 | United States | unknown | 21.88 | 6.25 |
| Candidozyma duobusheamuli | Candida duobushaemulonii | CBS 7800 | United States | unknown | 40.00 | 13.69 |
| Candidozyma haemuli | Candida haemulonii | CBS 12437 | Spain | unknown | 22.92 | 5.10 |
| Candidozyma haemuli | Candida haemulonii | CBS 12439 | Spain | unknown | 20.83 | 6.46 |
| Candidozyma haemuli | Candida intermedia | CBS 572 | Puerto Rico | unknown | 22.92 | 5.10 |
| Clavispora lusitaniae | Candida lusitaniae | CBS 6936 | Israel | unknown | 20.83 | 6.46 |
| Clavispora lusitaniae | Candida lusitaniae | CMC 1944 | Italy | Human | 45.83 | 10.21 |
| Diutina rugosa | Candida rugosa | CBS 613 | unknown | unknown | 22.50 | 5.59 |
| Diutina rugosa | Candida rugosa | CBS 7138 | Netherlands | unknown | 22.50 | 5.59 |
| Meyerozyma guilliermondii | Candida guilliermondii | CBS 2030 | United States | unknown | 45.00 | 11.18 |
| Nakaeomyces glabratus | Candida glabrata | CBS 138 | Italy | unknown | 22.73 | 5.06 |
| Nakaeomyces glabratus | Candida glabrata | LUMC | Netherlands | Human | 22.92 | 5.10 |
| Nakaseomyces bracarensis | Candida bracarensis | CBS 10154 | Portugal | unknown | 21.88 | 7.66 |
| Nakaseomyces bracarensis | Candida glabrata | CMC 1933 | Italy | Human | 23.21 | 4.73 |
| Nakaseomyces nivariensis | Candida nivariensis | CBS 9983 | Spain | unknown | 22.92 | 5.10 |
| Pichia inconspicua | Candida inconspicua | CBS 180 | Netherlands | unknown | 27.08 | 12.29 |
| Pichia inconspicua | Candida inconspicua | CBS 1735 | Norway | unknown | 20.83 | 6.46 |
| Pichia kudriavsevii | Candida krusei | LUMC | Netherlands | Human | 23.21 | 4.73 |
| Pichia kudriavsevii | Candida krusei | CMC 2002 | Italy | Human | 22.92 | 5.10 |
| Pseudolindnera jadinii | Candida jadinii | CBS 1600 | France | unknown | 22.92 | 5.10 |
| Species | Strain | MIC-Mean |
|---|---|---|
| Candida albicans | ATCC 90028 | >100 |
| Candida albicans | ATTC 10231 | >100 |
| Candida albicans | Y01-19 | >100 |
| Candida parapsilosis * | ATTC 22019 | >100 |
| Candida parapsilosis * | LUMC | >100 |
| Candida tropicalis | LUMC | >100 |
| Candidozyma auris | CBS 10913 | >100 |
| Candidozyma auris | CBS 12372 | >100 |
| Candidozyma auris | CBS 15279 | >100 |
| Nakaseomyces glabratus | LUMC | >100 |
| Pichia kudriavsevii | LUMC | >100 |
| Species | Strain | Country | Mean | St. Dev |
|---|---|---|---|---|
| hLF1-11 MIC | ||||
| Cz. auris | 2MG-1491 | unknown | 21.25 | 6.04 |
| Cz. auris | CBS 10913 | Japan | 21.25 | 5.88 |
| Cz. auris | CBS 12372 | Korea | 19.38 | 6.38 |
| Cz. auris | CBS 12373 | Korea | 21.88 | 5.55 |
| Cz. auris | CBS 12805 | India | 20.00 | 6.45 |
| Cz. auris | CBS 12806 | India | 21.25 | 6.04 |
| Cz. auris | CBS 12807 | India | 20.00 | 6.45 |
| Cz. auris | CBS 12874 | India | 20.00 | 6.45 |
| Cz. auris | CBS 12875 | India | 20.00 | 6.45 |
| Cz. auris | CBS 12883 | India | 20.00 | 6.45 |
| Cz. auris | CBS 12884 | India | 21.25 | 6.04 |
| Cz. auris | CBS 12885 | India | 22.50 | 5.27 |
| Cz. auris | CBS 14144 | Kuwait | 18.75 | 6.59 |
| Cz. auris | CBS 1491 | unknown | 20.00 | 6.85 |
| Cz. auris | CBS 14916 | Oman | 18.75 | 6.59 |
| Cz. auris | CBS 14918 | Oman | 20.00 | 6.45 |
| Cz. auris | CBS 1492 | unknown | 20.00 | 6.85 |
| Cz. auris | CBS 15108 | Oman | 20,00 | 6.45 |
| Cz. auris | CBS 15109 | Oman | 20.00 | 6.45 |
| Species | Strain | FLU MIC | ANI MIC | hLF (1-11) MIC | FLU + hLF MIC | ANI + hLF MIC | hLF + FLU MIC | hLF + ANI MIC | FIC hLF + FLU | FIC hLF + ANI |
|---|---|---|---|---|---|---|---|---|---|---|
| Eucast | Combination Antifungal + Peptide | Combination Peptide + Antifungal | ||||||||
| Candida albicans | CBS 562 | 1 | 0.25 | 50 | 0.5 | 0.13 | 12.5 | 12.5 | 0.75 | 0.63 |
| Candida dubliniensis | CBS 7987 | 128 | 0.03 | 25 | 64 | 0.03 | 12.5 | 6.25 | >1 | 1 |
| Candida parapsilosis | CBS 604 | 2 | 8 | 12.5 | 1 | 8 | 3.125 | 12.5 | 0.75 | >1 |
| Candida tropicalis | CBS 1920 | 0.25 | 0.5 | 6.25 | 0.25 | 0.13 | 3.13 | 6.25 | 0.75 | 1 |
| Candidozyma auris | CBS 15279 | 16 | 1 | 25 | 4 | 0.25 | 12.5 | 3.13 | 0.75 | 0.5 |
| Candidozyma duobushaemuli | CBS 7800 | 128 | 4 | 50 | 32 | 2 | 12.5 | 12.5 | 0.75 | 0.75 |
| Candidozyma duobushaemuli | CBS 12371 | 64 | 2 | 12.5 | 16 | 0.25 | 3.13 | 6.25 | 0.75 | 0.63 |
| Candidozyma haemuli | CBS 12439 | 128 | 4 | 12.5 | 128 | 0.5 | 12.5 | 3.13 | >1 | 0.63 |
| Candidozyma haemuli | CBS 180 | 64 | 1 | 12.5 | 16 | 0.25 | 6.25 | 6.25 | 1 | 0.63 |
| Clavispora lusitaniae | CBS 6936 | 4 | 1 | 12.5 | 1 | 0.25 | 3.13 | 3.13 | 0.75 | 0.63 |
| Cyberlindnera jadinii | CBS 1600 | 8 | 0.01 | 12.5 | 4 | 0 | 3.13 | 6.25 | 0.75–1.0 | >1 |
| Diotuna intermedia | CBS 572 | 16 | 0.5 | 25 | 8 | 0.25 | 6.25 | 12.5 | 0.75 | 1 |
| Diutina rugosa | CBS 7138 | 16 | 0.5 | 25 | 4 | 0.25 | 1.56 | 6.25 | 0.5 | 1 |
| Meyerozyma guilliermondii | CBS 2030 | 32 | 2 | 50 | 16 | 0.25 | 3.13 | 3.13 | 0.75 | 0.63 |
| Nakaeomyces glabratus | CBS 138 | 8 | 0.25 | 12.5 | 2 | 2 | 6.25 | 6.25 | 0.75 | 1 |
| Nakaseomyces bracarensis | CBS 10154 | 4 | 8 | 6.25 | 1 | 4 | 3.125 | 6.25 | 0.75 | >1 |
| Nakaseomyces nivariensis | CBS 9983 | 16 | 1 | 12.5 | 4 | 0.05 | 6.25 | 3.13 | 0.75 | 0.5 |
| Pichia kudriavsevii | CMC 2002 | 32 | 0.06 | 25 | 8 | 0.03 | 12.5 | 12.5 | 0.75 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brouwer, C.; van der Linden, Y.; Carrasco, M.R.; Alwasel, S.; Abalkhail, T.; Al-Otibi, F.O.; Boekhout, T.; Welling, M.M. Synthetic Human Lactoferrin Peptide hLF(1-11) Shows Antifungal Activity and Synergism with Fluconazole and Anidulafungin Towards Candida albicans and Various Non-Albicans Candida Species, Including Candidozyma auris. Antibiotics 2025, 14, 671. https://doi.org/10.3390/antibiotics14070671
Brouwer C, van der Linden Y, Carrasco MR, Alwasel S, Abalkhail T, Al-Otibi FO, Boekhout T, Welling MM. Synthetic Human Lactoferrin Peptide hLF(1-11) Shows Antifungal Activity and Synergism with Fluconazole and Anidulafungin Towards Candida albicans and Various Non-Albicans Candida Species, Including Candidozyma auris. Antibiotics. 2025; 14(7):671. https://doi.org/10.3390/antibiotics14070671
Chicago/Turabian StyleBrouwer, Carlo, Youp van der Linden, Maria Rios Carrasco, Saleh Alwasel, Tarad Abalkhail, Fatimah O. Al-Otibi, Teun Boekhout, and Mick M. Welling. 2025. "Synthetic Human Lactoferrin Peptide hLF(1-11) Shows Antifungal Activity and Synergism with Fluconazole and Anidulafungin Towards Candida albicans and Various Non-Albicans Candida Species, Including Candidozyma auris" Antibiotics 14, no. 7: 671. https://doi.org/10.3390/antibiotics14070671
APA StyleBrouwer, C., van der Linden, Y., Carrasco, M. R., Alwasel, S., Abalkhail, T., Al-Otibi, F. O., Boekhout, T., & Welling, M. M. (2025). Synthetic Human Lactoferrin Peptide hLF(1-11) Shows Antifungal Activity and Synergism with Fluconazole and Anidulafungin Towards Candida albicans and Various Non-Albicans Candida Species, Including Candidozyma auris. Antibiotics, 14(7), 671. https://doi.org/10.3390/antibiotics14070671







