Phenotypic Detection of Extended-Spectrum β-Lactamase and Carbapenemase-Producing Enterobacteriaceae from Wastewater Treatment Plants in Ouagadougou, Burkina Faso
Abstract
1. Introduction
2. Results
2.1. Bacterial Isolation
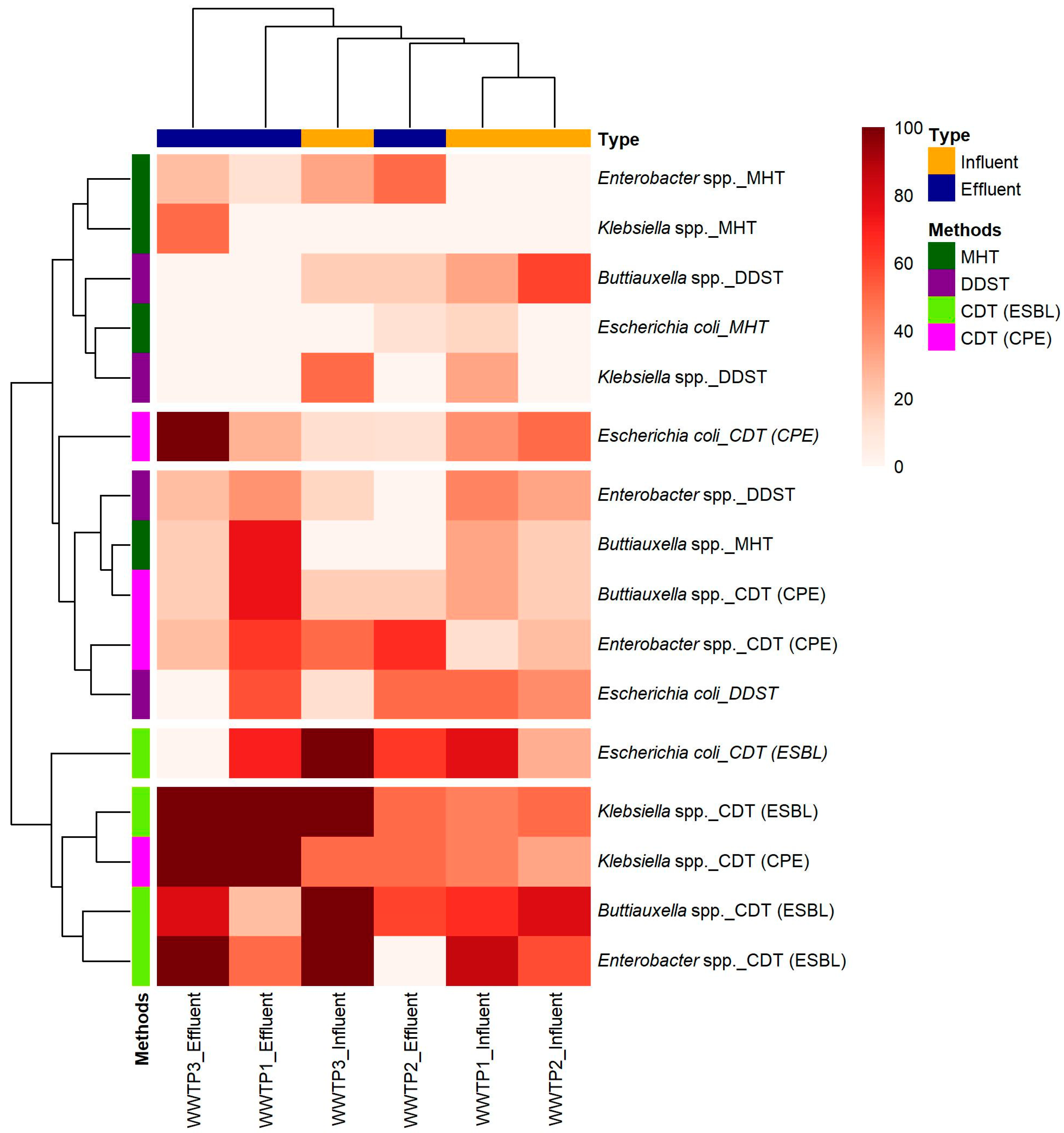
2.2. Phenotypic Detection from Three WWTPs
2.3. Co-Occurrence of ESBL-E and CPE
2.4. Determination of the Antimicrobial Susceptibility Pattern of Enterobacteriaceae
2.5. Multidrug Resistance
3. Discussion
3.1. Bacterial Isolation
3.2. Phenotypic Detection
3.3. Comparison of Techniques and Taxa Prevalence
3.4. Dual Resistance
3.5. Antibiotic Susceptibility
3.6. Multidrug Resistance
3.7. Limitations of the Study
4. Materials and Methods
4.1. Sites and Sampling
4.2. Bacterial Isolation
4.3. Identification
4.4. Antibiotic Susceptibility Test
4.5. Phenotypic Detection of β-Lactamases
4.5.1. Screening Enterobacteriaceae for ESBL Production
4.5.2. Screening Enterobacteriaceae for Carbapenemase Production
4.6. Quality Control
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Njeru, J.; Odero, J.; Chebore, S.; Ndung’u, M.; Tanui, E.; Wesangula, E.; Ndanyi, R.; Githii, S.; Gunturu, R.; Mwangi, W.; et al. Development, Roll-out and Implementation of an Antimicrobial Resistance Training Curriculum Harmonizes Delivery of in-Service Training to Healthcare Workers in Kenya. Front. Microbiol. 2023, 14, 1142622. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef]
- Godman, B.; Egwuenu, A.; Wesangula, E.; Schellack, N.; Kalungia, A.C.; Tiroyakgosi, C.; Kgatlwane, J.; Mwita, J.C.; Patrick, O.; Niba, L.L.; et al. Tackling Antimicrobial Resistance across Sub-Saharan Africa: Current Challenges and Implications for the Future. Expert. Opin. Drug Saf. 2022, 21, 1089–1111. [Google Scholar] [CrossRef]
- Siachalinga, L.; Godman, B.; Mwita, J.C.; Sefah, I.A.; Ogunleye, O.O.; Massele, A.; Lee, I.-H. Current Antibiotic Use Among Hospitals in the Sub-Saharan Africa Region; Findings and Implications. Infect. Drug Resist. 2023, 16, 2179–2190. [Google Scholar] [CrossRef]
- Kowalski, M.; Obama, B.M.; Catho, G.; Dewez, J.E.; Merglen, A.; Ruef, M.; Andrey, D.O.; Hassoun-Kheir, N.; de Kraker, M.E.A.; Combescure, C.; et al. Antimicrobial Resistance in Enterobacterales Infections among Children in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. eClinicalMedicine 2024, 70, 102512. [Google Scholar] [CrossRef]
- Ruef, M.; Emonet, S.; Merglen, A.; Dewez, J.E.; Obama, B.M.; Catho, G.; Andrey, D.O.; Kowalski, M.; Harbarth, S.; Combescure, C.; et al. Carriage of Third-Generation Cephalosporin-Resistant and Carbapenem-Resistant Enterobacterales among Children in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. eClinicalMedicine 2024, 70, 102508. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. The Changing Face of the Family Enterobacteriaceae (Order: “Enterobacterales”): New Members, Taxonomic Issues, Geographic Expansion, and New Diseases and Disease Syndromes. Clin. Microbiol. Rev. 2021, 34, e00174-20. [Google Scholar] [CrossRef]
- WHO Bacterial Priority Pathogens List 2024: Bacterial Pathogens of Public Health Importance, to Guide Research, Development, and Strategies to Prevent and Control Antimicrobial Resistance, 1st ed.; World Health Organization: Geneva, Switzerland, 2024; ISBN 978-92-4-009346-1.
- Bouniounou, D.Y.P.; Metuor Dabiré, A.; Ouedraogo, N.; Tiemtore, R.Y.; Sougué, S.; Bonkoungou, P.R.; Simporé, J. Multiresistance to Carbapenems by Production of Verona Integron-Encoded Metallo-β-Lactamase (VIM)-Type Carbapenemases in Gram-Negative Bacilli Isolated at the Centre Hospitalier Universitaire de Tengandogo (CHU-T) and the Hôpital Saint Camille de Ouagadougou (HOSCO) in Burkina Faso. Microbes Infect. Dis. 2024, 5, 759–769. [Google Scholar] [CrossRef]
- Soubeiga, A.P.; Kpoda, D.S.; Compaoré, M.K.A.; Somda-Belemlougri, A.; Kaseko, N.; Rouamba, S.S.; Ouedraogo, S.; Traoré, R.; Karfo, P.; Nezien, D.; et al. Molecular Characterization and the Antimicrobial Resistance Profile of Salmonella Spp. Isolated from Ready-to-Eat Foods in Ouagadougou, Burkina Faso. Int. J. Microbiol. 2022, 2022, 9640828. [Google Scholar] [CrossRef]
- Al-Riyami, I.M.; Ahmed, M.; Al-Busaidi, A.; Choudri, B.S. Antibiotics in Wastewaters: A Review with Focus on Oman. Appl. Water Sci. 2018, 8, 199. [Google Scholar] [CrossRef]
- Omuferen, L.O.; Maseko, B.; Olowoyo, J.O. Occurrence of Antibiotics in Wastewater from Hospital and Convectional Wastewater Treatment Plants and Their Impact on the Effluent Receiving Rivers: Current Knowledge between 2010 and 2019. Environ. Monit. Assess. 2022, 194, 306. [Google Scholar] [CrossRef]
- Ouédraogo, G.A.; Cissé, H.; Ouédraogo, H.S.; Kaboré, B.; Traoré, R.; Traoré, Y.; Bassolé, I.H.N.; Tchoumbougnang, F.; Savadogo, A. Research of Antibiotic Residues and Bacterial Strain’s Antibiotic Resistance Profile in the Liquid Effluents Evacuated in Nature by Two CHUs and a Mixed WWTP of Ouagadougou (Burkina Faso). Infect. Drug Resist. 2023, 16, 2537–2547. [Google Scholar] [CrossRef]
- Irfan, M.; Almotiri, A.; AlZeyadi, Z.A. Antimicrobial Resistance and β-Lactamase Production in Clinically Significant Gram-Negative Bacteria Isolated from Hospital and Municipal Wastewater. Antibiotics 2023, 12, 653. [Google Scholar] [CrossRef]
- Cho, S.; Jackson, C.R.; Frye, J.G. Freshwater Environment as a Reservoir of Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae. J. Appl. Microbiol. 2023, 134, lxad034. [Google Scholar] [CrossRef]
- Iovleva, A.; Doi, Y. Carbapenem-Resistant Enterobacteriaceae. Clin. Lab. Med. 2017, 37, 303. [Google Scholar] [CrossRef]
- Wang, H.; Wang, T.; Zhang, B.; Li, F.; Toure, B.; Omosa, I.B.; Chiramba, T.; Abdel-Monem, M.; Pradhan, M. Water and Wastewater Treatment in Africa–Current Practices and Challenges. CLEAN–Soil Air Water 2014, 42, 1029–1035. [Google Scholar] [CrossRef]
- WHO Antimicrobial Resistance: Accelerating National and Global Responses; World Health Organization: Geneva, Switzerland, 2023.
- Owojori, G.O.; Lateef, S.A.; Ana, G.R.E.E. Effectiveness of Wastewater Treatment Plant at the Removal of Nutrients, Pathogenic Bacteria, and Antibiotic-Resistant Bacteria in Wastewater from Hospital Source. Environ. Sci. Pollut. Res. 2024, 31, 10785–10801. [Google Scholar] [CrossRef]
- Drane, K.; Sheehan, M.; Whelan, A.; Ariel, E.; Kinobe, R. The Role of Wastewater Treatment Plants in Dissemination of Antibiotic Resistance: Source, Measurement, Removal and Risk Assessment. Antibiotics 2024, 13, 668. [Google Scholar] [CrossRef]
- Barathan, M.; Ng, S.-L.; Lokanathan, Y.; Ng, M.H.; Law, J.X. Unseen Weapons: Bacterial Extracellular Vesicles and the Spread of Antibiotic Resistance in Aquatic Environments. Int. J. Mol. Sci. 2024, 25, 3080. [Google Scholar] [CrossRef]
- Urzua-Abad, M.M.; Aquino-Andrade, A.; Castelan-Vega, J.A.; Merida-Vieyra, J.; Ribas-Aparicio, R.M.; Belmont-Monroy, L.; Jimenez-Alberto, A.; Aparicio-Ozores, G. Detection of Carbapenemases in Enterobacterales and Other Gram-Negative Bacilli Recovered from Hospital and Municipal Wastewater in Mexico City. Sci. Rep. 2024, 14, 26576. [Google Scholar] [CrossRef]
- Nasser-Ali, M.; Aja-Macaya, P.; Conde-Pérez, K.; Trigo-Tasende, N.; Rumbo-Feal, S.; Fernández-González, A.; Bou, G.; Poza, M.; Vallejo, J.A. Emergence of Carbapenemase Genes in Gram-Negative Bacteria Isolated from the Wastewater Treatment Plant in A Coruña, Spain. Antibiotics 2024, 13, 194. [Google Scholar] [CrossRef]
- Patra, N.; Prakash, M.R.; Patil, S.; Rao, M.B. First Case Report of Surgical Site Infection Due to Buttiauxella agrestis in a Neurocare Center in India. Arch. Med. Health Sci. 2018, 6, 117. [Google Scholar] [CrossRef]
- Wójcik-Fatla, A.; Farian, E.; Kowalczyk, K.; Sroka, J.; Skowron, P.; Siebielec, G.; Zdybel, J.M.; Jadczyszyn, T.; Cencek, T. Enterobacteriaceae in Sewage Sludge and Digestate Intended for Soil Fertilization. Pathogens 2024, 13, 1056. [Google Scholar] [CrossRef]
- Wilson, H.; Török, M.E. Extended-Spectrum β-Lactamase-Producing and Carbapenemase-Producing Enterobacteriaceae. Microb. Genom. 2018, 4, e000197. [Google Scholar] [CrossRef]
- Garba, Z.; Bonkoungou, I.O.J.; Millogo, N.O.; Natama, H.M.; Vokouma, P.A.P.; Bonko, M.d.A.; Karama, I.; Tiendrebeogo, L.A.W.; Haukka, K.; Tinto, H.; et al. Wastewater from Healthcare Centers in Burkina Faso Is a Source of ESBL, AmpC-β-Lactamase and Carbapenemase-Producing Escherichia Coli and Klebsiella Pneumoniae. BMC Microbiol. 2023, 23, 351. [Google Scholar] [CrossRef]
- Hubeny, J.; Ciesielski, S.; Harnisz, M.; Korzeniewska, E.; Dulski, T.; Jałowiecki, Ł.; Płaza, G. Impact of Hospital Wastewater on the Occurrence and Diversity of Beta-Lactamase Genes During Wastewater Treatment with an Emphasis on Carbapenemase Genes: A Metagenomic Approach. Front. Environ. Sci. 2021, 9, 738158. [Google Scholar] [CrossRef]
- Fouz, N.; Pangesti, K.N.A.; Yasir, M.; Al-Malki, A.L.; Azhar, E.I.; Hill-Cawthorne, G.A.; Abd El Ghany, M. The Contribution of Wastewater to the Transmission of Antimicrobial Resistance in the Environment: Implications of Mass Gathering Settings. Trop. Med. Infect. Dis. 2020, 5, 33. [Google Scholar] [CrossRef]
- Liu, Y.; Tong, Z.; Shi, J.; Jia, Y.; Yang, K.; Wang, Z. Correlation between Exogenous Compounds and the Horizontal Transfer of Plasmid-Borne Antibiotic Resistance Genes. Microorganisms 2020, 8, 1211. [Google Scholar] [CrossRef]
- Wu, Y.; Gong, Z.; Wang, S.; Song, L. Occurrence and Prevalence of Antibiotic Resistance Genes and Pathogens in an Industrial Park Wastewater Treatment Plant. Sci. Total Environ. 2023, 880, 163278. [Google Scholar] [CrossRef]
- Nyirenda, T. Antibiotic Resistance Causes More Deaths than Malaria and HIV/Aids Combined. What Africa Is Doing to Fight This Silent Epidemic. Available online: http://theconversation.com/antibiotic-resistance-causes-more-deaths-than-malaria-and-hiv-aids-combined-what-africa-is-doing-to-fight-this-silent-epidemic-217689 (accessed on 25 November 2024).
- Kaur, J.; Chopra, S.; Sheevani; Mahajan, G. Modified Double Disc Synergy Test to Detect ESBL Production in Urinary Isolates of Escherichia Coli and Klebsiella Pneumoniae. J. Clin. Diagn. Res. JCDR 2013, 7, 229. [Google Scholar] [CrossRef]
- Husna, A.; Rahman, M.M.; Badruzzaman, A.T.M.; Sikder, M.H.; Islam, M.R.; Rahman, M.T.; Alam, J.; Ashour, H.M. Extended-Spectrum β-Lactamases (ESBL): Challenges and Opportunities. Biomedicines 2023, 11, 2937. [Google Scholar] [CrossRef]
- Sageerabanoo, S.; Malini, A.; Mangaiyarkarasi, T.; Hemalatha, G. Phenotypic Detection of Extended Spectrum β-Lactamase and Amp-C β-Lactamase Producing Clinical Isolates in a Tertiary Care Hospital: A Preliminary Study. J. Nat. Sci. Biol. Med. 2015, 6, 383–387. [Google Scholar] [CrossRef]
- Sachdeva, R.; Sharma, B.; Sharma, R. Evaluation of Different Phenotypic Tests for Detection of Metallo-β-Lactamases in Imipenem-Resistant Pseudomonas Aeruginosa. J. Lab. Physicians 2017, 9, 249–253. [Google Scholar] [CrossRef]
- Kumudunie, W.G.M.; Wijesooriya, L.I.; Wijayasinghe, Y.S. Comparison of Four Low-Cost Carbapenemase Detection Tests and a Proposal of an Algorithm for Early Detection of Carbapenemase-Producing Enterobacteriaceae in Resource-Limited Settings. PLoS ONE 2021, 16, e0245290. [Google Scholar] [CrossRef]
- Haller, L.; Chen, H.; Ng, C.; Le, T.H.; Koh, T.H.; Barkham, T.; Sobsey, M.; Gin, K.Y.-H. Occurrence and Characteristics of Extended-Spectrum β-Lactamase- and Carbapenemase-Producing Bacteria from Hospital Effluents in Singapore. Sci. Total Environ. 2018, 615, 1119–1125. [Google Scholar] [CrossRef]
- Popa, L.I.; Gheorghe, I.; Barbu, I.C.; Surleac, M.; Paraschiv, S.; Măruţescu, L.; Popa, M.; Pîrcălăbioru, G.G.; Talapan, D.; Niţă, M.; et al. Multidrug Resistant Klebsiella Pneumoniae ST101 Clone Survival Chain from Inpatients to Hospital Effluent After Chlorine Treatment. Front. Microbiol. 2021, 11, 610296. [Google Scholar] [CrossRef]
- Onyedibe, K.I.; Shobowale, E.O.; Okolo, M.O.; Iroezindu, M.O.; Afolaranmi, T.O.; Nwaokorie, F.O.; Opajobi, S.O.; Isa, S.E.; Egah, D.Z. Low Prevalence of Carbapenem Resistance in Clinical Isolates of Extended Spectrum Beta Lactamase (ESBL) Producing Escherichia Coli in North Central, Nigeria. Adv. Infect. Dis. 2018, 8, 109–120. [Google Scholar] [CrossRef]
- Ebomah, K.E.; Okoh, A.I. An African Perspective on the Prevalence, Fate and Effects of Carbapenem Resistance Genes in Hospital Effluents and Wastewater Treatment Plant (WWTP) Final Effluents: A Critical Review. Heliyon 2020, 6, e03899. [Google Scholar] [CrossRef]
- Güzelaydın, K.; Yıldırım, M. The Bioavailability of Ampicillins in Some Animals. J. Istanb. Vet. Sci. 2024, 8, 1–4. [Google Scholar] [CrossRef]
- Belachew, T.; Mihret, A.; Legesse, T.; Million, Y.; Desta, K. High Level of Drug Resistance by Gram-Negative Bacteria from Selected Sewage Polluted Urban Rivers in Addis Ababa, Ethiopia. BMC Res. Notes 2018, 11, 524. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, X.; Luo, L.; Hu, N.; Duan, J.; Tang, Z.; Zhong, R.; Li, Y. The Prevalence and Characterization of Extended-Spectrum β-Lactamase- and Carbapenemase-Producing Bacteria from Hospital Sewage, Treated Effluents and Receiving Rivers. Int. J. Environ. Res. Public. Health 2020, 17, 1183. [Google Scholar] [CrossRef]
- Hanna, N.; Tamhankar, A.J.; Lundborg, C.S. Antibiotic Concentrations and Antibiotic Resistance in Aquatic Environments of the WHO Western Pacific and South-East Asia Regions: A Systematic Review and Probabilistic Environmental Hazard Assessment. Lancet Planet. Health 2023, 7, e45–e54. [Google Scholar] [CrossRef]
- Foxman, B.; Salzman, E.; Gesierich, C.; Gardner, S.; Ammerman, M.; Eisenberg, M.; Wigginton, K. Wastewater Surveillance of Antibiotic Resistant Bacteria for Public Health Action: Potential and Challenges. Am. J. Epidemiol. 2024, 194, kwae419. [Google Scholar] [CrossRef]
- Hoelle, J.; Johnson, J.R.; Johnston, B.D.; Kinkle, B.; Boczek, L.; Ryu, H.; Hayes, S. Survey of US Wastewater for Carbapenem-Resistant Enterobacteriaceae. J. Water Health 2019, 17, 219–226. [Google Scholar] [CrossRef]
- Valia, D.; Ingelbeen, B.; Kaboré, B.; Karama, I.; Peeters, M.; Lompo, P.; Vlieghe, E.; Post, A.; Cox, J.; de Mast, Q.; et al. Use of WATCH Antibiotics Prior to Presentation to the Hospital in Rural Burkina Faso. Antimicrob. Resist. Infect. Control 2022, 11, 59. [Google Scholar] [CrossRef]
- Nasrollahian, S.; Graham, J.P.; Halaji, M. A Review of the Mechanisms That Confer Antibiotic Resistance in Pathotypes of E. Coli. Front. Cell. Infect. Microbiol. 2024, 14, 1387497. [Google Scholar] [CrossRef]
- Lascols, C.; Cherney, B.; Conley, A.B.; Rishishwar, L.; Crawford, M.A.; Morse, S.A.; Fisher, D.J.; Anderson, K.; Hodge, D.R.; Pillai, S.P.; et al. Investigation of Multidrug-Resistant Plasmids from Carbapenemase-Producing Klebsiella Pneumoniae Clinical Isolates from Pakistan. Front. Microbiol. 2023, 14, 1192097. [Google Scholar] [CrossRef] [PubMed]
- Kopotsa, K.; Osei Sekyere, J.; Mbelle, N.M. Plasmid Evolution in Carbapenemase-Producing Enterobacteriaceae: A Review. Ann. N. Y. Acad. Sci. 2019, 1457, 61–91. [Google Scholar] [CrossRef]
- Jean, S.-S.; Harnod, D.; Hsueh, P.-R. Global Threat of Carbapenem-Resistant Gram-Negative Bacteria. Front. Cell. Infect. Microbiol. 2022, 12, 823684. [Google Scholar] [CrossRef]
- Akpan, S.N.; Odeniyi, O.A.; Adebowale, O.O.; Alarape, S.A.; Adeyemo, O.K. Antibiotic Resistance Profile of Gram-Negative Bacteria Isolated from Lafenwa Abattoir Effluent and Its Receiving Water (Ogun River) in Abeokuta, Ogun State, Nigeria. Onderstepoort J. Vet. Res. 2020, 87, 1854. [Google Scholar] [CrossRef] [PubMed]
- Whitman, W.B. Bergey’s Manual of Systematics of Archaea and Bacteria; Wiley: Hoboken, NJ, USA, 2015; ISBN 978-1-118-96060-8. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- CA-SFM/EUCAST. Comité de l’Antibiograme de la Société Française de Microbiologie; Société Française de Microbiologie: Paris, France, 2024. [Google Scholar]
- Lascols, C.; Legrand, P.; Mérens, A.; Leclercq, R.; Armand-Lefevre, L.; Drugeon, H.B.; Kitzis, M.D.; Muller-Serieys, C.; Reverdy, M.E.; Roussel-Delvallez, M.; et al. In Vitro Antibacterial Activity of Doripenem against Clinical Isolates from French Teaching Hospitals: Proposition of Zone Diameter Breakpoints. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 475–482. [Google Scholar] [CrossRef] [PubMed]
- M100; Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023.
- Chauhan, K.; Pandey, A.; Asthana, A.K.; Madan, M. Evaluation of Phenotypic Tests for Detection of Klebsiella Pneumoniae Carbapenemase and Metallo-Beta-Lactamase in Clinical Isolates of Escherichia Coli and Klebsiella Species. Indian. J. Pathol. Microbiol. 2015, 58, 31–35. [Google Scholar] [CrossRef]
- M100S; Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2016; Volume 26.

| WWTPs | ESBL-E and CPE Strains N (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Buttiauxella spp. | Enterobacter spp. | Escherichia Coli | Klebsiella spp. | |||||
| Influent | Effluent | Influent | Effluent | Influent | Effluent | Influent | Effluent | |
| WWTP 1 | 0/3 (0) | 1/4 (25) | 1/7 (14) | 4/8 (50) | 4/18 (22) | 1/7 (14) | 1/9 (11) | 1/1 (100) |
| WWTP 2 | 1/5 (20) | 1/5 (20) | 3/12 (25) | 0/6 (0) | 3/10 (30) | 1/8 (13) | 1/6 (17) | 1/2 (50) |
| WWTP 3 | 1/5 (20) | 0/5 (0) | 11/18 (61) | 1/4 (25) | 1/7 (14) | 1/1 (100) | 1/2 (50) | 2/2 (100) |
| Antibiotics | WWTP 1 | WWTP 2 | WWTP 3 | |||
|---|---|---|---|---|---|---|
| Influent | Effluent | Influent | Effluent | Influent | Effluent | |
| AMP | 100 | 90 | 80 | 80 | 100 | 100 |
| ATM | 70 | 70 | 60 | 70 | 90 | 80 |
| AUG | 70 | 50 | 50 | 50 | 80 | 100 |
| CAZ | 80 | 70 | 60 | 50 | 90 | 70 |
| CTR | 90 | 90 | 80 | 70 | 100 | 80 |
| CTX | 80 | 80 | 60 | 60 | 90 | 80 |
| DOR | 0 | 10 | 0 | 10 | 20 | 20 |
| ETP | 20 | 30 | 30 | 20 | 40 | 70 |
| FEP | 90 | 90 | 80 | 80 | 90 | 80 |
| IMP | 90 | 100 | 90 | 90 | 90 | 100 |
| MRP | 0 | 10 | 0 | 10 | 10 | 20 |
| TZP | 10 | 10 | 0 | 20 | 20 | 0 |
| Antibiotics | WWTP 1 | WWTP 2 | WWTP 3 | |||
|---|---|---|---|---|---|---|
| Influent | Effluent | Influent | Effluent | Influent | Effluent | |
| AMP | 80 | 80 | 70 | 70 | 100 | 100 |
| ATM | 60 | 40 | 30 | 30 | 90 | 70 |
| AUG | 60 | 60 | 50 | 60 | 70 | 80 |
| CAZ | 70 | 40 | 40 | 20 | 90 | 80 |
| CTR | 80 | 60 | 60 | 50 | 100 | 70 |
| CTX | 70 | 60 | 40 | 30 | 100 | 70 |
| DOR | 20 | 20 | 10 | 10 | 30 | 30 |
| ETP | 50 | 40 | 30 | 50 | 40 | 70 |
| FEP | 80 | 60 | 50 | 50 | 100 | 70 |
| IMP | 90 | 100 | 90 | 90 | 100 | 100 |
| MRP | 20 | 30 | 10 | 10 | 20 | 30 |
| TZP | 20 | 20 | 10 | 10 | 30 | 20 |
| Strains | WWTP 1 | WWTP 2 | WWTP 3 | |||
|---|---|---|---|---|---|---|
| Influent N (%) | Effluent N (%) | Influent N (%) | Effluent N (%) | Influent N (%) | Effluent N (%) | |
| ESBL-E | 40/41 (98) | 19/20 (95) | 22/26 (85) | 9/12 (82) | 56/56 (100) | 12/12 (100) |
| CPE | 22/25 (88) | 23/27 (85) | 10/15 (67) | 11/15 (73) | 21/21 (100) | 6/6 (100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamma Yacouba, I.; Konaté, Y.; Bazie, B.V.E.J.T.; Sawadogo, B. Phenotypic Detection of Extended-Spectrum β-Lactamase and Carbapenemase-Producing Enterobacteriaceae from Wastewater Treatment Plants in Ouagadougou, Burkina Faso. Antibiotics 2025, 14, 641. https://doi.org/10.3390/antibiotics14070641
Hamma Yacouba I, Konaté Y, Bazie BVEJT, Sawadogo B. Phenotypic Detection of Extended-Spectrum β-Lactamase and Carbapenemase-Producing Enterobacteriaceae from Wastewater Treatment Plants in Ouagadougou, Burkina Faso. Antibiotics. 2025; 14(7):641. https://doi.org/10.3390/antibiotics14070641
Chicago/Turabian StyleHamma Yacouba, Inayatou, Yacouba Konaté, Bapio Valérie Elvira Jean Télesphore Bazie, and Boukary Sawadogo. 2025. "Phenotypic Detection of Extended-Spectrum β-Lactamase and Carbapenemase-Producing Enterobacteriaceae from Wastewater Treatment Plants in Ouagadougou, Burkina Faso" Antibiotics 14, no. 7: 641. https://doi.org/10.3390/antibiotics14070641
APA StyleHamma Yacouba, I., Konaté, Y., Bazie, B. V. E. J. T., & Sawadogo, B. (2025). Phenotypic Detection of Extended-Spectrum β-Lactamase and Carbapenemase-Producing Enterobacteriaceae from Wastewater Treatment Plants in Ouagadougou, Burkina Faso. Antibiotics, 14(7), 641. https://doi.org/10.3390/antibiotics14070641






