Antimicrobial and Immunomodulatory Effects of Punicalagin and Meropenem in a Murine Model of Sublethal Sepsis
Abstract
1. Introduction
2. Results
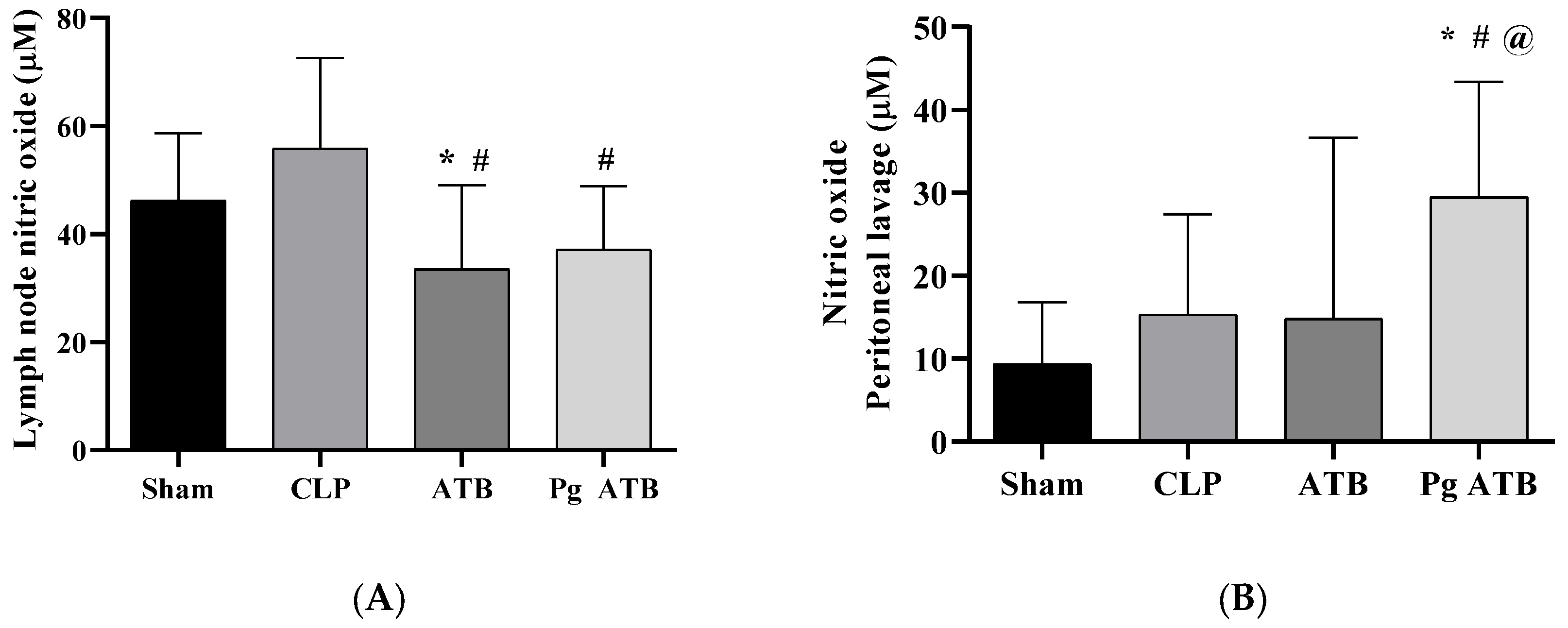
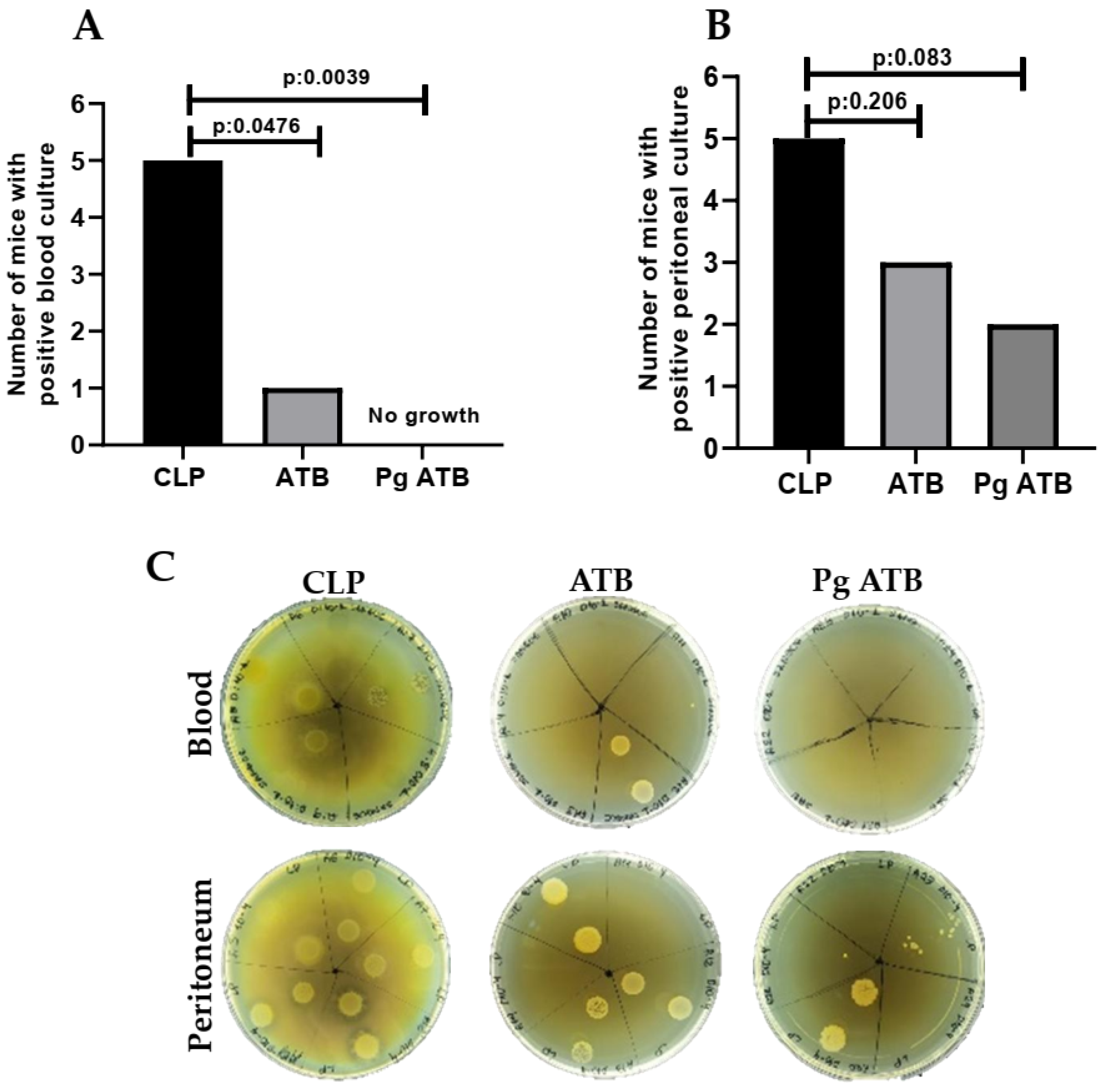
2.1. Effect of Associate Treatment (Pg ATB) on Nitric Oxide Levels and CFUs
2.2. Hematological Changes
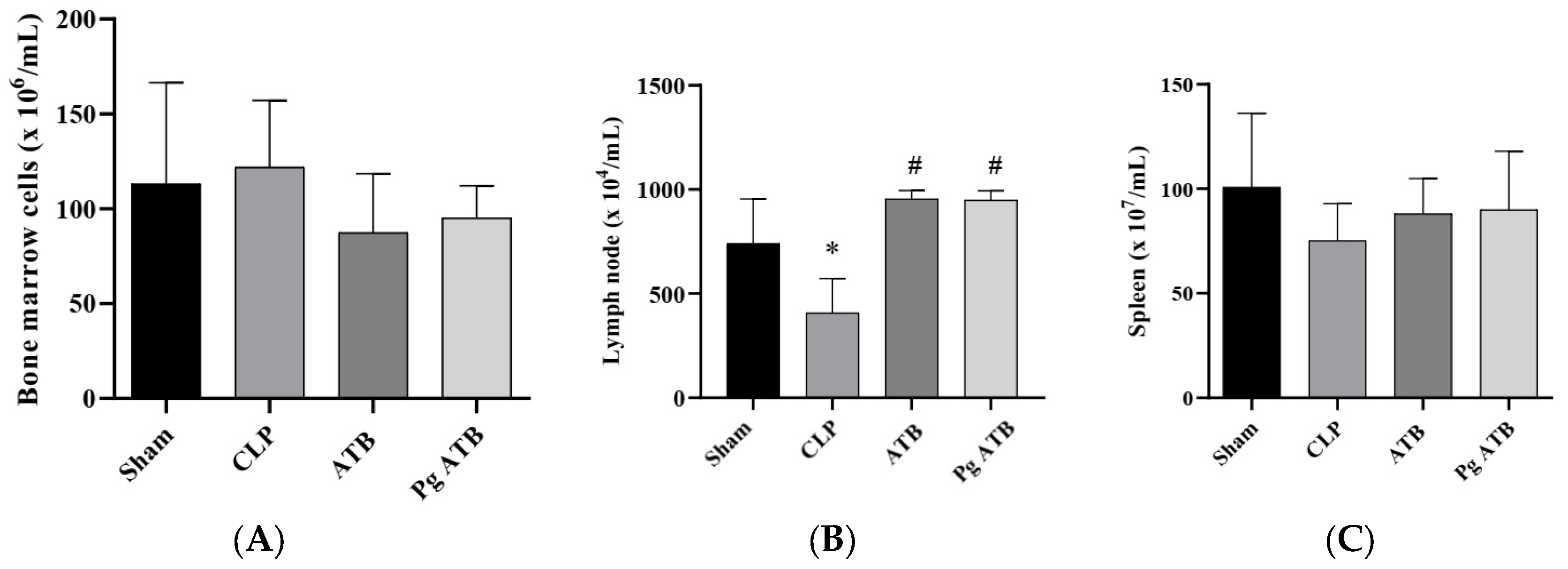
2.3. Effect of Pg ATB Treatment on Lymphoid Organs
2.4. Treatment with Pg ATB Influenced the Number of Leukocytes in the Peritoneum
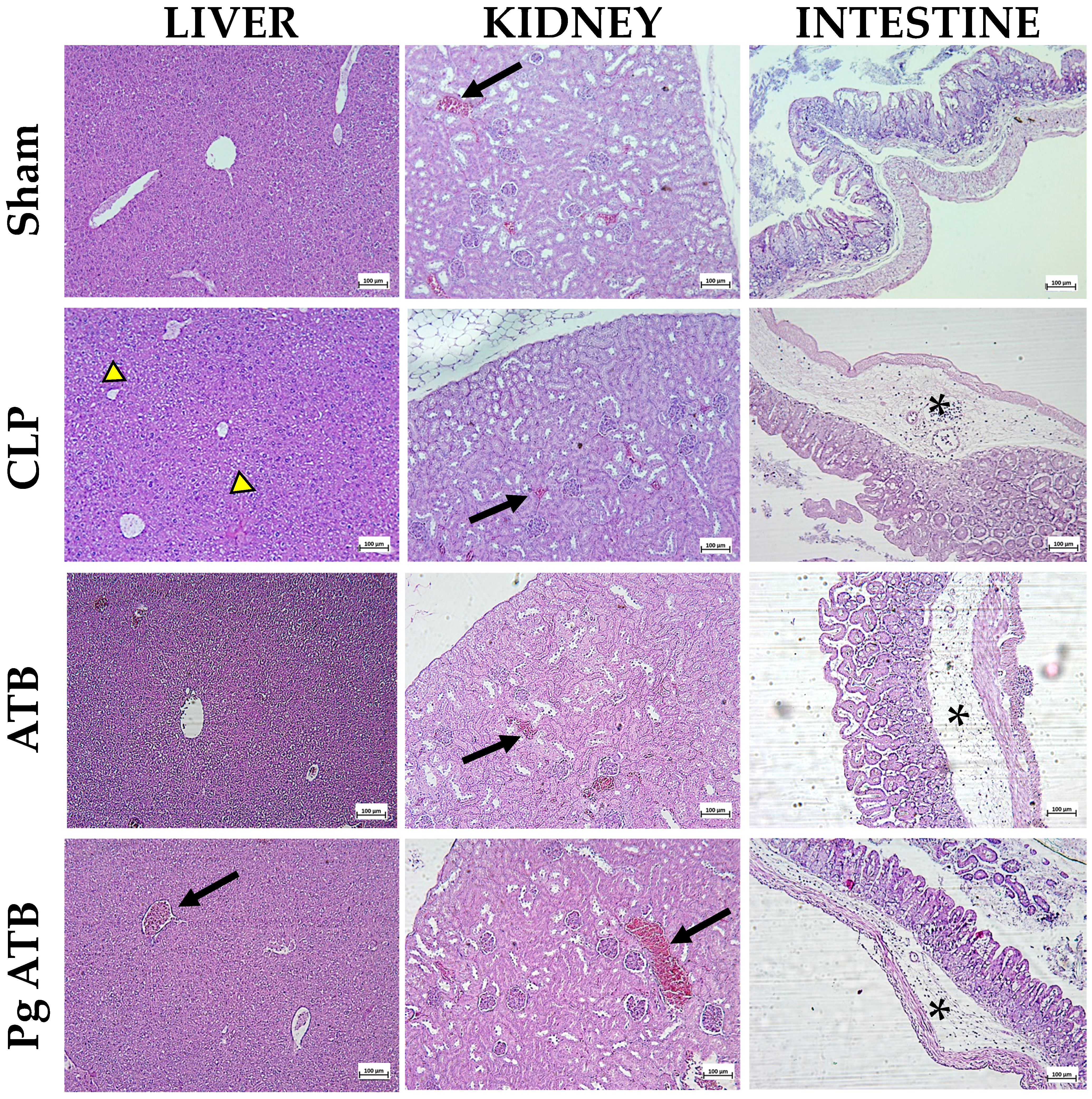
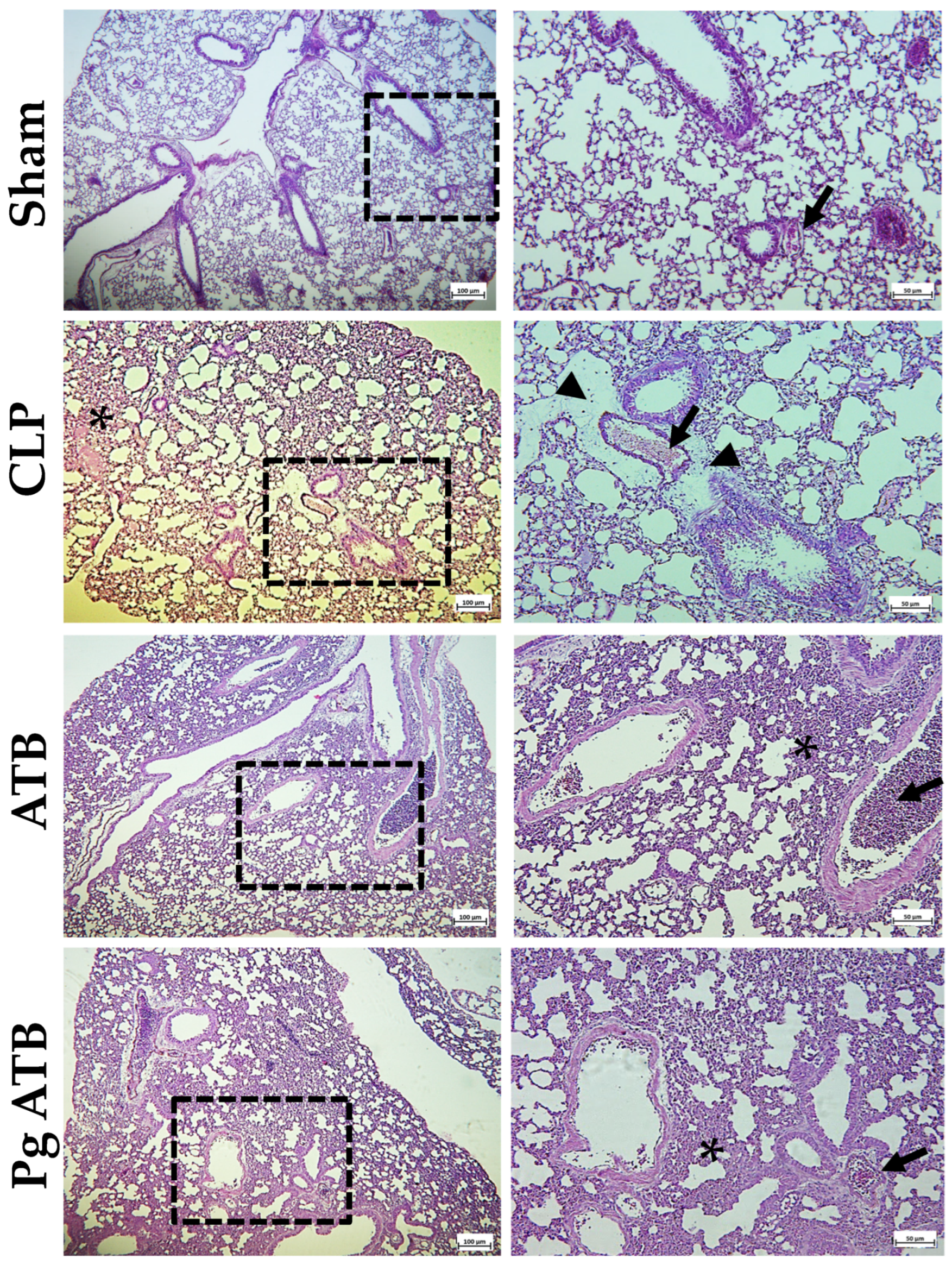
2.5. Effect of Pg ATB Treatment on Liver, Kidney, Intestine, and Lung
2.6. Cytokine Production
3. Discussion
4. Materials and Methods
4.1. Treatment
4.2. Animals
4.3. Experimental Design
4.4. Total and Differential Cell Count
4.5. Hematological Parameter Assessment
4.6. Histopathological Analyzes
4.7. Determination of Bacteria in Biological Samples
4.8. Spleen, Lymph Node, and Bone Marrow Cell Count
4.9. Determination of Serum Cytokines and Peritoneal Lavage
4.10. Determination of Nitric Oxide Production
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATB | Antibiotic |
| BHI | Brain Heart Infusion |
| CBA | Cytometric Bead Array |
| CFU | Colony-Forming Units |
| CLP | Cecal Ligation and Puncture |
| COX-2 | Cyclooxygenase-2 |
| FCS | Fetal Calf Serum |
| HCT | Hematocrit |
| HE | Hematoxylin and Eosin |
| HSV-1 | Herpes Simplex Virus 1 |
| ICU | Intensive Care Unit |
| IFN-γ | Interferon-Gamma |
| iNOS | Inducible Nitric Oxide Synthetase |
| IL | Interleukin |
| LPS | Lipopolysaccharid |
| MCV | Mean Corpuscular Volume |
| MPV | Mean Platelet Volume |
| MRSA | Oxacillin in Methicillin-Resistant Staphylococcus aureus |
| NF-κB | Nuclear Factor Kappa B |
| NK | Natural Killer |
| NO | Nitric Oxide |
| PBS | Phosphate-Buffered Saline |
| PCT | Plateletcrit |
| PDW | Platelet Distribution Width |
| P-LCR | Platelet–Large Cell Ratio |
| PLT | Platelet |
| Pg | Punicalagin |
| RBC | Red Blood Cell Count |
| RDW-CV | Red Cell Distribution Width Measured as Coefficient of Variation |
| RDW-SD | Red Cell Distribution Width Measured as Standard Deviation |
| ROS | Reactive Oxygen Species |
| RSV | Respiratory Syncytial Virus |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SBCAL | Brazilian Society for Laboratory Animal Science |
| SD | Standard Deviation |
| RPMI | Roswell Park Memorial Institute |
| TNF-α | Tumor Necrosis Factor |
References
- Huang, M.; Wu, K.; Zeng, S.; Liu, W.; Cui, T.; Chen, Z.; Lin, L.; Chen, D.; Ouyang, H. Punicalagin inhibited inflammation and migration of fibroblast-like synoviocytes through NF-κB pathway in the experimental study of rheumatoid arthritis. J. Inflamm. Res. 2021, 14, 1901–1913. [Google Scholar] [CrossRef] [PubMed]
- Pirzadeh, M.; Caporaso, N.; Rauf, A.; Shariati, M.A.; Yessimbekov, Z.; Khan, M.U.; Imran, M.; Mubarak, M.S. Pomegranate as a source of bioactive constituents: A review on their characterization, properties and applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 982–999. [Google Scholar] [CrossRef] [PubMed]
- Venusova, E.; Kolesarova, A.; Horky, P.; Slama, P. Physiological and immune functions of punicalagin. Nutrients 2021, 13, 2150. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Duan, Y.; Ma, F.; Lou, L. Punicalagin inhibits the viability, migration, invasion, and EMT by regulating GOLPH3 in breast cancer cells. J. Recept. Signal Transduct. 2020, 40, 173–180. [Google Scholar] [CrossRef]
- Xie, X.; Hu, L.; Liu, L.; Wang, J.; Liu, Y.; Ma, L.; Sun, G.; Li, C.; Aisa, H.A.; Meng, S. Punicalagin promotes autophagic degradation of human papillomavirus E6 and E7 proteins in cervical cancer through the ROS-JNK-BCL2 pathway. Transl. Oncol. 2022, 19, 101388. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, M.; Yang, X.; Yang, Z.; Li, L.; Mei, J. Supplementing punicalagin reduces oxidative stress markers and restores angiogenic balance in a rat model of pregnancy-induced hypertension. Korean J. Physiol. Pharmacol. 2018, 22, 409–417. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, X.; Cao, Y.; An, X.; Chen, J.; Yang, L. Punicalagin protects against diabetic liver injury by upregulating mitophagy and antioxidant enzyme activities. Nutrients 2022, 14, 2782. [Google Scholar] [CrossRef]
- Li, P.; Du, R.; Chen, Z.; Wang, Y.; Zhan, P.; Liu, X.; Kang, D.; Chen, Z.; Zhao, X.; Wang, L. Punicalagin is a neuraminidase inhibitor of influenza viruses. J. Med. Virol. 2021, 93, 3465–3472. [Google Scholar] [CrossRef]
- Lin, L.-T.; Chen, T.-Y.; Lin, S.-C.; Chung, C.-Y.; Lin, T.-C.; Wang, G.-H.; Anderson, R.; Lin, C.-C.; Richardson, C.D. Broad-spectrum antiviral activity of chebulagic acid and punicalagin against viruses that use glycosaminoglycans for entry. BMC Microbiol. 2013, 13, 187. [Google Scholar] [CrossRef]
- Li, G.; Xu, Y.; Pan, L.; Xia, X. Punicalagin damages the membrane of Salmonella typhimurium. J. Food Prot. 2020, 83, 2102–2106. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, C.; Wu, Q.; Zheng, Z.; Liu, P.; Li, G.; Peng, X.; Xia, X. Antimicrobial activity of punicalagin against Staphylococcus aureus and its effect on biofilm formation. Foodborne Pathog. Dis. 2017, 14, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Peng, Y.; Yao, H.; Wang, Y.; Li, J.; Yang, Y.; Lin, Z. Punicalagin as an allosteric NSP13 helicase inhibitor potently suppresses SARS-CoV-2 replication in vitro. Antivir. Res. 2022, 206, 105389. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.-H.; Kang, O.-H.; Kong, R.; Zhou, T.; Kim, S.-A.; Shin, D.-W.; Kwon, D.-Y. Punicalagin suppresses methicillin resistance of Staphylococcus aureus to oxacillin. J. Pharmacol. Sci. 2018, 137, 317–323. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Report on the Epidemiology and Burden of Sepsis: Current Evidence, Identifying Gaps and Future Directions; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Wiseman, L.R.; Wagstaff, A.J.; Brogden, R.N.; Bryson, H.M. Meropenem: A review of its antibacterial activity, pharmacokinetic properties and clinical efficacy. Drugs 1995, 50, 73–101. [Google Scholar] [CrossRef]
- Rello, J.; Ulldemolins, M.; Lisboa, T.; Koulenti, D.; Mañez, R.; Martin-Loeches, I.; De Waele, J.; Putensen, C.; Guven, M.; Deja, M. Determinants of prescription and choice of empirical therapy for hospital-acquired and ventilator-associated pneumonia. Eur. Respir. J. 2011, 37, 1332–1339. [Google Scholar] [CrossRef]
- Badawy, A.A.; Zaher, T.I.; Sharaf, S.M.; Emara, M.H.; Shaheen, N.E.; Aly, T.F. Effect of alternative antibiotics in treatment of cefotaxime resistant spontaneous bacterial peritonitis. World J. Gastroenterol. WJG 2013, 19, 1271. [Google Scholar] [CrossRef]
- Castagnola, E.; Bandettini, R.; Ginocchio, F.; Perotti, M.; Masa, D.L.; Ciucci, A.; Loy, A.; Caviglia, I.; Haupt, R.; Guida, E. Susceptibility to antibiotics of aerobic bacteria isolated from community acquired secondary peritonitis in children: Therapeutic guidelines might not always fit with and everyday experience. J. Chemother. 2013, 25, 213–216. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Winkler, M.S.; Kluge, S.; Holzmann, M.; Moritz, E.; Robbe, L.; Bauer, A.; Zahrte, C.; Priefler, M.; Schwedhelm, E.; Böger, R.H. Markers of nitric oxide are associated with sepsis severity: An observational study. Crit. Care 2017, 21, 189. [Google Scholar] [CrossRef]
- Lambden, S. Bench to bedside review: Therapeutic modulation of nitric oxide in sepsis—An update. Intensive Care Med. Exp. 2019, 7, 64. [Google Scholar] [CrossRef]
- Yeh, J.-C.; Hazam, P.K.; Hsieh, C.-Y.; Hsu, P.-H.; Lin, W.-C.; Chen, Y.-R.; Li, C.-C.; Chen, J.-Y. Rational design of stapled antimicrobial peptides to enhance stability and in vivo potency against polymicrobial sepsis. Microbiol. Spectr. 2023, 11, e03853-22. [Google Scholar] [CrossRef] [PubMed]
- Velho, T.R.; Raquel, H.; Figueiredo, N.; Neves-Costa, A.; Pedroso, D.; Santos, I.; Willmann, K.; Moita, L.F. Immunomodulatory Effects and Protection in Sepsis by the Antibiotic Moxifloxacin. Antibiotics 2024, 13, 742. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, C.V.; Denorme, F.; Stephens, W.Z.; Li, Q.; Cody, M.J.; Crandell, J.L.; Petrey, A.C.; Queisser, K.A.; Rustad, J.L.; Fulcher, J.M. Neonatal NET-Inhibitory Factor improves survival in the cecal ligation and puncture model of polymicrobial sepsis by inhibiting neutrophil extracellular traps. Front. Immunol. 2023, 13, 1046574. [Google Scholar] [CrossRef] [PubMed]
- Lertnimitphun, P.; Jiang, Y.; Kim, N.; Fu, W.; Zheng, C.; Tan, H.; Zhou, H.; Zhang, X.; Pei, W.; Lu, Y. Safranal alleviates dextran sulfate sodium-induced colitis and suppresses macrophage-mediated inflammation. Front. Pharmacol. 2019, 10, 1281. [Google Scholar] [CrossRef]
- Müller, A.J.; Aeschlimann, S.; Olekhnovitch, R.; Dacher, M.; Späth, G.F.; Bousso, P. Photoconvertible pathogen labeling reveals nitric oxide control of Leishmania major infection in vivo via dampening of parasite metabolism. Cell Host Microbe 2013, 14, 460–467. [Google Scholar] [CrossRef]
- Richardson, A.R.; Payne, E.C.; Younger, N.; Karlinsey, J.E.; Thomas, V.C.; Becker, L.A.; Navarre, W.W.; Castor, M.E.; Libby, S.J.; Fang, F.C. Multiple targets of nitric oxide in the tricarboxylic acid cycle of Salmonella enterica serovar typhimurium. Cell Host Microbe 2011, 10, 33–43. [Google Scholar] [CrossRef]
- Wiegand, S.B.; Traeger, L.; Nguyen, H.K.; Rouillard, K.R.; Fischbach, A.; Zadek, F.; Ichinose, F.; Schoenfisch, M.H.; Carroll, R.W.; Bloch, D.B. Antimicrobial effects of nitric oxide in murine models of Klebsiella pneumonia. Redox Biol. 2021, 39, 101826. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Antimicrobial potential of pomegranate peel: A review. Int. J. Food Sci. Technol. 2019, 54, 959–965. [Google Scholar] [CrossRef]
- Saini, S.; Mishra, P.; Balhara, M.; Dutta, D.; Ghosh, S.; Chaudhuri, S. Antimicrobial potency of Punica granatum peel extract: Against multidrug resistant clinical isolates. Gene Rep. 2023, 30, 101744. [Google Scholar] [CrossRef]
- Ikeda, M.; Shimizu, K.; Ogura, H.; Kurakawa, T.; Umemoto, E.; Motooka, D.; Nakamura, S.; Ichimaru, N.; Takeda, K.; Takahara, S. Hydrogen-rich saline regulates intestinal barrier dysfunction, dysbiosis, and bacterial translocation in a murine model of sepsis. Shock 2018, 50, 640–647. [Google Scholar] [CrossRef]
- Barroqueiro, E.S.; Prado, D.S.; Barcellos, P.S.; Silva, T.A.; Pereira, W.S.; Silva, L.A.; Maciel, M.C.; Barroqueiro, R.B.; Nascimento, F.R.; Gonçalves, A.G. Immunomodulatory and antimicrobial activity of babassu mesocarp improves the survival in lethal sepsis. Evid.-Based Complement. Altern. Med. 2016, 2016, 2859652. [Google Scholar] [CrossRef] [PubMed]
- Brady, J.; Horie, S.; Laffey, J.G. Role of the adaptive immune response in sepsis. Intensive Care Med. Exp. 2020, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Guclu, E.; Durmaz, Y.; Karabay, O. Effect of severe sepsis on platelet count and their indices. Afr. Health Sci. 2013, 13, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef]
- Ilham, D.; Syahputra, D.A.; Yusuf, M. The role of mean platelet volume (MPV) and platelet distribution width (PDW) pre-operating as sepsis indicators in laparotomy patients in Dr. Zainoel Abidin Hospital Banda Aceh, Indonesia. Bali Med. J. 2023, 12, 1390–1393. [Google Scholar] [CrossRef]
- Mangalesh, S.; Dudani, S.; Malik, A. Platelet indices and their kinetics predict mortality in patients of sepsis. Indian J. Hematol. Blood Transfus. 2021, 37, 600–608. [Google Scholar] [CrossRef]
- Faurschou, M.; Borregaard, N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003, 5, 1317–1327. [Google Scholar] [CrossRef]
- Maciel, M.C.; Farias, J.C.; Maluf, M.J.; Gomes, E.A.; Pereira, P.V.; Aragão-Filho, W.C.; Frazão, J.B.; Costa, G.C.; Sousa, S.M.; Silva, L.A. Syzygium jambolanum treatment improves survival in lethal sepsis induced in mice. BMC Complement. Altern. Med. 2008, 8, 57. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, J.; Ren, G.; Zhang, Y.; Tan, X.; Yang, L. Punicalagin prevents inflammation in LPS-induced RAW264. 7 macrophages by inhibiting FoxO3a/autophagy signaling pathway. Nutrients 2019, 11, 2794. [Google Scholar] [CrossRef]
- Lo, J.; Liu, C.-C.; Li, Y.-S.; Lee, P.-Y.; Liu, P.-L.; Wu, P.-C.; Lin, T.-C.; Chen, C.-S.; Chiu, C.-C.; Lai, Y.-H. Punicalagin attenuates LPS-induced inflammation and ROS production in microglia by inhibiting the MAPK/NF-κB signaling pathway and NLRP3 inflammasome activation. J. Inflamm. Res. 2022, 15, 5347–5359. [Google Scholar] [CrossRef]
- Makled, M.N.; El-Awady, M.S.; Abdelaziz, R.R.; Atwan, N.; Guns, E.T.; Gameil, N.M.; El-Din, A.B.S.; Ammar, E.M. Pomegranate protects liver against cecal ligation and puncture-induced oxidative stress and inflammation in rats through TLR4/NF-κB pathway inhibition. Environ. Toxicol. Pharmacol. 2016, 43, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, Q. Punicalagin suppresses inflammation in ventilator-induced lung injury through protease-activated receptor-2 inhibition-induced inhibition of NLR family pyrin domain containing-3 inflammasome activation. Chem. Biol. Drug Des. 2022, 100, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; O’garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Li, G.; Feng, Y.; Xu, Y.; Wu, Q.; Han, Q.a.; Liang, X.; Yang, B.; Wang, X.; Xia, X. The anti-infective activity of punicalagin against Salmonella enterica subsp. enterica serovar typhimurium in mice. Food Funct. 2015, 6, 2357–2364. [Google Scholar] [CrossRef]
- Silva, A.Y.; Amorim, É.A.; Barbosa-Silva, M.C.; Lima, M.N.; Oliveira, H.A.; Granja, M.G.; Oliveira, K.S.; Fagundes, P.M.; Neris, R.L.; Campos, R.M. Mesenchymal stromal cells protect the blood-brain barrier, reduce astrogliosis, and prevent cognitive and behavioral alterations in surviving septic mice. Crit. Care Med. 2020, 48, e290–e298. [Google Scholar] [CrossRef]
- de, O. Trovão, L.; dos S. Rodrigues, L.; Mendes, P.M.; Alves, P.C.; da S. Oliveira, A.; Brito, J.M.; Vale, A.A.; de O. Garbis, D.V.; Simão, G.; Dos Santos, A.P.S. The Immunomodulatory Activity of Punica granatum L. Peel Extract Increases the Lifespan of Mice with Lethal Sepsis. J. Immunol. Res. 2023, 2023, 2868707. [Google Scholar]
- Ceron, C.S.; do Vale, G.T.; Simplicio, J.A.; Ricci, S.T.; De Martinis, B.S.; de Freitas, A.; Tirapelli, C.R. Chronic ethanol consumption increases vascular oxidative stress and the mortality induced by sub-lethal sepsis: Potential role of iNOS. Eur. J. Pharmacol. 2018, 825, 39–47. [Google Scholar] [CrossRef]
- Thomas, P.; Sekhar, A.C.; Upreti, R.; Mujawar, M.M.; Pasha, S.S. Optimization of single plate-serial dilution spotting (SP-SDS) with sample anchoring as an assured method for bacterial and yeast cfu enumeration and single colony isolation from diverse samples. Biotechnol. Rep. 2015, 8, 45–55. [Google Scholar] [CrossRef]
- Rios, C.E.; Abreu, A.G.; Braga Filho, J.A.; Nascimento, J.R.; Guerra, R.N.; Amaral, F.M.; Maciel, M.C.; Nascimento, F.R. Chenopodium ambrosioides L. improves phagocytic activity and decreases bacterial growth and the systemic inflammatory response in sepsis induced by cecal ligation and puncture. Front. Microbiol. 2017, 8, 148. [Google Scholar] [CrossRef]

| Hematological Parameters # | Sham | CLP | ATB | Pg ATB |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| RBC (×106/µL) | 1.44 ± 2.33 | 5.38 ± 1.16 * | 3.22 ± 2.03 | 4.44 ± 1.23 * |
| MCV (fL) | 41.95 ± 1.47 | 41.80 ± 0.48 | 43.76 ± 5.21 | 41.32 ± 0.73 |
| RDW-CV (%) | 14.55 ± 1.29 | 16.26 ± 1.80 | 13.44 ± 2.89 | 16.10 ± 0.63 * |
| RDW-SD (fL) | 30.53 ± 3.19 | 33.98 ± 3.68 | 28.76 ± 3.97 | 33.28 ± 1.30 † |
| HCT (%) | 6.23 ± 10.19 | 22.56 ± 5.12 * | 13.36 ± 8.31 | 18.32 ± 4.98 |
| PLT (×103/µL) | 297.25 ± 315.46 | 528.40 ± 152.53 | 286.6 ± 141.7 | 489.00 ± 65.51 † |
| MPV (fL) | 5.68 ± 0.46 | 6.02 ± 0.24 | 5.84 ± 0.31 | 5.72 ± 0.24 |
| PDW (fL) | 5.08 ± 1.86 | 7.54 ± 0.72 * | 7.38 ± 0.67 * | 6.72 ± 0.62 |
| PCT (%) | 0.17 ± 0.19 | 0.32 ± 0.09 | 0.17 ± 0.08 | 0.28 ± 0.04 |
| P-LCR (%) | 7.57 ± 4.57 | 6.00 ± 1.84 | 4.64 ± 0.41 | 4.04 ± 1.41 |
| Cytokines † | Sham | CLP | ATB | Pg ATB |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| IL-2 | 1.90 ± 3.80 | 0.00 ± 0.00 | 1.64 ± 3.28 | 0.00 ± 0.00 |
| IL-4 | 1.02 ± 1.91 | 0.00 ± 0.00 | 0.26 ± 0.41 | 0.00 ± 0.00 |
| IL-6 | 18.55 ± 13.50 | 269.7 ± 442.8 | 4710 ± 4920 * | 2423 ± 4913 |
| IFN-γ | 0.57 ± 1.10 | 5.21 ± 9.17 | 128.5 ± 159.7 * | 3.10 ± 3.21 |
| TNF-α | 10.69 ± 8.14 | 39.83 ± 49.48 | 834.4 ± 843.4 * | 116.4 ± 142.3 |
| IL-17 | 0.31 ± 0.63 | 0.56 ± 0.80 | 3.88 ± 3.25 | 0.77 ± 1.72 |
| IL-10 | 2.92 ± 5.41 | 14.76 ± 24.21 | 1710 ± 1933 | 39.7 ± 15.9 *,# |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, L.d.S.; Mendes, P.M.; Vale, A.A.M.; Pereira-Filho, J.L.; Fernandes, E.S.; Sousa, J.C.d.S.; Maciel, M.C.G.; Monteiro-Neto, V. Antimicrobial and Immunomodulatory Effects of Punicalagin and Meropenem in a Murine Model of Sublethal Sepsis. Antibiotics 2025, 14, 626. https://doi.org/10.3390/antibiotics14070626
Rodrigues LdS, Mendes PM, Vale AAM, Pereira-Filho JL, Fernandes ES, Sousa JCdS, Maciel MCG, Monteiro-Neto V. Antimicrobial and Immunomodulatory Effects of Punicalagin and Meropenem in a Murine Model of Sublethal Sepsis. Antibiotics. 2025; 14(7):626. https://doi.org/10.3390/antibiotics14070626
Chicago/Turabian StyleRodrigues, Liliane dos Santos, Priscila Mendonça Mendes, André Alvares Marques Vale, José Lima Pereira-Filho, Elizabeth Soares Fernandes, Joicy Cortez de Sá Sousa, Márcia Cristina Gonçalves Maciel, and Valério Monteiro-Neto. 2025. "Antimicrobial and Immunomodulatory Effects of Punicalagin and Meropenem in a Murine Model of Sublethal Sepsis" Antibiotics 14, no. 7: 626. https://doi.org/10.3390/antibiotics14070626
APA StyleRodrigues, L. d. S., Mendes, P. M., Vale, A. A. M., Pereira-Filho, J. L., Fernandes, E. S., Sousa, J. C. d. S., Maciel, M. C. G., & Monteiro-Neto, V. (2025). Antimicrobial and Immunomodulatory Effects of Punicalagin and Meropenem in a Murine Model of Sublethal Sepsis. Antibiotics, 14(7), 626. https://doi.org/10.3390/antibiotics14070626








