Agave amica (Medik.) Thiede & Govaerts (Asparagaceae)—Insights into Its Valuable Phenolic Profile and In Vitro Antimicrobial, Antibiofilm, Antioxidative, and Antiproliferative Properties
Abstract
1. Introduction
2. Results
2.1. Quantitative Evaluation of Phenolic Compounds and Antioxidant Activity
2.2. LC–MS Analysis
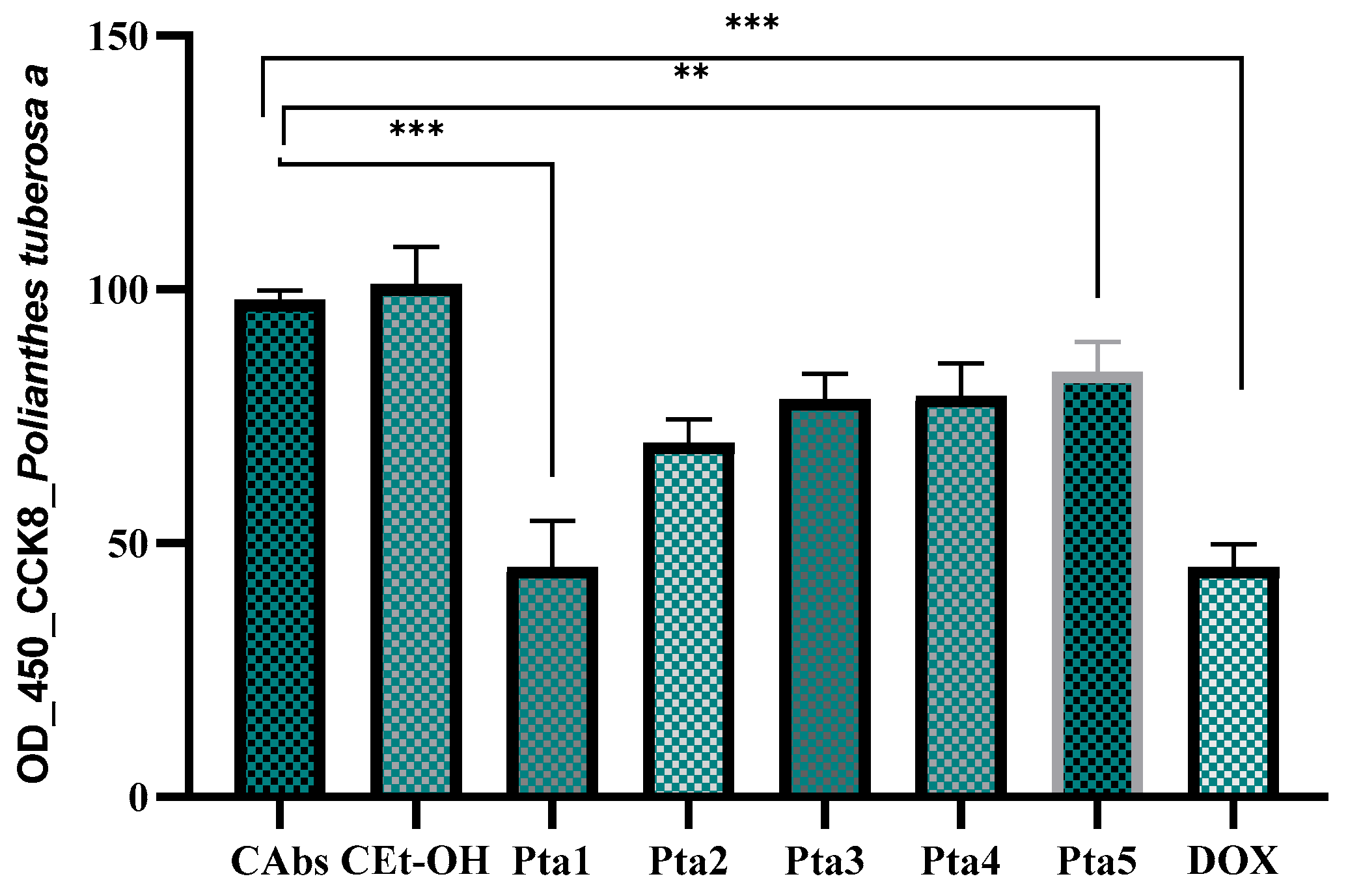
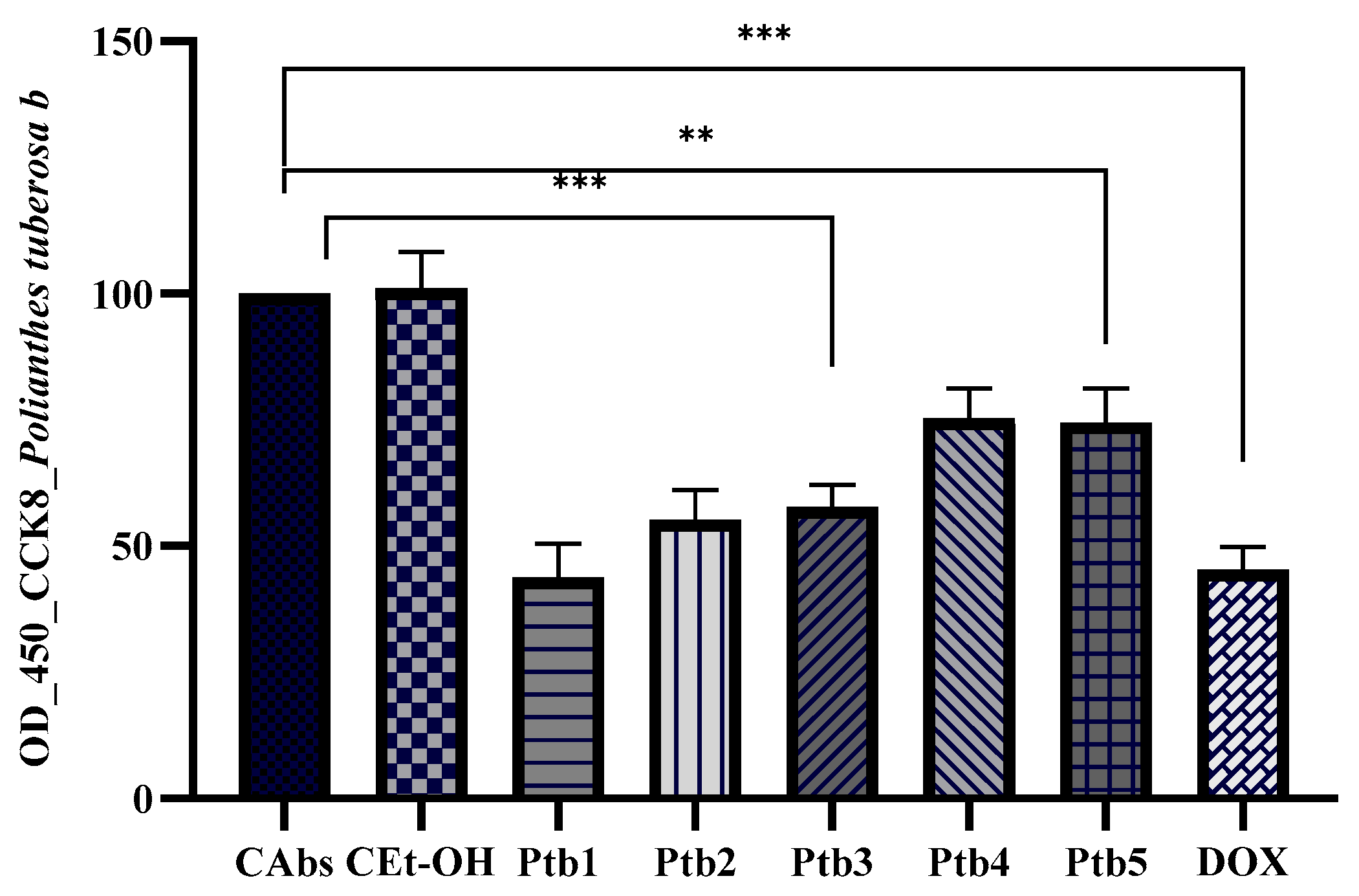
2.3. Antiproliferative Assays
2.4. Antimicrobial Activity Assays
2.4.1. The Agar-Well Diffusion Method
2.4.2. The Broth Microdilution Method
2.4.3. The Antibiofilm Assay
3. Discussion
4. Materials and Methods
4.1. Vegetal Material
4.2. Chemical Agents
4.3. Extraction Method
4.4. Total Polyphenolic Content (TPC) Quantification Method
4.5. Flavonoid Content (FC) Quantification Method
4.6. Caffeic Acid Derivatives (CADC) Quantification Method
4.7. Tannin Content (TC) Quantification Method
4.8. LC–MS Analysis
4.9. Antioxidant Assays
4.9.1. DPPH Test
4.9.2. FRAP Test
4.10. Cell Line and Cytotoxicity Assay
4.11. Antimicrobial Activity Assays
4.11.1. Agar-Well Diffusion Method
4.11.2. Broth Microdilution Method
4.11.3. Antibiofilm Assay
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial Resistance: Impacts, Challenges, and Future Prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Liu, H.Y.; Prentice, E.L.; Webber, M.A. Mechanisms of Antimicrobial Resistance in Biofilms. npj Antimicrob. Resist. 2024, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Antunes, W.; Mota, S.; Madureira-Carvalho, Á.; Dinis-Oliveira, R.J.; Dias da Silva, D. An Overview of the Recent Advances in Antimicrobial Resistance. Microorganisms 2024, 12, 1920. [Google Scholar] [CrossRef]
- Zouine, N.; Ghachtouli, N.E.; Abed, S.E.; Koraichi, S.I. A Comprehensive Review on Medicinal Plant Extracts as Antibacterial Agents: Factors, Mechanism Insights and Future Prospects. Sci. Afr. 2024, 26, e02395. [Google Scholar] [CrossRef]
- Lahiri, D.; Dash, S.; Dutta, R.; Nag, M. Elucidating the Effect of Anti-Biofilm Activity of Bioactive Compounds Extracted from Plants. J. Biosci. 2019, 44, 52. [Google Scholar] [CrossRef]
- Ahmadian, M.; Ahmadi, N.; Babaei, A.; Naghavi, M.R.; Ayyari, M. Comparison of Volatile Compounds at Various Developmental Stages of Tuberose (Polianthes tuberosa l. Cv. Mahallati) Flower with Different Extraction Methods. J. Essent. Oil Res. 2018, 30, 197–206. [Google Scholar] [CrossRef]
- Srinivasa Suryakoppa, K.; Appadurai, R.; Byrappa, K.; Khan, M.H.M. Phytochemical Analysis of UV Active and Inactive Bioactive Compounds Present in Polianthes tuberosa (Linn.) Flower. J. Sep. Sci. 2021, 44, 3376–3385. [Google Scholar] [CrossRef]
- Thiede, J. Agave (Agavaceae). In Monocotyledons; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Thiede, J.; Eggli, U. Agavaceae. In Monocotyledons; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Thiede, J.; Govaerts, R. New Combinations in Agave (Asparagaceae): A. Amica, A. Nanchititlensis, and A. Quilae. Phytotaxa 2017, 306, 237–240. [Google Scholar] [CrossRef]
- Sadhukhan, R.; Chowdhuri, T.K.; Datta, S.K. Tuberose (Polyanthes tuberosa Linn./Agave amica); Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Salomé-Abarca, L.F.; Márquez-López, R.E.; López, M.G. Agave amica a Potential Model for the Study of Agavins Metabolism. Sci. Rep. 2023, 13, 19888. [Google Scholar] [CrossRef]
- Barghout, N.; Chebata, N.; Moumene, S.; Khennouf, S.; Gharbi, A.; El Hadi, D. Antioxidant and Antimicrobial Effect of Alkaloid Bulbs Extract of Polianthes tuberosa L. (Amaryllidaceae) Cultivated in Algeria. J. Drug Deliv. Ther. 2020, 10, 173–180. [Google Scholar] [CrossRef]
- Barghout, N.; Khennouf, S.; Yekrelef, A.; El-Hadi, D. Polyphenols from Polianthes tuberosa L.(Amaryllidaceae) Leaves and Their Antioxidant Properties. AgroBiologia 2018, 8, 902–912. [Google Scholar]
- Bharathi, T.U.; Lallawmzuali, R.; Kirthishree, S.P. Diversity in Flower Morphology of the Single-Type Tuberose (Agave amica (Medik.) Thiede & Govaerts). Genet. Resour. Crop Evol. 2024, 71, 4239–4254. [Google Scholar] [CrossRef]
- Copetta, A.; Marchioni, I.; Mascarello, C.; Pistelli, L.; Cambournac, L.; Dimita, R.; Ruffoni, B. Polianthes tuberosa as Edible Flower: In Vitro Propagation and Nutritional Properties. Int. J. Food Eng. 2020, 6, 57–62. [Google Scholar] [CrossRef]
- Kutty, N.N.; Mitra, A. Profiling of Volatile and Non-Volatile Metabolites in Polianthes tuberosa L. Flowers Reveals Intraspecific Variation among Cultivars. Phytochemistry 2019, 162, 10–20. [Google Scholar] [CrossRef]
- Rumi, F.; Kuddus, M.; Das, S. Evaluation of Antioxidant, Cytotoxic, Antimicrobial, Membrane Stabilizing and Thrombolytic Activities of Polianthes Tuberose Linn. Br. J. Pharm. Res. 2014, 4, 2106–2115. [Google Scholar] [CrossRef]
- Lim, T.K. Edible Medicinal And Non-Medicinal Plants—Volume 7: Flowers; Springer: Berlin/Heidelberg, Germany, 2014; Volume 7. [Google Scholar] [CrossRef]
- Kumaran, S.; Santhiyaa, R.V.; Prakaesh, U.; Sivasankari, B.; Kokila, D. Biosynthesis of Silver Nanoparticles Using Aqueous Flower Extracts of Polianthes tuberosa and Their Antibacterial and Cytotoxicity Activity. Int. J. Res. Anal. Rev. 2019, 5, 407–414. [Google Scholar]
- Maiti, S.; Moon, U.R.; Bera, P.; Samanta, T.; Mitra, A. The in Vitro Antioxidant Capacities of Polianthes tuberosa L. Flower Extracts. Acta Physiol. Plant. 2014, 36, 2597–2605. [Google Scholar] [CrossRef]
- Mimaki, Y.; Yokosuka, A.; Sashida, Y. Steroidal Glycosides from the Aerial Parts of Polianthes tuberosa. J. Nat. Prod. 2000, 63, 1519–1523. [Google Scholar] [CrossRef] [PubMed]
- Rahmatullah, N.R.; Jannat, K.; Islam, M.; Rahman, T.; Jahan, R.; Rahmatullah, M. A Short Review of Polianthes tuberosa L. Considered a Medicinal Plant in Bangladesh. J. Med. Plants Stud. 2019, 7, 1–4. [Google Scholar]
- Lydia, J.; Kannan, M.; Aruna, P.; Jeyakumar, P.; Vetrivelkaalai, P.; Muralidharan, B. Determination of Bioactive Compounds in Agave amica L. Cv Arka Prajwal. Biol. Forum–Int. J. 2022, 14, 366–372. [Google Scholar]
- Fragoso-Jiménez, J.C.; Tapia-Campos, E.; Estarron-Espinosa, M.; Barba-Gonzalez, R.; Castañeda-Saucedo, M.C.; Castillo-Herrera, G.A. Effect of Supercritical Fluid Extraction Process on Chemical Composition of Polianthes tuberosa Flower Extracts. Processes 2019, 7, 60. [Google Scholar] [CrossRef]
- Yadav, R.; Mohapatra, D.; Kate, A.; Giri, S.K.; Modhera, B. Sequential Ultrasound-Microwave-Assisted Extraction of Tuberose (Polianthes tuberosa L.) Concrete: The Effect of Processing Parameters on Yield, Volatile Metabolite Profiles, and Functional Groups. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; Patil, S.; Rajkuberan, C.; Krishnan, M.; Krishnan, U.; Abd-Elsalam, K.A. Polianthes tuberosa-Mediated Silver Nanoparticles from Flower Extract and Assessment of Their Antibacterial and Anticancer Potential: An In Vitro Approach. Plants 2023, 12, 1261. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; Rajkuberan, C.; Santhiya, T.; Krejcar, O.; Kuča, K.; Periakaruppan, R.; Prabukumar, S. Green Synthesis of Gold Nanoparticles Using Polianthes tuberosa L. Floral Extract. Plants 2021, 10, 2370. [Google Scholar] [CrossRef] [PubMed]
- Christy, J.J.A.; Begum, S.B.; Revathy, M.; Harisma, B.R.; Murugappan, R.M. Antimicrobial and Anti-Inflammatory Efficiency of Green Synthesized Zinc Oxide Nanoparticles Using Polianthes tuberosa Flower Concentrate. J. Environ. Biol. 2024, 45, 243–252. [Google Scholar] [CrossRef]
- Ghosh, P.K.; Bhattacharjee, P.; Das, S. Antimicrobial Activity of Supercritical Carbon Dioxide Extracts of Tuberose (Polianthes tuberosa Linn.) Flowers against Common Pathogens. Int. J. Pharm. Sci. Res. 2014, 5, 1279–1289. [Google Scholar] [CrossRef]
- Sundar, R.D.V.; Arunachalam, S. Anti-MRSA Activity of Pollianthes Tuberosa Leaf Extracts. Bangladesh J. Pharmacol. 2022, 17, 11–13. [Google Scholar] [CrossRef]
- Setiani, N.A.; Aulifa, D.L.; Selynita; Septiningsih, E. Phytochemical Screening and Antibacterial Activity of Flower, Stem, and Tuber of Polianthes tuberosa L. Against Acne-Inducing Bacteria. Adv. Heal. Sci. Res. 2020, 26, 92–95. [Google Scholar] [CrossRef]
- Nidiry, E.S.J.; Babu, C.S.B. Antifungal Activity of Tuberose Absolute and Some of Its Constituents. Phyther. Res. 2005, 19, 447–449. [Google Scholar] [CrossRef]
- Toma, F.; Georgescu, M.I.; Petra, S.; Dobrescu, E. Some Aspects Concerning the Rest Period of Tuberose Bulbs. Agric. Agric. Sci. Procedia 2015, 6, 179–183. [Google Scholar] [CrossRef]
- Moldovan, I.; Cantor, M.; Sabo, R.A.; Lukacs, L.; Somsai, P.A. Research Concerning the Influence of Culture Substrate on the Main Characteristics of Tuberose. Curr. Trends Nat. Sci. 2018, 7, 54–64. [Google Scholar]
- Brînză, M.; Draghia, L.; Ciobănică, M.; Chelaru, E.L.; Bernardis, R. The Influence of Bulb Size on the Morphological and Ornamental Characters of the Polyanthes tuberosa L. In Proceedings of the Conferința “Horticultură, Viticultură şi Vinificaţie, Silvicultură şi Grădini Publice, Protecţia Plantelor”, Chișinău, Moldova, 1–2 October 2018; pp. 534–539. [Google Scholar]
- Olawuwo, O.S.; Famuyide, I.M.; McGaw, L.J. Antibacterial and Antibiofilm Activity of Selected Medicinal Plant Leaf Extracts Against Pathogens Implicated in Poultry Diseases. Front. Vet. Sci. 2022, 9, 820304. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, J.; Venkataraman, S.; Meera, R.; Chidambaranathan, N.; Devi Devisree, P. Phyto-Physico Chemical Investigation, Anti-Inflammatory and Antimicrobial Activities of Pollianthes Tuberosa Linn. Res. J. Pharm. Technol. 2009, 2, 738–742. [Google Scholar]
- Neamțu, A.A.; Maghiar, T.A.; Turcuș, V.; Maghiar, P.B.; Căpraru, A.M.; Lazar, B.A.; Dehelean, C.A.; Pop, O.L.; Neamțu, C.; Totolici, B.D.; et al. A Comprehensive View on the Impact of Chlorogenic Acids on Colorectal Cancer. Curr. Issues Mol. Biol. 2024, 46, 6783–6804. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.; Guo, R.; Yi, Z.; Wang, Y.; Wang, H.; Li, Y.; Li, X.; Song, J. The Multiple Biological Activities of Hyperoside: From Molecular Mechanisms to Therapeutic Perspectives in Neoplastic and Non-Neoplastic Diseases. Front. Pharmacol. 2025, 16, 1538601. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Wilairatana, P.; Joshi, P.B.; Ahmad, Z.; Olatunde, A.; Hafeez, N.; Hemeg, H.A.; Mubarak, M.S. Revisiting Luteolin: An Updated Review on Its Anticancer Potential. Heliyon 2024, 10, e26701. [Google Scholar] [CrossRef]
- Naumowicz, M.; Kusaczuk, M.; Zając, M.; Jabłońska-Trypuć, A.; Mikłosz, A.; Gál, M.; Worobiczuk, M.; Kotyńska, J. The Influence of the PH on the Incorporation of Caffeic Acid into Biomimetic Membranes and Cancer Cells. Sci. Rep. 2022, 12, 3692. [Google Scholar] [CrossRef]
- Salari, N.; Faraji, F.; Jafarpour, S.; Faraji, F.; Rasoulpoor, S.; Dokaneheifard, S.; Mohammadi, M. Anti-Cancer Activity of Chrysin in Cancer Therapy: A Systematic Review. Indian J. Surg. Oncol. 2022, 13, 681–690. [Google Scholar] [CrossRef]
- Garcia-Ochoa, E.Y.; León-Morales, J.; Lugo-Cervantes, E.C.; Tapia-Campos, E. In Vitro Biological Activity of Metabolic Extracts of Wild and Cultivated Species of the Genus Polianthes. Acta Hortic. 2020, 1288, 149–152. [Google Scholar] [CrossRef]
- Wang, L.; Cao, X.; Pei, H.; Liu, P.; Song, Y.; Wu, Y. Anti-Biofilm Activity of Chlorogenic Acid against Pseudomonas Using Quorum Sensing System. Foods 2023, 12, 3601. [Google Scholar] [CrossRef]
- Davidova, S.; Galabov, A.S.; Satchanska, G. Antibacterial, Antifungal, Antiviral Activity, and Mechanisms of Action of Plant Polyphenols. Microorganisms 2024, 12, 2502. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Liu, M.; Fu, Y.; Zhang, J.; Liu, W.; Li, J.; Li, X.; Li, Y.; Wang, T. Antimicrobial Mechanism of Luteolin against Staphylococcus Aureus and Listeria Monocytogenes and Its Antibiofilm Properties. Microb. Pathog. 2020, 142, 104056. [Google Scholar] [CrossRef]
- Wang, G.; Li, L.; Wang, X.; Li, X.; Zhang, Y.; Yu, J.; Jiang, J.; You, X.; Xiong, Y.Q. Hypericin Enhances β-Lactam Antibiotics Activity by Inhibiting SarA Expression in Methicillin-Resistant Staphylococcus Aureus. Acta Pharm. Sin. B 2019, 9, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; He, Q.; Li, L.; Yu, X.; Sun, S.; Zhu, H. Exploring the Quorum Sensing Inhibition of Isolated Chrysin from Penicillium Chrysogenum DXY-1. Bioorg. Chem. 2021, 111, 104894. [Google Scholar] [CrossRef]
- Hanganu, D.; Niculae, M.; Ielciu, I.; Olah, N.-K.; Munteanu, M.; Burtescu, R.; Ştefan, R.; Olar, L.; Pall, E.; Andrei, S.; et al. Chemical Profile, Cytotoxic Activity and Oxidative Stress Reduction of Different Syringa vulgaris L. Extracts. Molecules 2021, 26, 3104. [Google Scholar] [CrossRef]
- Ielciu, I.; Niculae, M.; Pall, E.; Barbălată, C.; Tomuţă, I.; Olah, N.K.; Burtescu, R.F.; Benedec, D.; Oniga, I.; Hanganu, D. Antiproliferative and Antimicrobial Effects of Rosmarinus officinalis L. Loaded Liposomes. Molecules 2022, 27, 3988. [Google Scholar] [CrossRef]
- Aitynova, A.; Sevastre, B.; Ielciu, I.; Hanganu, D.; Olah, N.K.; Ibragimova, N.; Shalakhmetova, T.; Benedec, D.; Lyu, M.; Krasnoshtanov, A.; et al. Hepatoprotective Activity and Oxidative Stress Reduction of an Arctium tomentosum Mill. Root Extract in Mice with Experimentally Induced Hepatotoxicity. Livers 2024, 4, 696–710. [Google Scholar] [CrossRef]
- Ielciu, I.; Filip, G.A.; Sevastre-Berghian, A.C.; Bâldea, I.; Olah, N.K.; Burtescu, R.F.; Toma, V.A.; Moldovan, R.; Oniga, I.; Hanganu, D. Effects of a Rosmarinus officinalis L. Extract and Rosmarinic Acid in Improving Streptozotocin-Induced Aortic Tissue Damages in Rats. Nutrients 2025, 17, 158. [Google Scholar] [CrossRef]
- Benedec, D.; Oniga, I.; Hanganu, D.; Vlase, A.M.; Ielciu, I.; Crisan, G.; Fit, N.; Niculae, M.; Bab, T.; Pall, E.; et al. Revealing the Phenolic Composition and the Antioxidant, Antimicrobial and Antiproliferative Activities of Two Euphrasia Sp. Extracts. Plants 2024, 13, 1790. [Google Scholar] [CrossRef]
- Sevastre-Berghian, A.C.; Ielciu, I.; Mitre, A.O.; Filip, G.A.; Oniga, I.; Vlase, L.; Benedec, D.; Gheldiu, A.-M.; Toma, V.A.; Mihart, B.; et al. Targeting Oxidative Stress Reduction and Inhibition of HDAC1, MECP2, and NF-KB Pathways in Rats With Experimentally Induced Hyperglycemia by Administration of Thymus Marshallianus Willd. Extracts. Front. Pharmacol. 2020, 11, 581470. [Google Scholar] [CrossRef]
- Ielciu, I.; Filip, G.A.; Oniga, I.; Olah, N.K.; Bâldea, I.; Olteanu, D.; Burtescu, R.F.; Turcuș, V.; Sevastre-Berghian, A.C.; Benedec, D.; et al. Oxidative Stress and DNA Lesion Reduction of a Polyphenolic Enriched Extract of Thymus Marschallianus Willd. In Endothelial Vascular Cells Exposed to Hyperglycemia. Plants 2021, 10, 2810. [Google Scholar] [CrossRef]
- EDQM Council of Europe. European Pharmacopoeia, 11th ed.; EDQM Council of Europe: Strasbourg, France, 2025. [Google Scholar]
- Editura, Medicală. Romanian Pharmacopoeia, 10th ed.; Editura, Medicală: Bucureşti, Romania, 2005. [Google Scholar]
- Moldovan, M.L.; Iurian, S.; Puscas, C.; Silaghi-Dumitrescu, R.; Hanganu, D.; Bogdan, C.; Vlase, L.; Oniga, I.; Benedec, D. A Design of Experiments Strategy to Enhance the Recovery of Polyphenolic Compounds from Vitis Vinifera By-Products through Heat Reflux Extraction. Biomolecules 2019, 9, 529. [Google Scholar] [CrossRef] [PubMed]
- Galvão, M.A.M.; Arruda, A.O.d.; Bezerra, I.C.F.; Ferreira, M.R.A.; Soares, L.A.L. Evaluation of the Folin-Ciocalteu Method and Quantification of Total Tannins in Stem Barks and Pods from Libidibia Ferrea (Mart. Ex Tul) L. P. Queiroz. Braz. Arch. Biol. Technol. 2018, 61, e18170586. [Google Scholar] [CrossRef]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of Antiradical Properties of Antioxidants Using DPPH- Assay: A Critical Review and Results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant Activity of Dietary Polyphenols as Determined by a Modified Ferric Reducing/Antioxidant Power Assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef]
- Lupșe, I.; Pall, E.; Tudoran, L.B.; Bulboacă, A.E.; Ciurea, A.; Micu, I.C.; Roman, A.; Delean, A.G.; Muntean, A.; Soancă, A. Cytotoxic Effects on Gingival Mesenchymal Stromal Cells and Root Surface Modifications Induced by Some Local Antimicrobial Products Used in Periodontitis Treatment. Materials 2021, 14, 5049. [Google Scholar] [CrossRef]
- Pall, E.; Roman, A.; Olah, D.; Beteg, F.I.; Cenariu, M.; Spînu, M. Enhanced Bioactive Potential of Functionalized Injectable Platelet-Rich Plasma. Molecules 2023, 28, 1943. [Google Scholar] [CrossRef]
- Păltinean, R.; Ielciu, I.; Hanganu, D.; Niculae, M.; Pall, E.; Angenot, L.; Tits, M.; Mocan, A.; Babotă, M.; Frumuzachi, O.; et al. Biological Activities of Some Isoquinoline Alkaloids from Fumaria Schleicheri Soy. Will. Plants 2022, 11, 1202. [Google Scholar] [CrossRef]
- Simea, S.; Ielciu, I.; Hanganu, D.; Niculae, M.; Pall, E.; Burtescu, R.F.; Olah, N.; Cenariu, M.; Oniga, I.; Benedec, D.; et al. Evaluation of the Cytotoxic, Antioxidative and Antimicrobial Effects of Dracocephalum Moldavica L. Cultivars. Molecules 2023, 28, 1604. [Google Scholar] [CrossRef]
- Niculae, M.; Spinu, M.; Sandru, C.D.; Brudasca, F.; Cadar, D.; Szakacs, B.; Scurtu, I.; Bolfa, P.; Mates, C.I. Antimicrobial Potential of Some Lamiaceae Essential Oils against Animal Multiresistant Bacteria. Lucr. Științifice Med. Vet. 2009, 42, 170–175. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing, (EUCAST). Antimicrobial Susceptibility Testing EUCAST Disk Diffusion Method; EUCAST: Växjö, Sweden, 2020. [Google Scholar]
- Benedec, D.; Oniga, I.; Hanganu, D.; Tiperciuc, B.; Nistor, A.; Vlase, A.M.; Vlase, L.; Pușcaș, C.; Duma, M.; Login, C.C.; et al. Stachys Species: Comparative Evaluation of Phenolic Profile and Antimicrobial and Antioxidant Potential. Antibiotics 2023, 12, 1644. [Google Scholar] [CrossRef] [PubMed]
- Meccatti, V.M.; Santos, L.F.; Carvalho, L.S.d.; Souza, C.B.; Carvalho, C.A.T.; Marcucci, M.C.; Hasna, A.A.; de Oliveira, L.D. Antifungal Action of Herbal Plants’ Glycolic Extracts against Candida Species. Molecules 2023, 28, 2857. [Google Scholar] [CrossRef] [PubMed]
| A. amica Extracts | TPC (mg GAE/g) | TC (mg GAE/g) | FC (mg RE/g) | CADC (mg CAE/g) | DPPH (IC50 mg/mL) | FRAP (µM TE/g) |
|---|---|---|---|---|---|---|
| Pta | 44.25 ± 1.08 e | 12.55 ± 0.34 | 9.20 ± 0.19 | 19.95 ± 0.05 | 0.82 ± 0.02 c | 79.75 ± 1.80 |
| Ptb | 29.60 ± 0.89 a,e | 9.85 ± 0.15 a | 5.65 ± 0.13 a | 8.08 ± 0.12 a | 1.65 ± 0.01 b,c | 29.62 ± 0.37 d |
| Trolox | - | - | - | - | 0.011 ± 0.002 | - |
| Compound | Retention Time, min | m/z and Main Transition | A. amica Content (μg/g) | |||
|---|---|---|---|---|---|---|
| Reference | Separated Compound | Reference | Separated Compound | Ptb | Pta | |
| Caffeic acid | 13.8 | 13.6 | 179.0 > 135.0 | 179.0 > 135.0 | 4230 ± 10.40 | 8890 ± 22.20 |
| Chlorogenic acid | 12.0 | 12.0 | 353.0 > 191.0 | 353.0 > 191.0 | 1501 ± 3.70 | 4000 ± 9.90 |
| Salicylic acid | 23.5 | 23.4 | 137.0 > 93.0 | 137.0 > 93.0 | - | 3400 ± 8.40 |
| Carnosol | 30.7 | 30.4 | 329.1 > 285.1 | 329.1 > 285.1 | 37 ± 0.20 | 34 ± 0.20 |
| Chrysin | 29.7 | 29.8 | 253.0 > 143.0 | 253.0 > 253.0 | 1380 ± 3.40 | 1920 ± 4.70 |
| Hyperoside | 20.3 | 20.4 | 463.1 > 300.0 | 463.1 > 300.0 | 1510 ± 3.70 | 1640 ± 4.10 |
| Luteolin-7-O-glucoside | 19.9 | 19.8 | 447.0 > 284.9 | 447.0 > 284.9 | 72 ± 0.40 | 2290 ± 5.70 |
| Luteolin | 26.9 | 26.8 | 287.0 > 153.0 | 287.0 > 153.0 | - | 2300 ± 5.70 |
| Naringenin | 26.3 | 26.1 | 271.0 > 119.0 | 271.0 > 119.0 | - | 45 ± 0.20 |
| Quercetin | 25.4 | 25.4 | 300.9 > 151.0 | 300.9 > 151.0 | - | 20 ± 0.09 |
| Tested Samples | Diameters of Inhibition Zone (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| MSSA | MRSA | Bacillus cereus | Enterococcus faecalis | Listeria monocytogenes | Escherichia coli | Pseudomonas aeruginosa | Candida albicans | |
| Ptb | 13.25 ± 0.43 a | 10.75 ± 0.83 a | 12.75 ± 0.43 a | 0 | 14.25 ± 0.83 a | 10.5 ± 0.5 a | 0 | 19 ± 0.71 b |
| Pta | 13.50 ± 0.50 a | 11.50 ± 0.50 a | 11 ± 0.71 a | 0 | 14.45 ± 0.43 a | 10.25 ± 0.43 a | 0 | 18.5 ± 0.50 b |
| Gentamicin | 19 ± 0.00 | 17 ± 0.25 | 20 ± 0.00 | 10 ± 0.00 | 22 ± 0.50 | 19 ± 0.00 | 18 ± 0.00 | - |
| Fluconazole | - | - | - | - | - | - | - | 21 ± 0.00 |
| Samples | Microorganisms | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MSSA | MRSA | Bacillus cereus | Enterococcus faecalis | Listeria monocytogenes | Escherichia coli | Pseudomonas aeruginosa | Candida albicans | |||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MFC | |
| Ptb | 1.3 | 2.6 | 1.3 | 2.6 | 1.3 | 2.6 | >2.6 | >2.6 | 1.3 | 1.3 | 2.6 | > 2.6 | >2.6 | >2.6 | 0.325 | 0.325 |
| Pta | 1.3 | 2.6 | 1.3 | 2.6 | 2.6 | 2.6 | 2.6 | >2.6 | 1.3 | 1.3 | 2.6 | 2.6 | >2.6 | >2.6 | 0.325 | 0.325 |
| Gentamicin MIC (mg/L) | 3 | 4 | 3 | 3 | 3 | 4 | - | - | ||||||||
| Fluconazole MIC (mg/L) | - | - | - | - | - | - | - | 8 | ||||||||
| Inhibitory Activity Against Biofilm | ||||||
|---|---|---|---|---|---|---|
| Samples | MSSA | Listeria monocytogenes | Candida albicans | |||
| T0 | T24 | T0 | T24 | T0 | T24 | |
| Ptb | + | ++ | + | ++ | ++ | ++ |
| Pta | + | ++ | + | ++ | ++ | ++ |
| Gentamicin | + | ++ | + | ++ | - | - |
| Fluconazole | - | - | - | - | ++ | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niculae, M.; Hanganu, D.; Oniga, I.; Burcă, S.-A.; Tiperciuc, B.; Ielciu, I.; Pall, E.; Bab, T.; Burtescu, R.F.; Sava, M.A.; et al. Agave amica (Medik.) Thiede & Govaerts (Asparagaceae)—Insights into Its Valuable Phenolic Profile and In Vitro Antimicrobial, Antibiofilm, Antioxidative, and Antiproliferative Properties. Antibiotics 2025, 14, 638. https://doi.org/10.3390/antibiotics14070638
Niculae M, Hanganu D, Oniga I, Burcă S-A, Tiperciuc B, Ielciu I, Pall E, Bab T, Burtescu RF, Sava MA, et al. Agave amica (Medik.) Thiede & Govaerts (Asparagaceae)—Insights into Its Valuable Phenolic Profile and In Vitro Antimicrobial, Antibiofilm, Antioxidative, and Antiproliferative Properties. Antibiotics. 2025; 14(7):638. https://doi.org/10.3390/antibiotics14070638
Chicago/Turabian StyleNiculae, Mihaela, Daniela Hanganu, Ilioara Oniga, Sergiu-Alexandru Burcă, Brîndușa Tiperciuc, Irina Ielciu, Emoke Pall, Timea Bab, Ramona Flavia Burtescu, Mihaela Andreea Sava, and et al. 2025. "Agave amica (Medik.) Thiede & Govaerts (Asparagaceae)—Insights into Its Valuable Phenolic Profile and In Vitro Antimicrobial, Antibiofilm, Antioxidative, and Antiproliferative Properties" Antibiotics 14, no. 7: 638. https://doi.org/10.3390/antibiotics14070638
APA StyleNiculae, M., Hanganu, D., Oniga, I., Burcă, S.-A., Tiperciuc, B., Ielciu, I., Pall, E., Bab, T., Burtescu, R. F., Sava, M. A., & Benedec, D. (2025). Agave amica (Medik.) Thiede & Govaerts (Asparagaceae)—Insights into Its Valuable Phenolic Profile and In Vitro Antimicrobial, Antibiofilm, Antioxidative, and Antiproliferative Properties. Antibiotics, 14(7), 638. https://doi.org/10.3390/antibiotics14070638














