Oral Administration of Heat-Killed Multi-Strain Probiotics Confers Durable Protection Against Antibiotic-Resistant Primary and Recurrent Urinary Tract Infections in a Murine Model
Abstract
1. Introduction
2. Results
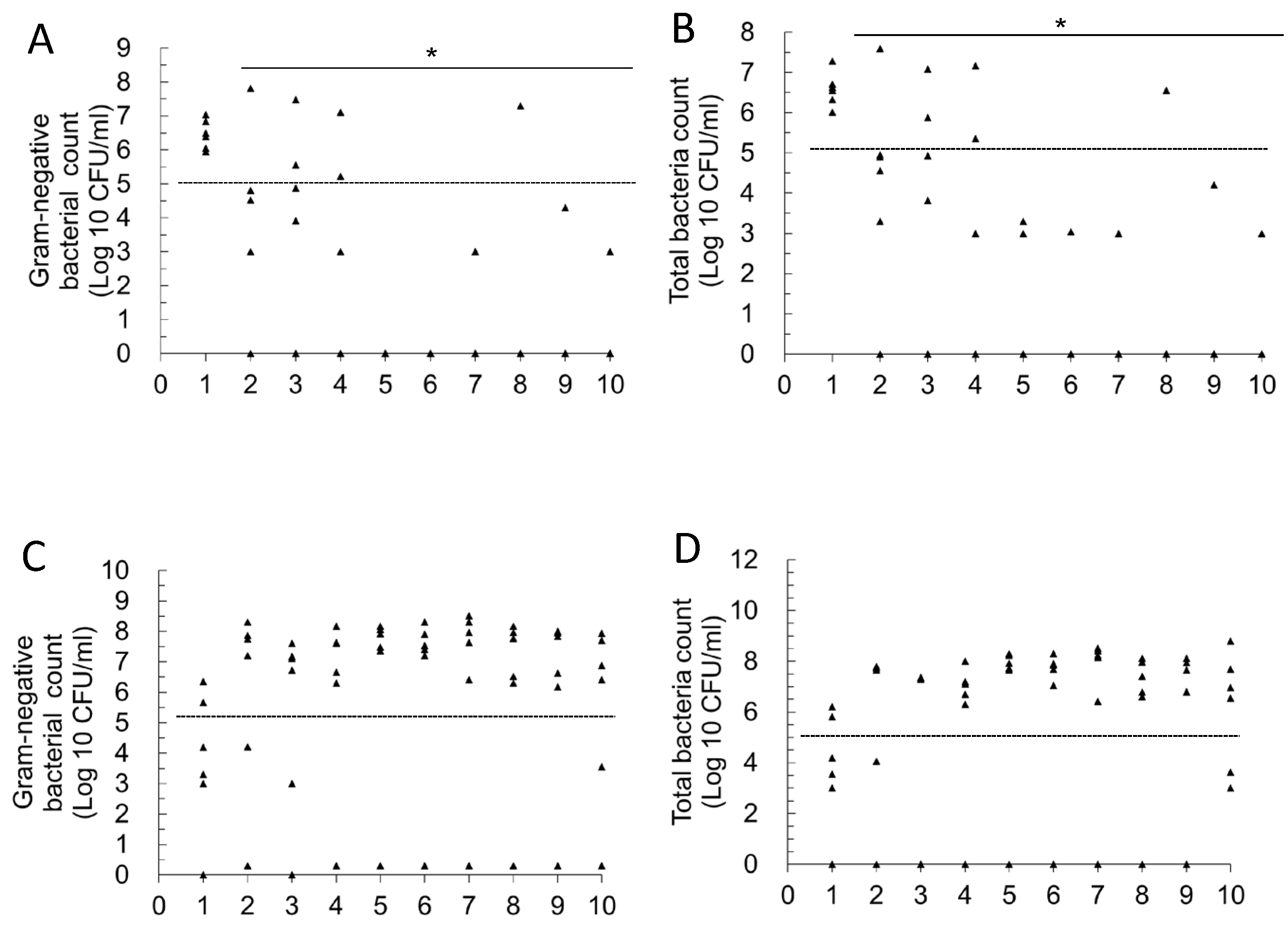
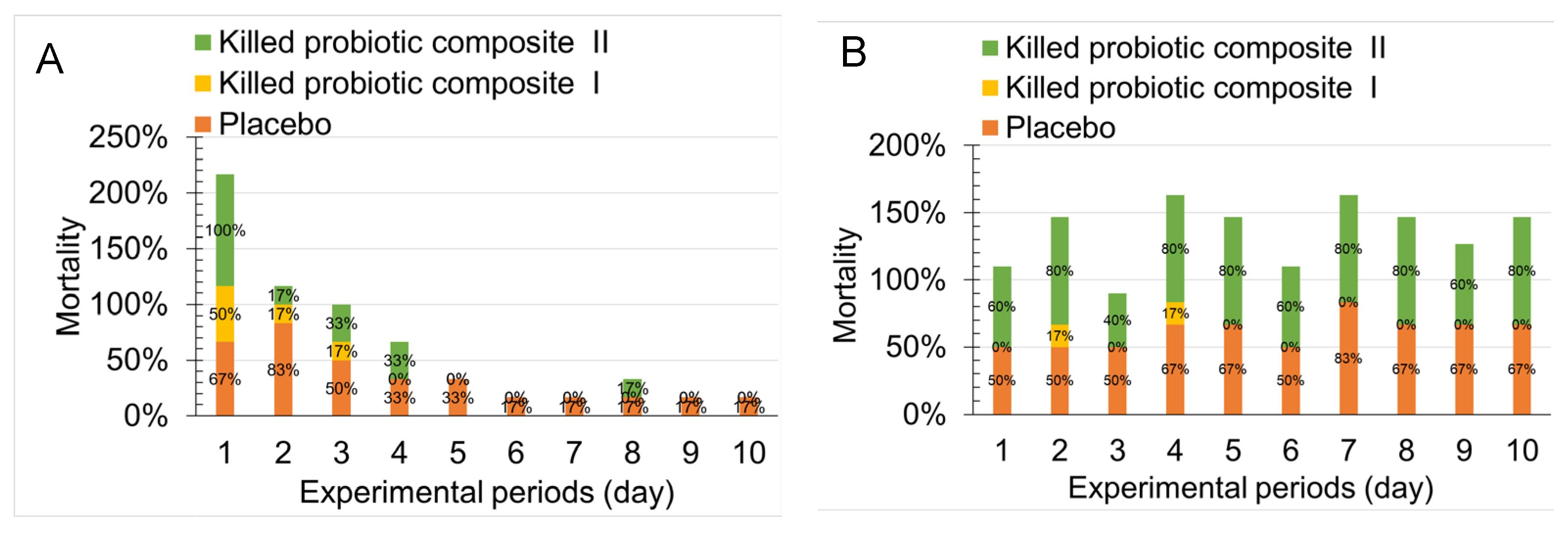
2.1. Effect of Single- and Multi-Strain Postbiotic Formulations on the Improvement of Primary Urinary Tract Infections in Mice
2.2. Effect of Oral Administration of Two Postbiotics on Improving High Bacterial Load and Recurrent UTIs
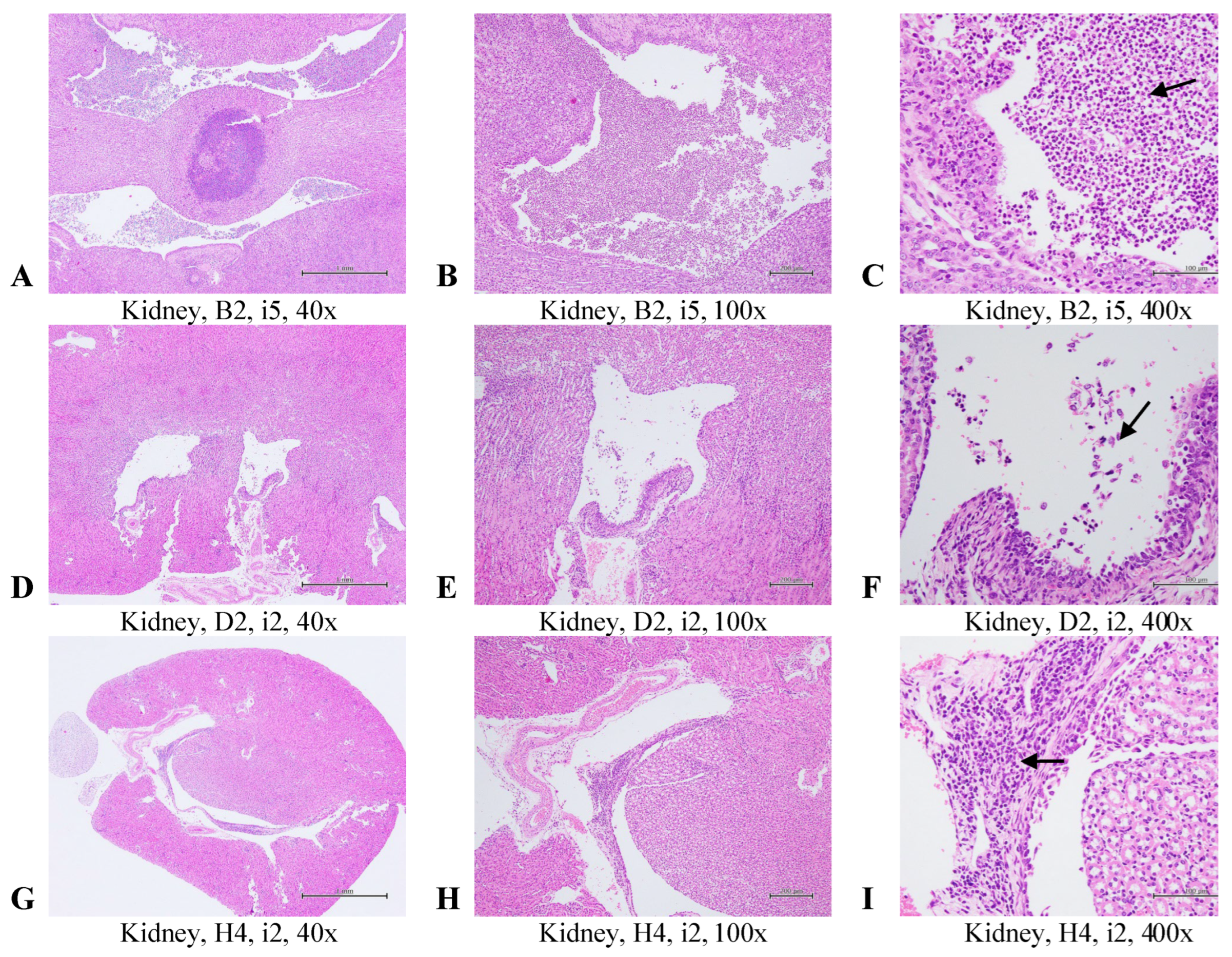
2.3. Histopathological Evaluation of the Selected Tissues in the Uropathogens-Induced Urinary Inflammation
3. Discussion
4. Materials and Methods
4.1. Uropathogens
4.2. Probiotic Strains
4.3. Preparation of Inactivated Probiotic Cells Through Heat Treatment
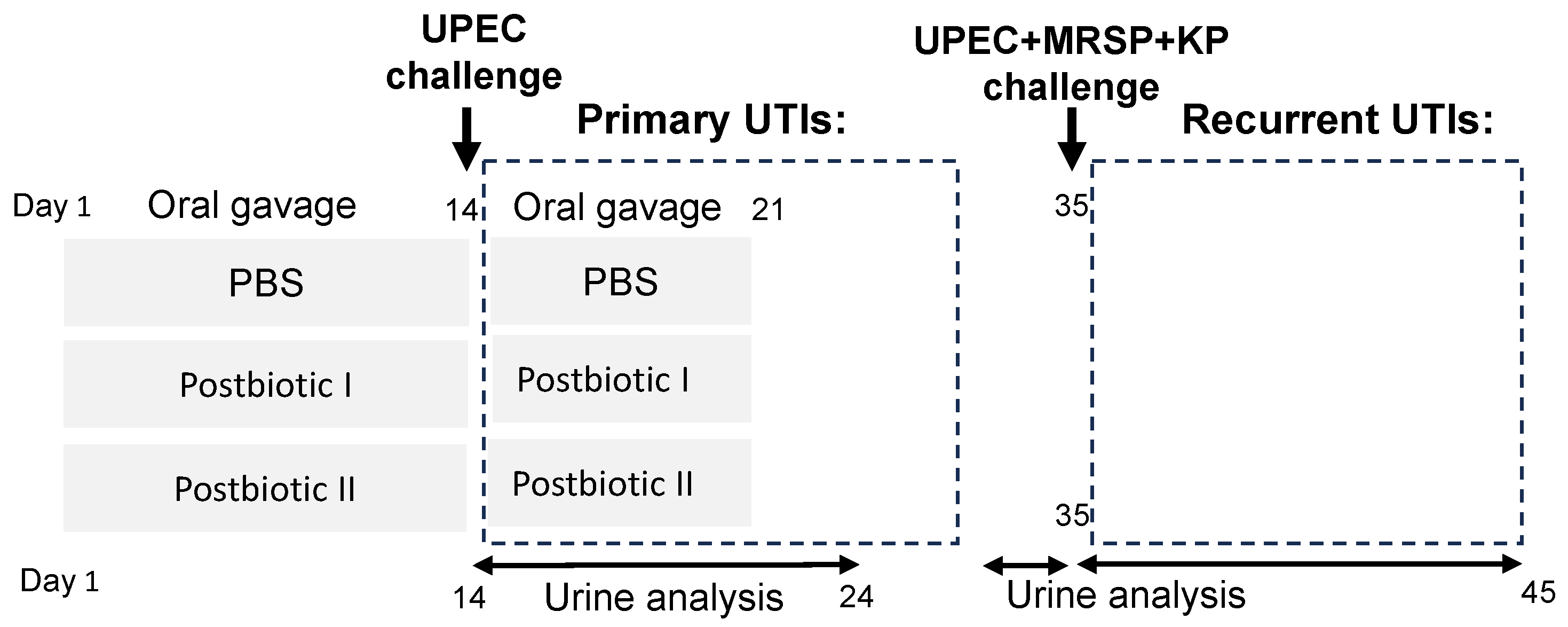
4.4. The Urinary Tract Infection Mouse Model
4.5. Histologic Analysis and Severity Scoring
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alam, M.S.; Anwar, M.J.; Akhtar, M.S.; Alam, P.; Mohammad, A.A.S.; Almutairy, A.F.; Nazmi, A.S.; Mukherjee, T.K. A systematic review of recent advances in urinary tract infection interventions and treatment technology. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 4238–4254. [Google Scholar] [CrossRef] [PubMed]
- Kalhori, R.P.; Faraji, A.; Yari, M.; Ganjabi, M.; Kazeminia, M. Global Prevalence of Urinary Tract Infections in the Older Persons: A Systematic Review and Meta-Analysis. Ageing Int. 2024, 49, 813–835. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Marra, M.; Zummo, S.; Biondo, C. Urinary Tract Infections: The Current Scenario and Future Prospects. Pathogens 2023, 12, 623. [Google Scholar] [CrossRef]
- Al Lawati, H.; Blair, B.M.; Larnard, J. Urinary Tract Infections: Core Curriculum 2024. Am. J. Kidney Dis. 2024, 83, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, Z.; Zheng, L.; Gong, Z.; Li, Y.; Jin, Y.; Huang, Y.; Chi, M. Urinary Tract Infections Caused by Uropathogenic Escherichia coli: Mechanisms of Infection and Treatment Options. Int. J. Mol. Sci. 2023, 24, 10537. [Google Scholar] [CrossRef]
- Kostakioti, M.; Hultgren, S.J.; Hadjifrangiskou, M. Molecular blueprint of uropathogenic Escherichia coli virulence provides clues toward the development of anti-virulence therapeutics. Virulence 2012, 3, 592–594. [Google Scholar] [CrossRef]
- Stapleton, P.J.; Lundon, D.J.; McWade, R.; Scanlon, N.; Hannan, M.M.; O’Kelly, F.; Lynch, M. Antibiotic resistance patterns of Escherichia coli urinary isolates and comparison with antibiotic consumption data over 10 years, 2005–2014. Ir. J. Med. Sci. 2017, 186, 733–741. [Google Scholar] [CrossRef]
- Albert, X.; Huertas, I.; Pereiro, I.; Sanfélix, J.; Gosalbes, V.; Perrotta, C. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst. Rev. 2004, 2004, CD001209. [Google Scholar] [CrossRef]
- Williams, G.; Craig, J.C. Long-term antibiotics for preventing recurrent urinary tract infection in children. Cochrane Database Syst. Rev. 2019, 2019, CD001534. [Google Scholar] [CrossRef]
- Nasrollahian, S.; Moradi, F.; Hadi, N.; Ranjbar, S.; Ranjbar, R. An update on alternative therapy for Escherichia coli causing urinary tract infections; a narrative review. Photodiagnosis Photodyn. Ther. 2024, 46, 104075. [Google Scholar] [CrossRef]
- Finney, E.L.; Pagura, E.J.; MacLachlan, L.S. Efficacy and safety of alternative treatments for the prevention of recurrent urinary tract infections. Curr. Bladder Dysfunct. Rep. 2023, 18, 42–50. [Google Scholar] [CrossRef]
- Hayward, G.; Mort, S.; Hay, A.D.; Moore, M.; Thomas, N.P.B.; Cook, J.; Robinson, J.; Williams, N.; Maeder, N.; Edeson, R.; et al. d-Mannose for Prevention of Recurrent Urinary Tract Infection Among Women: A Randomized Clinical Trial. JAMA Intern. Med. 2024, 184, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Kazi, S.; Rehman, S.U.; Khan, S.J.; Kazi, S.A.; Zainab, S.; Bano, S. The role of probiotic in preventing recurrent urinary tract infections in pregnant women. Biomedica 2024, 40, 130–134. [Google Scholar] [CrossRef]
- Schwenger, E.M.; Tejani, A.M.; Loewen, P.S. Probiotics for preventing urinary tract infections in adults and children. Cochrane Database Syst. Rev. 2015, 2015, CD008772. [Google Scholar] [CrossRef]
- Forster, C.S.; Hsieh, M.H.; Cabana, M.D. Perspectives from the Society for Pediatric Research: Probiotic use in urinary tract infections, atopic dermatitis, and antibiotic-associated diarrhea: An overview. Pediatr. Res. 2021, 90, 315–327. [Google Scholar] [CrossRef]
- Beyitler, I.; Kavukcu, S. Probiotics for prophylaxis and treatment of urinary tract infections in children. Iran. J. Pediatr. 2017, 27, e7695. [Google Scholar] [CrossRef]
- ter Riet, G.; Nys, S.; van der Wal, W.M.; de Borgie, C.A.; de Reijke, T.M.; Prins, J.M.; Koeijers, J.; Verbon, A.; Stobberingh, E.; Geerlings, S.E. Lactobacilli vs antibiotics to prevent urinary tract infections: A randomized, double-blind, noninferiority trial in postmenopausal women. Arch. Intern. Med. 2012, 172, 704–712. [Google Scholar]
- Qasemi, A.; Lagzian, M.; Rahimi, F.; Majd, F.K.; Bayat, Z. The power of probiotics to combat urinary tract infections: A comprehensive review. Res. Biotechnol. Environ. Sci. 2023, 2, 1–11. [Google Scholar] [CrossRef]
- Thorakkattu, P.; Khanashyam, A.C.; Shah, K.; Babu, K.S.; Mundanat, A.S.; Deliephan, A.; Deokar, G.S.; Santivarangkna, C.; Nirmal, N.P. Postbiotics: Current Trends in Food and Pharmaceutical Industry. Foods 2022, 11, 3094. [Google Scholar] [CrossRef]
- Van, V.T.H.; Liu, Z.-S.; Hsieh, Y.J.; Shiu, W.-C.; Chen, B.-Y.; Ku, Y.-W.; Chen, P.-W. Therapeutic effects of orally administration of viable and inactivated probiotic strains against murine urinary tract infection. J. Food Drug Anal. 2023, 31, 2. [Google Scholar] [CrossRef]
- Stamm, W.E.; Counts, G.W.; Running, K.R.; Fihn, S.; Turck, M.; Holmes, K.K. Diagnosis of coliform infection in acutely dysuric women. N. Engl. J. Med. 1982, 307, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Kass, E.H. Asymptomatic infections of the urinary tract. J. Urol. 2002, 167, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Smelt, M.J.; de Haan, B.J.; Bron, P.A.; van Swam, I.; Meijerink, M.; Wells, J.M.; Kleerebezem, M.; Faas, M.M.; de Vos, P. The impact of Lactobacillus plantarum WCFS1 teichoic acid D-alanylation on the generation of effector and regulatory T-cells in healthy mice. PLoS ONE 2013, 8, e63099. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.W.; Kim, J.K.; Han, M.J.; Kim, D.H. Lacticaseibacillus paracasei NK112 mitigates Escherichia coli-induced depression and cognitive impairment in mice by regulating IL-6 expression and gut microbiota. Benef. Microbes 2021, 12, 541–551. [Google Scholar] [CrossRef]
- Armbruster, C.E.; Smith, S.N.; Mody, L.; Mobley, H.L.T. Urine Cytokine and Chemokine Levels Predict Urinary Tract Infection Severity Independent of Uropathogen, Urine Bacterial Burden, Host Genetics, and Host Age. Infect. Immun. 2018, 86, e00327-18. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Liu, Z.-S.; Chen, B.-Y.; Tam, H.-M.-H.; Shia, W.-Y.; Yu, H.-H.; Chen, P.-W. Effects of Heat-Killed Probiotic Strains on Biofilm Formation, Transcription of Virulence-Associated Genes, and Prevention of UTIs in Mice. Probiotics Antimicrob. Proteins 2024, 1–16. [Google Scholar] [CrossRef]
- Hishiki, H.; Kawashima, T.; Tsuji, N.M.; Ikari, N.; Takemura, R.; Kido, H.; Shimojo, N. A double-blind, randomized, placebo-controlled trial of heat-killed Pediococcus acidilactici K15 for prevention of respiratory tract infections among preschool children. Nutrients 2020, 12, 1989. [Google Scholar] [CrossRef]
- Takeshita, K.; Hishiki, H.; Takei, H.; Ikari, N.; Tanaka, S.; Iijima, Y.; Ogata, H.; Fujishiro, K.; Tominaga, T.; Konno, Y.; et al. The Effectiveness of Heat-Killed Pediococcus acidilactici K15 in Preventing Respiratory Tract Infections in Preterm Infants: A Pilot Double-Blind, Randomized, Placebo-Controlled Study. Nutrients 2024, 16, 3635. [Google Scholar] [CrossRef]
- Pique, N.; Berlanga, M.; Minana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef]
- Sanders, M.E.; Akkermans, L.M.; Haller, D.; Hammerman, C.; Heimbach, J.; Hormannsperger, G.; Huys, G.; Levy, D.D.; Lutgendorff, F.; Mack, D.; et al. Safety assessment of probiotics for human use. Gut Microbes 2010, 1, 164–185. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Khawcharoenporn, T.; Pruetpongpun, N.; Tiamsak, P.; Rutchanawech, S.; Mundy, L.M.; Apisarnthanarak, A. Colistin-based treatment for extensively drug-resistant Acinetobacter baumannii pneumonia. Int. J. Antimicrob. Agents 2014, 43, 378–382. [Google Scholar] [CrossRef]
- Hopkins, W.J.; Gendron-Fitzpatrick, A.; Balish, E.; Uehling, D.T. Time course and host responses to Escherichia coli urinary tract infection in genetically distinct mouse strains. Infect. Immun. 1998, 66, 2798–2802. [Google Scholar] [CrossRef] [PubMed]
- Taur, Y.; Smith, M.A. Adherence to the Infectious Diseases Society of America guidelines in the treatment of uncomplicated urinary tract infection. Clin. Infect. Dis. 2007, 44, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Smani, Y.; Fabrega, A.; Roca, I.; Sanchez-Encinales, V.; Vila, J.; Pachon, J. Role of OmpA in the multidrug resistance phenotype of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2014, 58, 1806–1808. [Google Scholar] [CrossRef]
- Karam, M.R.A.; Habibi, M.; Bouzari, S. Urinary tract infection: Pathogenicity, antibiotic resistance and development of effective vaccines against Uropathogenic Escherichia coli. Mol. Immunol. 2019, 108, 56–67. [Google Scholar] [CrossRef]
- Asahara, T.; Nomoto, K.; Watanuki, M.; Yokokura, T. Antimicrobial activity of intraurethrally administered probiotic Lactobacillus casei in a murine model of Escherichia coli urinary tract infection. Antimicrob. Agents Chemother. 2001, 45, 1751–1760. [Google Scholar] [CrossRef]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef]



| Placebo 1 | Postbiotic I 1 | Postbiotic II 1 | p-Value | |
|---|---|---|---|---|
| Bladder -Inflammation, multifocal | 2.3 ± 1.9 a | 0 ± 02.1 b | 3.7 ± 1.9 a | 0.003 |
| Bladder -Hyperplasia, mucosa, diffuse | 2.3 ± 2.1 a | 0 ± 0 b | 2.3 ± 1.2 a | 0.014 |
| Kidney -Inflammation, pelvis, multifocal | 2.5 ± 2.1 a | 0 ± 0 b | 0.3 ± 0.8 b | 0.008 |
| Ileum | 0.3 ± 0.5 a | 0 ± 0 a | 0 ± 0 a | 0.116 |
| Liver -Inflammation, multifocal | 0.3 ± 0.8 a | 0 ± 0 a | 0 ± 0 a | 0.391 |
| Spleen -Hyperplasia, lymphocyte, multifocal | 0.7 ± 1.6 a | 0 ± 0 a | 0 ± 0 a | 0.391 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.-Y.; Liu, Z.-S.; Lin, Y.-S.; Lin, H.C.; Chen, P.-W. Oral Administration of Heat-Killed Multi-Strain Probiotics Confers Durable Protection Against Antibiotic-Resistant Primary and Recurrent Urinary Tract Infections in a Murine Model. Antibiotics 2025, 14, 634. https://doi.org/10.3390/antibiotics14070634
Chen B-Y, Liu Z-S, Lin Y-S, Lin HC, Chen P-W. Oral Administration of Heat-Killed Multi-Strain Probiotics Confers Durable Protection Against Antibiotic-Resistant Primary and Recurrent Urinary Tract Infections in a Murine Model. Antibiotics. 2025; 14(7):634. https://doi.org/10.3390/antibiotics14070634
Chicago/Turabian StyleChen, Bo-Yuan, Zhen-Shu Liu, Yu-Syuan Lin, Hsiao Chin Lin, and Po-Wen Chen. 2025. "Oral Administration of Heat-Killed Multi-Strain Probiotics Confers Durable Protection Against Antibiotic-Resistant Primary and Recurrent Urinary Tract Infections in a Murine Model" Antibiotics 14, no. 7: 634. https://doi.org/10.3390/antibiotics14070634
APA StyleChen, B.-Y., Liu, Z.-S., Lin, Y.-S., Lin, H. C., & Chen, P.-W. (2025). Oral Administration of Heat-Killed Multi-Strain Probiotics Confers Durable Protection Against Antibiotic-Resistant Primary and Recurrent Urinary Tract Infections in a Murine Model. Antibiotics, 14(7), 634. https://doi.org/10.3390/antibiotics14070634








