Sentinel-Site-Based Surveillance of Mycobacterium tuberculosis Drug Resistance and Epidemiology in Sichuan, China
Abstract
1. Introduction
2. Results
2.1. Demographic and Clinical Characteristics
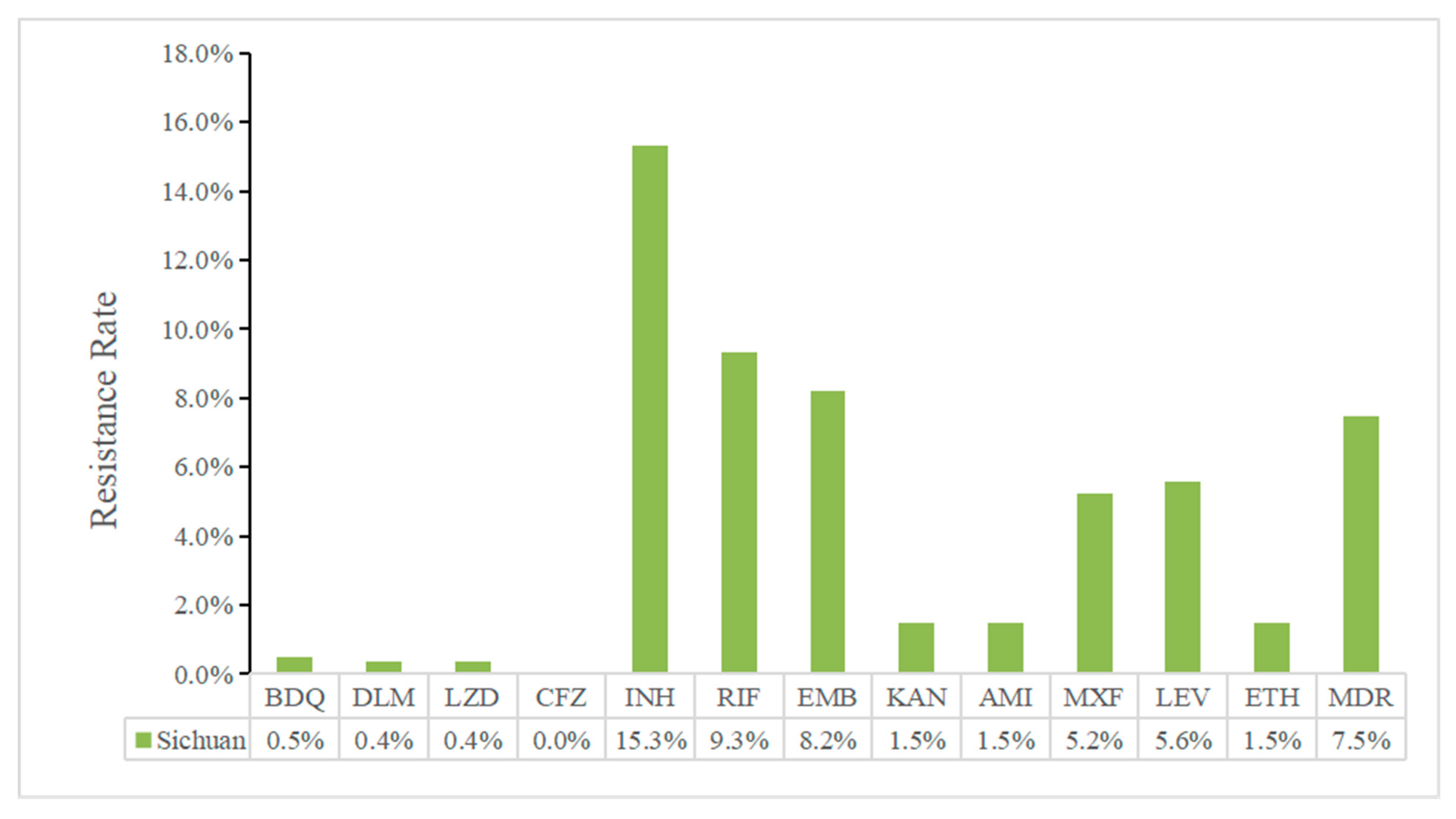
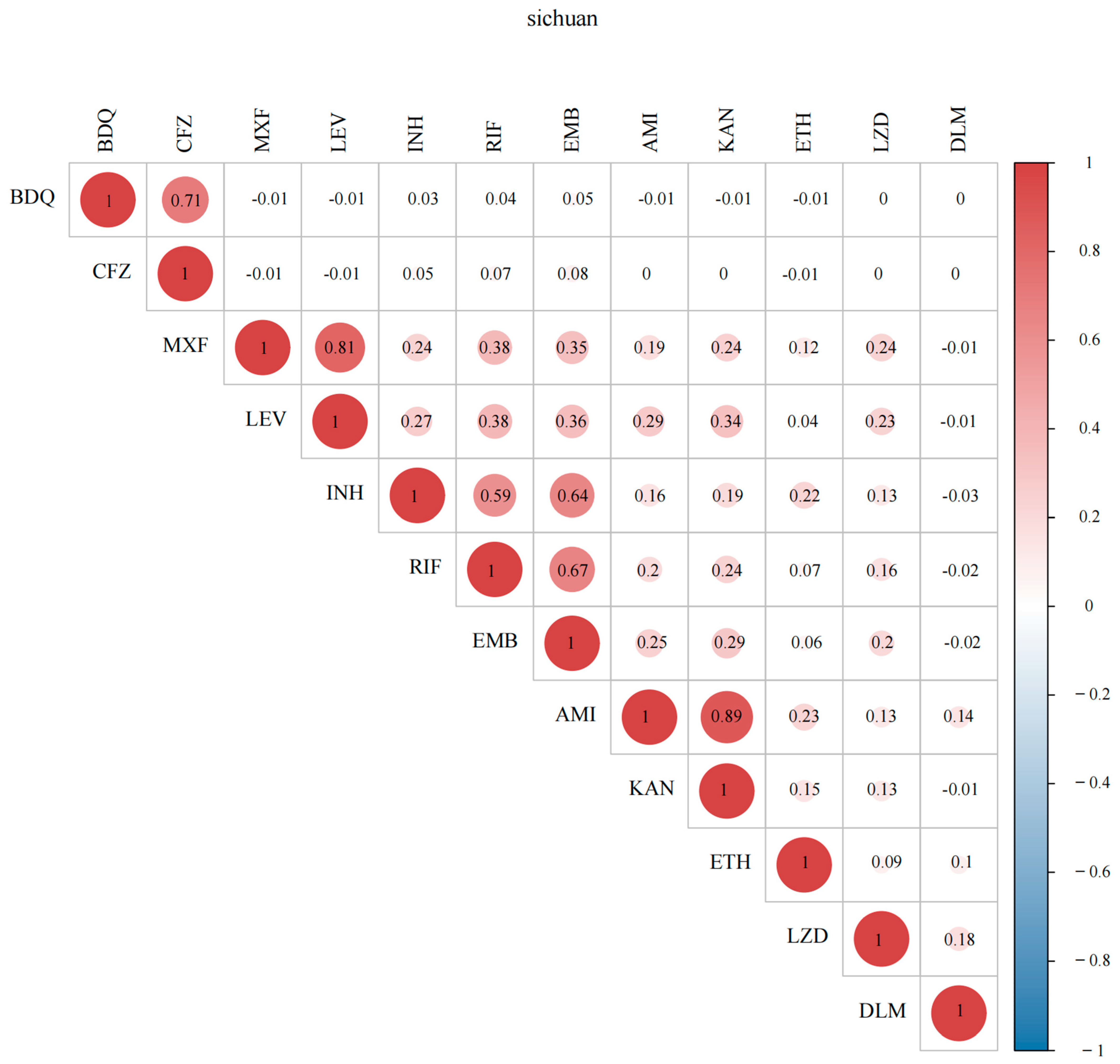
2.2. Drug-Resistance Characteristics
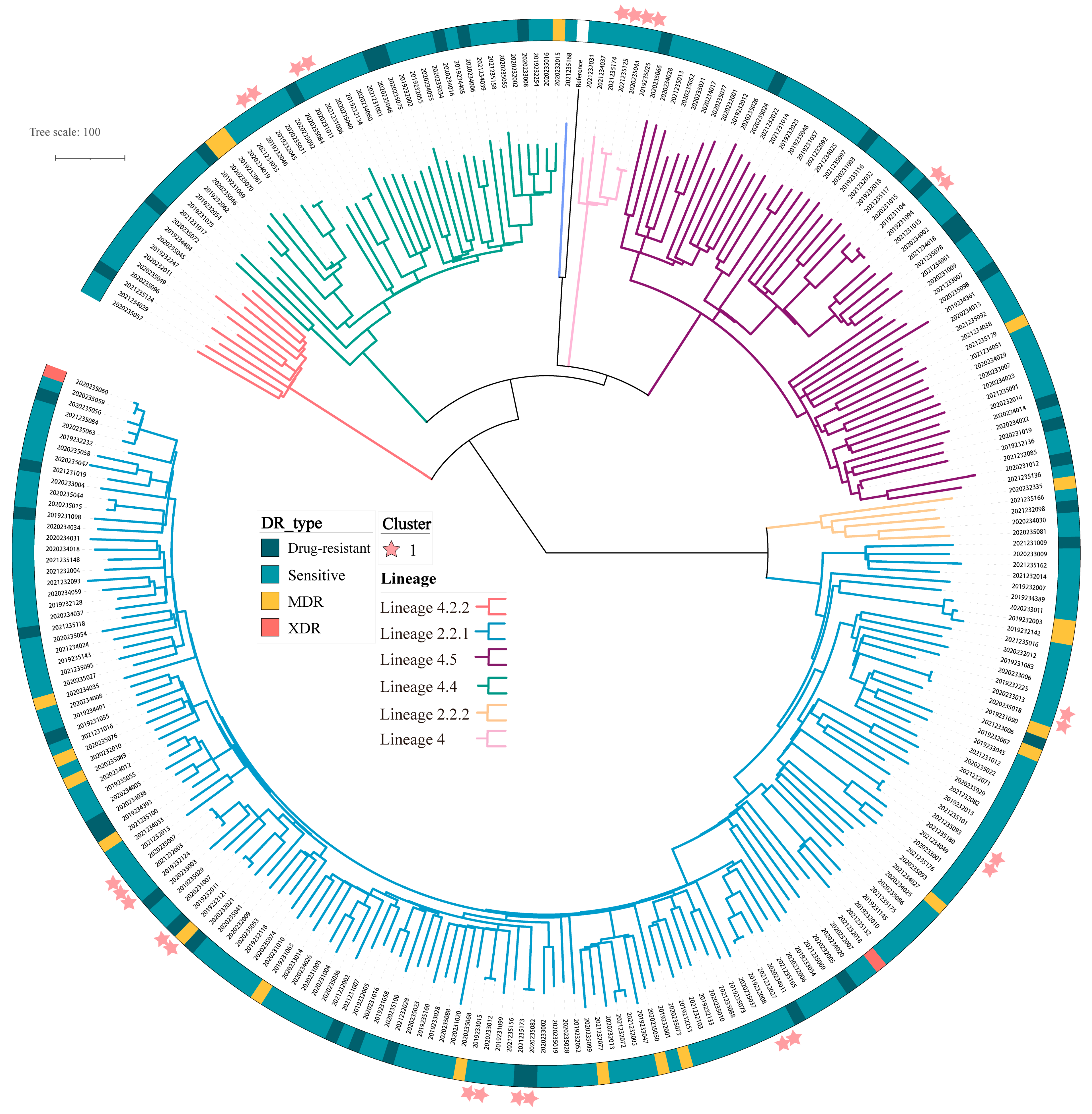
2.3. Genetic Determinants of Resistance
2.4. Transmission of MTB and Associated Risk Factors
3. Discussion
4. Conclusions
5. Methods
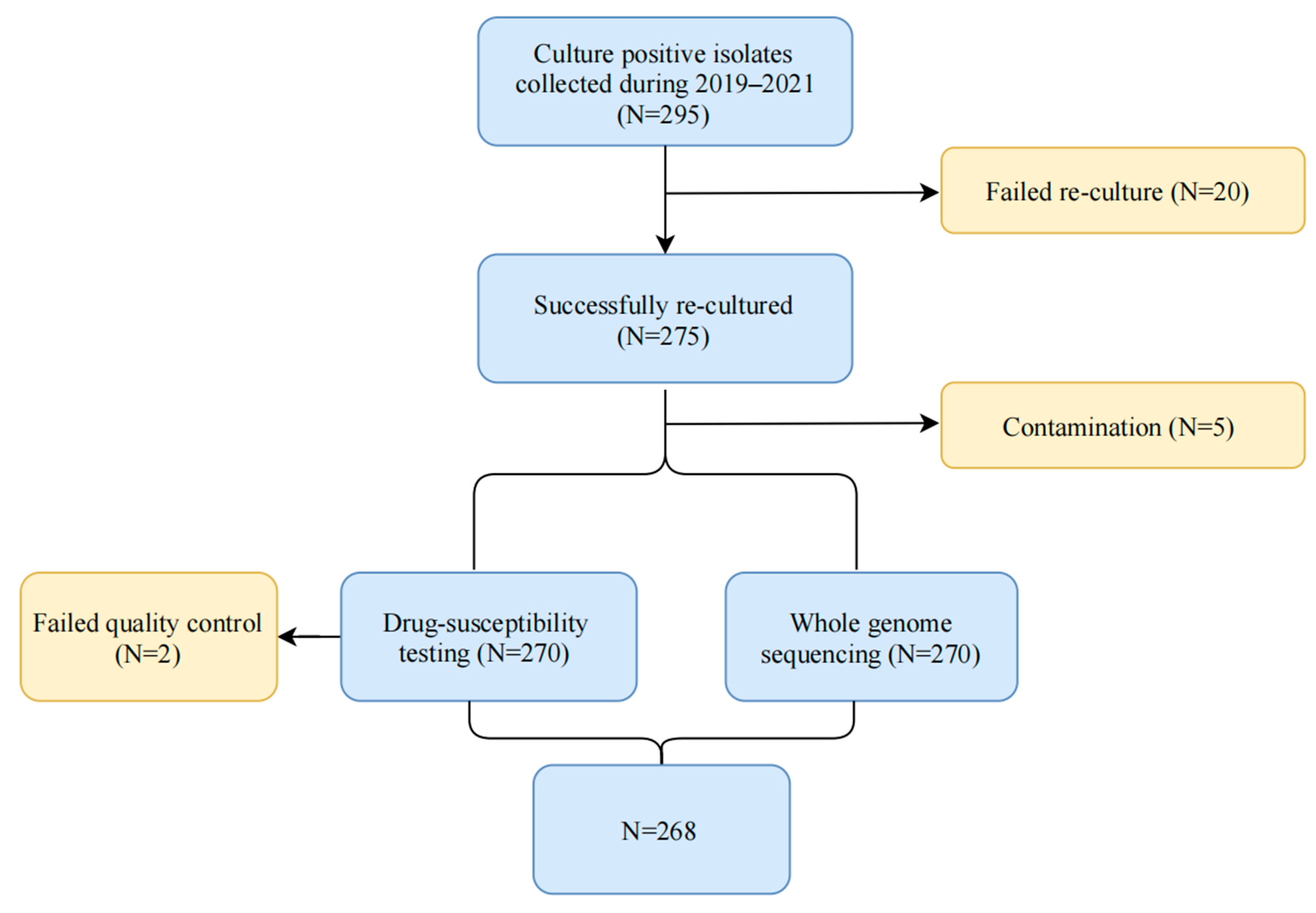
5.1. Isolate Collection
5.2. Drug Susceptibility Testing
5.3. Genomic DNA Preparation
5.4. Whole-Genome Sequencing Analysis
5.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gygli, S.M.; Borrell, S.; Trauner, A.; Gagneux, S. Antimicrobial Resistance in Mycobacterium tuberculosis: Mechanistic and Evolutionary Perspectives. FEMS Microbiol. Rev. 2017, 41, 354–373. [Google Scholar] [CrossRef] [PubMed]
- Alame Emane, A.K.; Guo, X.; Takiff, H.E.; Liu, S. Highly Transmitted M. Tuberculosis Strains Are More Likely to Evolve MDR/XDR and Cause Outbreaks, but What Makes Them Highly Transmitted? Tuberculosis 2021, 129, 102092. [Google Scholar] [CrossRef] [PubMed]
- Bagcchi, S. WHO’s Global Tuberculosis Report 2022. Lancet Microbe 2023, 4, e20. [Google Scholar] [CrossRef]
- Kendall, E.A.; Fofana, M.O.; Dowdy, D.W. Burden of Transmitted Multidrug Resistance in Epidemics of Tuberculosis: A Transmission Modelling Analysis. Lancet Respir. Med. 2015, 3, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Shen, X.; Peng, Y.; Lan, R.; Zhao, Y.; Long, B.; Luo, T.; Sun, G.; Li, X.; Qiao, K.; et al. Transmission of Mycobacterium tuberculosis in China: A Population-Based Molecular Epidemiologic Study. Clin. Infect. Dis. 2015, 61, 219–227. [Google Scholar] [CrossRef]
- Loiseau, C.; Windels, E.M.; Gygli, S.M.; Jugheli, L.; Maghradze, N.; Brites, D.; Ross, A.; Goig, G.; Reinhard, M.; Borrell, S.; et al. The Relative Transmission Fitness of Multidrug-Resistant Mycobacterium tuberculosis in a Drug Resistance Hotspot. Nat. Commun. 2023, 14, 1988. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, S.; Wang, L.; Chin, D.P.; Wang, S.; Jiang, G.; Xia, H.; Zhou, Y.; Li, Q.; Ou, X.; et al. National Survey of Drug-Resistant Tuberculosis in China. N. Engl. J. Med. 2012, 366, 2161–2170. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, X.; Yin, J.; Walley, J.; Beanland, R.; Zou, G.; Zhang, H.; Li, F.; Liu, Z.; Zee, B.C.Y.; et al. Changes in Pulmonary Tuberculosis Prevalence: Evidence from the 2010 Population Survey in a Populous Province of China. BMC Infect. Dis. 2014, 14, 21. [Google Scholar] [CrossRef]
- Chen, X.; Emam, M.; Zhang, L.; Rifhat, R.; Zhang, L.; Zheng, Y. Analysis of Spatial Characteristics and Geographic Weighted Regression of Tuberculosis Prevalence in Kashgar, China. Prev. Med. Rep. 2023, 35, 102362. [Google Scholar] [CrossRef]
- Mijiti, P.; Yuehua, L.; Feng, X.; Milligan, P.J.; Merle, C.; Gang, W.; Nianqiang, L.; Upur, H. Prevalence of Pulmonary Tuberculosis in Western China in 2010-11: A Population-Based, Cross-Sectional Survey. Lancet Glob. Health 2016, 4, e485–e494. [Google Scholar] [CrossRef]
- Wang, X.; Yin, S.; Li, Y.; Wang, W.; Du, M.; Guo, W.; Xue, M.; Wu, J.; Liang, D.; Wang, R.; et al. Spatiotemporal Epidemiology of, and Factors Associated with, the Tuberculosis Prevalence in Northern China, 2010–2014. BMC Infect. Dis. 2019, 19, 365. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Yang, J.; Wang, W.; Zhang, S.; Zhang, L.; Chen, C.; Rao, Z.; Wang, D.; Long, B. Analysis of the Drug Resistance Situation of Tuberculosis at 10 Drug Resistance Surveillance Sites in Sichuan Province from 2016 to 2017. J. Prev. Med. Inf. 2019, 35, 681–686. [Google Scholar]
- Zhou, R.; Zheng, T.; Luo, D.; Zhu, M.; Li, Q.; Xu, Y.; Wang, D.; Luo, J.; Zeng, C.; Wei, G.; et al. Drug Resistance Characteristics of Mycobacterium tuberculosis Isolates Obtained between 2018 and 2020 in Sichuan, China. Epidemiol. Infect. 2022, 150, e27. [Google Scholar] [CrossRef]
- Wang, D.-M.; Li, Q.-F.; Zhu, M.; Wu, G.-H.; Li, X.; Xu, Y.-H.; Zhong, J.; Luo, J.; Li, Y.-J.; Ying, B.-W.; et al. Epidemiological, Clinical Characteristics and Drug Resistance Situation of Culture-Confirmed Children TBM in Southwest of China: A 6-Year Retrospective Study. BMC Infect. Dis. 2020, 20, 318. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Q.; Zhu, M.; Wu, X.; Wang, D.; Luo, J.; Li, Y.; Zhong, J.; Zeng, P. The Epidemiological Characteristics and Profile of Drug-Resistant Tuberculosis among Children with Tuberculosis in Sichuan, China, 2015–2018: A Retrospective Study. Medicine 2020, 99, e22608. [Google Scholar] [CrossRef]
- Pfyffer, G.E.; Wittwer, F. Incubation Time of Mycobacterial Cultures: How Long Is Long Enough to Issue a Final Negative Report to the Clinician? J. Clin. Microbiol. 2012, 50, 4188–4189. [Google Scholar] [CrossRef]
- Bwanga, F.; Hoffner, S.; Haile, M.; Joloba, M.L. Direct Susceptibility Testing for Multi Drug Resistant Tuberculosis: A Meta-Analysis. BMC Infect. Dis. 2009, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Weinrick, B. Genotyping of Mycobacterium tuberculosis Rifampin Resistance-Associated Mutations by Use of Data from Xpert MTB/RIF Ultra Enables Large-Scale Tuberculosis Molecular Epidemiology Studies. J. Clin. Microbiol. 2019, 58, e01504-19. [Google Scholar] [CrossRef]
- Dorman, S.E.; Chihota, V.N.; Lewis, J.J.; van der Meulen, M.; Mathema, B.; Beylis, N.; Fielding, K.L.; Grant, A.D.; Churchyard, G.J. Genotype MTBDRplus for Direct Detection of Mycobacterium tuberculosis and Drug Resistance in Strains from Gold Miners in South Africa. J. Clin. Microbiol. 2012, 50, 1189–1194. [Google Scholar] [CrossRef]
- WHO Consolidated Guidelines on Tuberculosis: Module 3: Diagnosis—Tests for Tuberculosis Infection; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-005608-4.
- Meehan, C.J.; Goig, G.A.; Kohl, T.A.; Verboven, L.; Dippenaar, A.; Ezewudo, M.; Farhat, M.R.; Guthrie, J.L.; Laukens, K.; Miotto, P.; et al. Whole Genome Sequencing of Mycobacterium tuberculosis: Current Standards and Open Issues. Nat. Rev. Microbiol. 2019, 17, 533–545. [Google Scholar] [CrossRef]
- McNerney, R.; Zignol, M.; Clark, T.G. Use of Whole Genome Sequencing in Surveillance of Drug Resistant Tuberculosis. Expert Rev. Anti Infect. Ther. 2018, 16, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.; Acharya, A.; Gautam, S.; Ghimire, S.P.; Mishra, G.; Parajuli, N.; Sapkota, B. Advances in Diagnosis of Tuberculosis: An Update into Molecular Diagnosis of Mycobacterium tuberculosis. Mol. Biol. Rep. 2020, 47, 4065–4075. [Google Scholar] [CrossRef] [PubMed]
- Nikolayevskyy, V.; Niemann, S.; Anthony, R.; van Soolingen, D.; Tagliani, E.; Ködmön, C.; van der Werf, M.J.; Cirillo, D.M. Role and Value of Whole Genome Sequencing in Studying Tuberculosis Transmission. Clin. Microbiol. Infect. 2019, 25, 1377–1382. [Google Scholar] [CrossRef]
- The CRyPTIC Consortium. Epidemiological Cut-off Values for a 96-Well Broth Microdilution Plate for High-Throughput Research Antibiotic Susceptibility Testing of M. tuberculosis. Eur. Respir. J. 2022, 60, 2200239. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, A.; Wei, L.; Pang, Y.; Wu, B.; Luo, T.; Zhou, Y.; Zheng, H.-X.; Jiang, Q.; Gan, M.; et al. China’s Tuberculosis Epidemic Stems from Historical Expansion of Four Strains of Mycobacterium tuberculosis. Nat. Ecol. Evol. 2018, 2, 1982–1992. [Google Scholar] [CrossRef]
- Li, Y.-F.; Kong, X.-L.; Song, W.-M.; Li, Y.-M.; Li, Y.-Y.; Fang, W.-W.; Yang, J.-Y.; Yu, C.-B.; Li, H.-C.; Liu, Y. Genomic Analysis of Lineage-Specific Transmission of Multidrug Resistance Tuberculosis in China. Emerg. Microbes Infect. 2024, 13, 2294858. [Google Scholar] [CrossRef]
- Gao, W.; Wang, W.; Li, J.; Gao, Y.; Zhang, S.; Lei, H.; He, L.; Li, T.; He, J. Drug-Resistance Characteristics, Genetic Diversity, and Transmission Dynamics of Multidrug-Resistant or Rifampicin-Resistant Mycobacterium tuberculosis from 2019 to 2021 in Sichuan, China. Antimicrob. Resist. Infect. Control 2024, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Dye, C.; Espinal, M.A. Will Tuberculosis Become Resistant to All Antibiotics? Proc. Biol. Sci. 2001, 268, 45–52. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Wang, J.; Chen, S.; Wu, B.; Zhou, L.; Pan, A.; Wang, W.; Wang, X. Longitudinal Analysis of Prevalence and Risk Factors of Rifampicin-Resistant Tuberculosis in Zhejiang, China. Biomed. Res. Int. 2020, 2020, 3159482. [Google Scholar] [CrossRef]
- Gegia, M.; Winters, N.; Benedetti, A.; van Soolingen, D.; Menzies, D. Treatment of Isoniazid-Resistant Tuberculosis with First-Line Drugs: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2017, 17, 223–234. [Google Scholar] [CrossRef]
- Chen, H.; He, L.; Huang, H.; Shi, C.; Ni, X.; Dai, G.; Ma, L.; Li, W. Mycobacterium tuberculosis Lineage Distribution in Xinjiang and Gansu Provinces, China. Sci. Rep. 2017, 7, 1068. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, C.; Xu, Y.; Chen, Z.; Qiu, W.; Chen, S.; Pei, H.; Zhong, Y. Analysis of Drug-Resistance Characteristics and Genetic Diversity of Multidrug-Resistant Tuberculosis Based on Whole-Genome Sequencing on the Hainan Island, China. Infect. Drug Resist. 2023, 16, 5783–5798. [Google Scholar] [CrossRef]
- Shao, Y.; Li, Y.; Song, H.; Li, G.; Li, Y.; Zhu, L.; Lu, W.; Chen, C. A Retrospective Cohort Study of Isoniazid-Resistant Tuberculosis Treatment Outcomes and Isoniazid Resistance-Associated Mutations in Eastern China from 2013 to 2018. J. Glob. Antimicrob. Resist. 2020, 22, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Menzies, D.; Benedetti, A.; Paydar, A.; Royce, S.; Madhukar, P.; Burman, W.; Vernon, A.; Lienhardt, C. Standardized Treatment of Active Tuberculosis in Patients with Previous Treatment and/or with Mono-Resistance to Isoniazid: A Systematic Review and Meta-Analysis. PLoS Med. 2009, 6, e1000150. [Google Scholar] [CrossRef]
- CRyPTIC Consortium and the 100,000 Genomes Project; Allix-Béguec, C.; Arandjelovic, I.; Bi, L.; Beckert, P.; Bonnet, M.; Bradley, P.; Cabibbe, A.M.; Cancino-Muñoz, I.; Caulfield, M.J.; et al. Prediction of Susceptibility to First-Line Tuberculosis Drugs by DNA Sequencing. N. Engl. J. Med. 2018, 379, 1403–1415. [Google Scholar] [CrossRef]
- Liu, D.; Huang, F.; Zhang, G.; He, W.; Ou, X.; He, P.; Zhao, B.; Zhu, B.; Liu, F.; Li, Z.; et al. Whole-Genome Sequencing for Surveillance of Tuberculosis Drug Resistance and Determination of Resistance Level in China. Clin. Microbiol. Infect. 2022, 28, 731.e9–731.e15. [Google Scholar] [CrossRef]
- Musser, J.M. Antimicrobial Agent Resistance in Mycobacteria: Molecular Genetic Insights. Clin. Microbiol. Rev. 1995, 8, 496–514. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Shin, J.-I.; Yoo, I.Y.; Jo, S.; Chu, J.; Cho, W.Y.; Shin, S.-H.; Chung, Y.-J.; Park, Y.-J.; Jung, S.-H. GenoMycAnalyzer: A Web-Based Tool for Species and Drug Resistance Prediction for Mycobacterium Genomes. BMC Genom. 2024, 25, 387. [Google Scholar] [CrossRef]
- Wu, X.; Gao, R.; Shen, X.; Guo, Y.; Yang, J.; Wu, Z.; Tan, G.; Wang, H.; Yu, F. Use of Whole-Genome Sequencing to Predict Mycobacterium tuberculosis Drug Resistance in Shanghai, China. Int. J. Infect. Dis. 2020, 96, 48–53. [Google Scholar] [CrossRef]
- Liu, D.; Huang, F.; Li, Y.; Mao, L.; He, W.; Wu, S.; Xia, H.; He, P.; Zheng, H.; Zhou, Y.; et al. Transmission Characteristics in Tuberculosis by WGS: Nationwide Cross-Sectional Surveillance in China. Emerg. Microbes Infect. 2024, 13, 2348505. [Google Scholar] [CrossRef]
- World Health Organization. Meeting Report of the WHO Expert Conslutation on the Definition of Extensively Drug Resistant Tuberculosis, 27–29 October 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Li, M.; Zhang, Y.; Wu, Z.; Jiang, Y.; Sun, R.; Yang, J.; Li, J.; Lin, H.; Zhang, R.; Jiang, Q.; et al. Transmission of Fluoroquinolones Resistance among Multidrug-Resistant Tuberculosis in Shanghai, China: A Retrospective Population-Based Genomic Epidemiology Study. Emerg. Microbes Infect. 2024, 13, 2302837. [Google Scholar] [CrossRef] [PubMed]
- The CRyPTIC Consortium. Genome-Wide Association Studies of Global Mycobacterium tuberculosis Resistance to 13 Antimicrobials in 10,228 Genomes Identify New Resistance Mechanisms. PLoS Biol. 2022, 20, e3001755. [Google Scholar] [CrossRef]
- Yang, C.; Luo, T.; Shen, X.; Wu, J.; Gan, M.; Xu, P.; Wu, Z.; Lin, S.; Tian, J.; Liu, Q.; et al. Transmission of Multidrug-Resistant Mycobacterium tuberculosis in Shanghai, China: A Retrospective Observational Study Using Whole-Genome Sequencing and Epidemiological Investigation. Lancet Infect. Dis. 2017, 17, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Coll, F.; McNerney, R.; Preston, M.D.; Guerra-Assunção, J.A.; Warry, A.; Hill-Cawthorne, G.; Mallard, K.; Nair, M.; Miranda, A.; Alves, A.; et al. Rapid Determination of Anti-Tuberculosis Drug Resistance from Whole-Genome Sequences. Genome Med. 2015, 7, 51. [Google Scholar] [CrossRef]

| Category | Factors | Count (n = 268) | Percentage (%) |
|---|---|---|---|
| Sex | |||
| male | 187 | 69.8 | |
| female | 81 | 30.2 | |
| Residence | |||
| rural | 196 | 73.1 | |
| urban | 72 | 26.9 | |
| Age (year) | |||
| ≤24 | 44 | 16.4 | |
| 25–44 | 96 | 35.8 | |
| 45–64 | 87 | 32.5 | |
| ≥65 | 41 | 15.3 | |
| Occupation | |||
| farmer | 154 | 57.5 | |
| employee | 21 | 7.8 | |
| retired | 52 | 19.4 | |
| student | 9 | 3.4 | |
| other | 32 | 11.9 | |
| Ethnicity | |||
| Han | 249 | 92.9 | |
| others | 19 | 7.1 | |
| Education | |||
| uneducated | 37 | 13.8 | |
| primary | 92 | 34.3 | |
| junior | 80 | 29.9 | |
| senior | 44 | 16.4 | |
| college or above | 15 | 5.6 | |
| Diabetes | |||
| Yes | 19 | 7.1 | |
| No | 249 | 92.9 | |
| TB exposure | |||
| Yes | 28 | 10.4 | |
| No | 240 | 89.6 | |
| Previous treatment | |||
| Yes | 38 | 14.2 | |
| No | 230 | 85.8 |
| Drug | WGS Results | Phenotypic DRS | Sensitivity (%) | Specificity (%) | Consistency (%) | |
|---|---|---|---|---|---|---|
| R | S | |||||
| Isoniazid | R | 40 | 1 | 97.6 | 99.6 | 99.3 |
| S | 1 | 226 | ||||
| Rifampicin | R | 24 | 2 | 96 | 99.2 | 98.9 |
| S | 1 | 241 | ||||
| Ethambutol | R | 19 | 2 | 86.4 | 99.2 | 98.1 |
| S | 3 | 244 | ||||
| Kanamycin | R | 3 | 0 | 75 | 100 | 99.6 |
| S | 1 | 264 | ||||
| Amikacin | R | 3 | 0 | 75 | 100 | 99.6 |
| S | 1 | 264 | ||||
| Moxifloxacin | R | 12 | 9 | 85.7 | 96.5 | 95.9 |
| S | 2 | 245 | ||||
| Levofloxacin | R | 12 | 9 | 80 | 96.4 | 95.5 |
| S | 3 | 244 | ||||
| Ethionamide | R | 3 | 5 | 75 | 98.1 | 97.8 |
| S | 1 | 259 | ||||
| Category | Factors | Cluster (n = 25) | Non-Cluster (n = 243) | cOR (95% CI) | p Value | aOR (95% CI) | p Value |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| male | 16 (64%) | 171 (70.4%) | 1.336 (0.564–3.163) | 0.51 | 1.172 (0.436–3.147) | 0.753 | |
| female | 9 (36%) | 72 (29.6%) | Ref | Ref | |||
| Residence | |||||||
| rural | 17 (68%) | 179 (73.7%) | 1.316 (0.542–3.197) | 0.544 | 1.426 (0.465–4.377) | 0.535 | |
| urban | 8 (32%) | 64 (26.3%) | Ref | Ref | |||
| Age | |||||||
| ≤24 | 10 (40%) | 34 (14%) | 11.765 (1.432–96.637) | 0.022 | 11.697 (0.817–167.463) | 0.07 | |
| 25–44 | 8 (32%) | 88 (36.2%) | 3.636 (0.44–30.059) | 0.231 | 5.455 (0.518–57.429) | 0.158 | |
| 45–64 | 6 (24%) | 81 (33.3%) | 2.963 (0.345–25.452) | 0.322 | 3.646 (0.386–34.408) | 0.259 | |
| ≥65 | 1 (4%) | 40 (16.5%) | Ref | Ref | |||
| Occupation | |||||||
| farmer | 10 (40%) | 144 (59.3%) | 0.375 (0.119–1.184) | 0.094 | 0.828 (0.196–3.505) | 0.798 | |
| employee | 1 (4%) | 20 (8.2%) | 0.27 (0.029–2.495) | 0.248 | 0.286 (0.028–2.954) | 0.294 | |
| retired | 5 (20%) | 47 (19.3%) | 0.574 (0.152–2.165) | 0.413 | 0.887 (0.199–3.951) | 0.875 | |
| student | 4 (16%) | 5 (2.1%) | 4.32 (0.851–21.929) | 0.078 | 2.985 (0.461–19.341) | 0.251 | |
| other | 5 (20%) | 27 (11.1%) | Ref | Ref | |||
| Ethnicity | |||||||
| Han | 24 (96%) | 225 (92.6%) | Ref | Ref | |||
| others | 1 (4%) | 18 (7.4%) | 1.92 (0.245–15.022) | 0.534 | 0.873 (0.098–7.806) | 0.904 | |
| Education | |||||||
| uneducated | 1 (4%) | 36 (14.8%) | Ref | Ref | |||
| primary | 7 (28%) | 85 (35%) | 0.181 (0.015–2.162) | 0.177 | 0.435 (0.024–7.963) | 0.574 | |
| junior | 5 (20%) | 75 (30.9%) | 0.535 (0.1–2.862) | 0.465 | 1.156 (0.131–10.189) | 0.896 | |
| senior | 10 (40%) | 34 (14%) | 0.433(0.076–2.475) | 0.347 | 0.59 (0.077–4.533) | 0.612 | |
| college or above | 2 (8%) | 13 (5.3%) | 1.912 (0.368–9.927) | 0.441 | 1.42 (0.222–9.064) | 0.711 | |
| Diabetes | |||||||
| yes | 3 (12%) | 16 (6.6%) | 0.517 (0.14–1.913) | 0.323 | 0.321 (0.065–1.581) | 0.163 | |
| no | 22 (88%) | 227 (93.4%) | Ref | Ref | |||
| TB exposure | |||||||
| yes | 1 (4%) | 27 (11.1%) | 3 (0.39–23.072) | 0.291 | 4.668 (0.522–41.771) | 0.168 | |
| no | 24 (96%) | 216 (88.9%) | Ref | Ref | |||
| Previous treatment | |||||||
| yes | 1 (4%) | 37 (15.2%) | 4.311 (0.566–32.847) | 0.158 | 2.629 (0.323–21.376) | 0.366 | |
| no | 24 (96%) | 206 (84.8%) | Ref | Ref | |||
| RR-TB | |||||||
| yes | 1 (4%) | 24 (9.9%) | 2.63 (0.341–20.314) | 0.354 | 4.77 (0.46–49.481) | 0.191 | |
| no | 24 (96%) | 219 (90.1%) | Ref | Ref | |||
| Lineage | |||||||
| lineage 2 | 14 (56%) | 145 (59.7%) | Ref | Ref | |||
| lineage 4 | 11 (44%) | 98 (40.3%) | 0.86 (0.375–1.973) | 0.722 | 0.835 (0.331–2.107) | 0.702 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, C.; Zhao, B.; Ou, X.; Xia, H.; Song, Y.; Zheng, Y.; Zhou, Y.; Xing, R.; Zhao, Y.; et al. Sentinel-Site-Based Surveillance of Mycobacterium tuberculosis Drug Resistance and Epidemiology in Sichuan, China. Antibiotics 2025, 14, 625. https://doi.org/10.3390/antibiotics14070625
Wang Y, Liu C, Zhao B, Ou X, Xia H, Song Y, Zheng Y, Zhou Y, Xing R, Zhao Y, et al. Sentinel-Site-Based Surveillance of Mycobacterium tuberculosis Drug Resistance and Epidemiology in Sichuan, China. Antibiotics. 2025; 14(7):625. https://doi.org/10.3390/antibiotics14070625
Chicago/Turabian StyleWang, Yiting, Chunfa Liu, Bing Zhao, Xichao Ou, Hui Xia, Yuanyuan Song, Yang Zheng, Yang Zhou, Ruida Xing, Yanlin Zhao, and et al. 2025. "Sentinel-Site-Based Surveillance of Mycobacterium tuberculosis Drug Resistance and Epidemiology in Sichuan, China" Antibiotics 14, no. 7: 625. https://doi.org/10.3390/antibiotics14070625
APA StyleWang, Y., Liu, C., Zhao, B., Ou, X., Xia, H., Song, Y., Zheng, Y., Zhou, Y., Xing, R., Zhao, Y., & Zheng, H. (2025). Sentinel-Site-Based Surveillance of Mycobacterium tuberculosis Drug Resistance and Epidemiology in Sichuan, China. Antibiotics, 14(7), 625. https://doi.org/10.3390/antibiotics14070625







