Ability of Linezolid to Combat Staphylococcus aureus and Pseudomonas aeruginosa Isolated from Polymicrobial Wound Infections
Abstract
1. Introduction
2. Results
2.1. Time-Killing Experiments
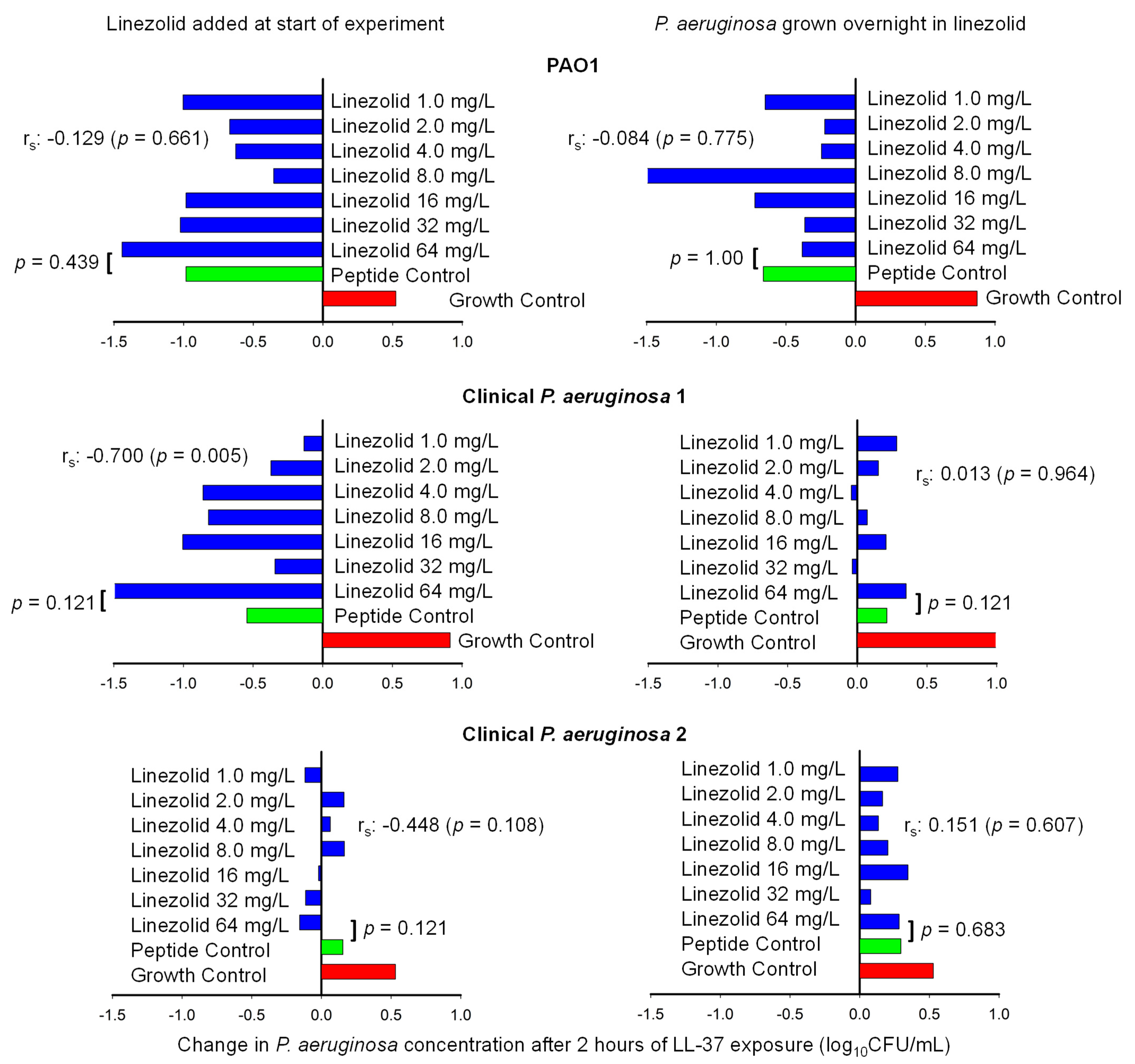
2.2. Host Defense Peptide Assay
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates
4.2. Time-Killing Experiments
4.3. Time-Killing Data Analysis
4.4. Host Defense Peptide Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MRSA | Methicillin-resistant S. aureus |
| MSSA | Methicillin-susceptible S. aureus |
References
- Centers for Disease Control. Antibiotic Resistance Threats in the United States. 2019. Available online: https://www.cdc.gov/antimicrobial-resistance/media/pdfs/2019-ar-threats-report-508.pdf (accessed on 31 October 2024).
- Phan, S.; Feng, C.H.; Huang, R.; Lee, Z.X.; Moua, Y.; Phung, O.J.; Lenhard, J.R. Relative Abundance and Detection of Pseudomonas aeruginosa from Chronic Wound Infections Globally. Microorganisms 2023, 11, 1210. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; de Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef]
- Keim, K.; Bhattacharya, M.; Crosby, H.A.; Jenul, C.; Mills, K.; Schurr, M.; Horswill, A. Polymicrobial interactions between Staphylococcus aureus and Pseudomonas aeruginosa promote biofilm formation and persistence in chronic wound infections. bioRxiv 2024, bioRxiv:2024.11.04.621402. [Google Scholar] [CrossRef]
- Radlinski, L.; Rowe, S.E.; Kartchner, L.B.; Maile, R.; Cairns, B.A.; Vitko, N.P.; Gode, C.J.; Lachiewicz, A.M.; Wolfgang, M.C.; Conlon, B.P. Pseudomonas aeruginosa exoproducts determine antibiotic efficacy against Staphylococcus aureus. PLoS Biol. 2017, 15, e2003981. [Google Scholar] [CrossRef]
- Trizna, E.Y.; Yarullina, M.N.; Baidamshina, D.R.; Mironova, A.V.; Akhatova, F.S.; Rozhina, E.V.; Fakhrullin, R.F.; Khabibrakhmanova, A.M.; Kurbangalieva, A.R.; Bogachev, M.I.; et al. Bidirectional alterations in antibiotics susceptibility in Staphylococcus aureus-Pseudomonas aeruginosa dual-species biofilm. Sci. Rep. 2020, 10, 14849. [Google Scholar] [CrossRef]
- Orazi, G.; Jean-Pierre, F.; O’Toole, G.A. Pseudomonas aeruginosa PA14 Enhances the Efficacy of Norfloxacin Against Staphylococcus aureus Newman Biofilms. J. Bacteriol. 2020, 202, e00159-20. [Google Scholar] [CrossRef] [PubMed]
- Orazi, G.; O’Toole, G.A. Pseudomonas aeruginosa Alters Staphylococcus aureus Sensitivity to Vancomycin in a Biofilm Model of Cystic Fibrosis Infection. mBio 2017, 8, e00873-17. [Google Scholar] [CrossRef] [PubMed]
- Orazi, G.; Ruoff, K.L.; O’Toole, G.A. Pseudomonas aeruginosa Increases the Sensitivity of Biofilm-Grown Staphylococcus aureus to Membrane-Targeting Antiseptics and Antibiotics. mBio 2019, 10, e01501-19. [Google Scholar] [CrossRef]
- DeLeon, S.; Clinton, A.; Fowler, H.; Everett, J.; Horswill, A.R.; Rumbaugh, K.P. Synergistic interactions of Pseudomonas aeruginosa and Staphylococcus aureus in an in vitro wound model. Infect. Immun. 2014, 82, 4718–4728. [Google Scholar] [CrossRef]
- Barraza, J.P.; Whiteley, M. A Pseudomonas aeruginosa Antimicrobial Affects the Biogeography but Not Fitness of Staphylococcus aureus During Coculture. mBio 2021, 12, e00047-21. [Google Scholar] [CrossRef]
- Ibberson, C.B.; Barraza, J.P.; Holmes, A.L.; Cao, P.; Whiteley, M. Precise spatial structure impacts antimicrobial susceptibility of S. aureus in polymicrobial wound infections. Proc. Natl. Acad. Sci. United States Am. 2022, 119, e2212340119. [Google Scholar] [CrossRef]
- Tahmasebi, H.; Dehbashi, S.; Arabestani, M.R. Antibiotic resistance alters through iron-regulating Sigma factors during the interaction of Staphylococcus aureus and Pseudomonas aeruginosa. Sci. Rep. 2021, 11, 18509. [Google Scholar] [CrossRef] [PubMed]
- Lenhard, J.R.; Smith, N.M.; Quach, C.D.; Nguyen, T.Q.; Doan, L.H.; Chau, J. Bacterial brothers in arms: Cooperation of Staphylococcus aureus and Pseudomonas aeruginosa during antimicrobial exposure. J. Antimicrob. Chemother. 2019, 74, 2657–2665. [Google Scholar] [CrossRef] [PubMed]
- Leach, K.L.; Brickner, S.J.; Noe, M.C.; Miller, P.F. Linezolid, the first oxazolidinone antibacterial agent. Ann. N. Y. Acad. Sci. 2011, 1222, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, e10-52. [Google Scholar] [CrossRef]
- Dighriri, I.M.; Alanazi, S.; AlMutairi, K.; Alhusayni, S.J.; Balharith, F.M.; Aljuwaie, R.A.; Alfayez, H.K.; Althubaiti, G.M.; Alosaimi, G.A.; Jameel, O.W.; et al. Efficacy and Safety of Vancomycin, Linezolid, and Ceftaroline in the Treatment of Methicillin-Resistant Staphylococcus aureus (MRSA): A Systematic Review and Meta-Analysis. Cureus 2025, 17, e77949. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Itani, K.; Norden, C. Treating foot infections in diabetic patients: A randomized, multicenter, open-label trial of linezolid versus ampicillin-sulbactam/amoxicillin-clavulanate. Clin. Infect. Dis. 2004, 38, 17–24. [Google Scholar] [CrossRef]
- Kai-Larsen, Y.; Agerberth, B. The role of the multifunctional peptide LL-37 in host defense. Front. Biosci. A J. Virtual Libr. 2008, 13, 3760–3767. [Google Scholar] [CrossRef]
- Turner, J.; Cho, Y.; Dinh, N.N.; Waring, A.J.; Lehrer, R.I. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob. Agents Chemother. 1998, 42, 2206–2214. [Google Scholar] [CrossRef]
- Xie, Y.; Shi, Y.H.; Wang, L.L.; Li, C.W.; Wu, M.; Xu, J.F. Outer membrane vesicle contributes to the Pseudomonas aeruginosa resistance to antimicrobial peptides in the acidic airway of bronchiectasis patients. MedComm 2025, 6, e70084. [Google Scholar] [CrossRef]
- Song, Y.Q.; Kyung, S.M.; Kim, S.; Kim, G.; Lee, S.Y.; Yoo, H.S. Effects of synthetic peptide RP557 and its origin, LL-37, on carbapenem-resistant Pseudomonas aeruginosa. Microbiol. Spectr. 2023, 11, e0043023. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Luo, Y.; Shi, W.; Lu, Y.; Xiong, R.; Wu, X.; Huang, H.; Zhao, C.; Zeng, J.; Chen, C. The effects of LL-37 on virulence factors related to the quorum sensing system of Pseudomonas aeruginosa. Ann. Transl. Med. 2022, 10, 284. [Google Scholar] [CrossRef] [PubMed]
- Geitani, R.; Ayoub Moubareck, C.; Touqui, L.; Karam Sarkis, D. Cationic antimicrobial peptides: Alternatives and/or adjuvants to antibiotics active against methicillin-resistant Staphylococcus aureus and multidrug-resistant Pseudomonas aeruginosa. BMC Microbiol. 2019, 19, 54. [Google Scholar] [CrossRef]
- Sibila, O.; Perea, L.; Cantó, E.; Shoemark, A.; Cassidy, D.; Smith, A.H.; Suarez-Cuartin, G.; Rodrigo-Troyano, A.; Keir, H.R.; Oriano, M.; et al. Antimicrobial peptides, disease severity and exacerbations in bronchiectasis. Thorax 2019, 74, 835–842. [Google Scholar] [CrossRef]
- Ridyard, K.E.; Elsawy, M.; Mattrasingh, D.; Klein, D.; Strehmel, J.; Beaulieu, C.; Wong, A.; Overhage, J. Synergy Between Human Peptide LL-37 and Polymyxin B Against Planktonic and Biofilm Cells of Escherichia coli and Pseudomonas aeruginosa. Antibiotics 2023, 1, 389. [Google Scholar] [CrossRef] [PubMed]
- Sakoulas, G.; Bayer, A.S.; Pogliano, J.; Tsuji, B.T.; Yang, S.J.; Mishra, N.N.; Nizet, V.; Yeaman, M.R.; Moise, P.A. Ampicillin enhances daptomycin- and cationic host defense peptide-mediated killing of ampicillin- and vancomycin-resistant Enterococcus faecium. Antimicrob. Agents Chemother. 2012, 56, 838–844. [Google Scholar] [CrossRef]
- Sakoulas, G.; Okumura, C.Y.; Thienphrapa, W.; Olson, J.; Nonejuie, P.; Dam, Q.; Dhand, A.; Pogliano, J.; Yeaman, M.R.; Hensler, M.E.; et al. Nafcillin enhances innate immune-mediated killing of methicillin-resistant Staphylococcus aureus. J. Mol. Med. 2014, 92, 139–149. [Google Scholar] [CrossRef]
- Senneville, É.; Albalawi, Z.; van Asten, S.A.; Abbas, Z.G.; Allison, G.; Aragón-Sánchez, J.; Embil, J.M.; Lavery, L.A.; Alhasan, M.; Oz, O.; et al. IWGDF/IDSA Guidelines on the Diagnosis and Treatment of Diabetes-related Foot Infections (IWGDF/IDSA 2023). Clin. Infect. Dis. 2023, 40, e3687. [Google Scholar] [CrossRef]
- Tsuji, B.T.; Rybak, M.J.; Cheung, C.M.; Amjad, M.; Kaatz, G.W. Community- and health care-associated methicillin-resistant Staphylococcus aureus: A comparison of molecular epidemiology and antimicrobial activities of various agents. Diagn. Microbiol. Infect. Dis. 2007, 58, 41–47. [Google Scholar] [CrossRef]
- Bawankar, N.S.; Agrawal, G.N.; Zodpey Shrikhande, S.S. Unmasking a looming crisis: Escalating MIC of last resort drugs against MRSA isolates from a tertiary care hospital in Central India. Indian. J. Med. Microbiol. 2024, 51, 100707. [Google Scholar] [CrossRef]
- Zelmer, A.R.; Yang, D.; Gunn, N.J.; Solomon, L.B.; Nelson, R.; Kidd, S.P.; Richter, K.; Atkins, G.J. Osteomyelitis-relevant antibiotics at clinical concentrations show limited effectivity against acute and chronic intracellular S. aureus infections in osteocytes. Antimicrob. Agents Chemother. 2024, 68, e0080824. [Google Scholar] [CrossRef] [PubMed]
- Natsumoto, B.; Yokota, K.; Omata, F.; Furukawa, K. Risk factors for linezolid-associated thrombocytopenia in adult patients. Infection 2014, 42, 1007–1012. [Google Scholar] [CrossRef]
- Filkins, L.M.; Graber, J.A.; Olson, D.G.; Dolben, E.L.; Lynd, L.R.; Bhuju, S.; O’Toole, G.A. Coculture of Staphylococcus aureus with Pseudomonas aeruginosa Drives S. aureus Towards Fermentative Metabolism and Reduced Viability in a Cystic Fibrosis Model. J. Bacteriol. 2015, 197, 2252–2264. [Google Scholar] [CrossRef]
- Sánchez-Peña, A.; Winans, J.B.; Nadell, C.D.; Limoli, D.H. Pseudomonas aeruginosa surface motility and invasion into competing communities enhance interspecies antagonism. mBio 2024, 15, e0095624. [Google Scholar] [CrossRef] [PubMed]
- Wulkersdorfer, B.; Wicha, S.G.; Kurdina, E.; Carrion Carrera, S.F.; Matzneller, P.; Al Jalali, V.; Vossen, M.G.; Riesenhuber, S.; Lackner, E.; Dorn, C.; et al. Protein binding of clindamycin in vivo by means of intravascular microdialysis in healthy volunteers. J. Antimicrob. Chemother. 2021, 76, 2106–2113. [Google Scholar] [CrossRef] [PubMed]
- Begic, D.; von Eiff, C.; Tsuji, B.T. Daptomycin pharmacodynamics against Staphylococcus aureus hemB mutants displaying the small colony variant phenotype. J. Antimicrob. Chemother. 2009, 63, 977–981. [Google Scholar] [CrossRef]
- Sakoulas, G.; Moise, P.A.; Casapao, A.M.; Nonejuie, P.; Olson, J.; Okumura, C.Y.; Rybak, M.J.; Kullar, R.; Dhand, A.; Rose, W.E.; et al. Antimicrobial salvage therapy for persistent staphylococcal bacteremia using daptomycin plus ceftaroline. Clin. Ther. 2014, 36, 1317–1333. [Google Scholar] [CrossRef]
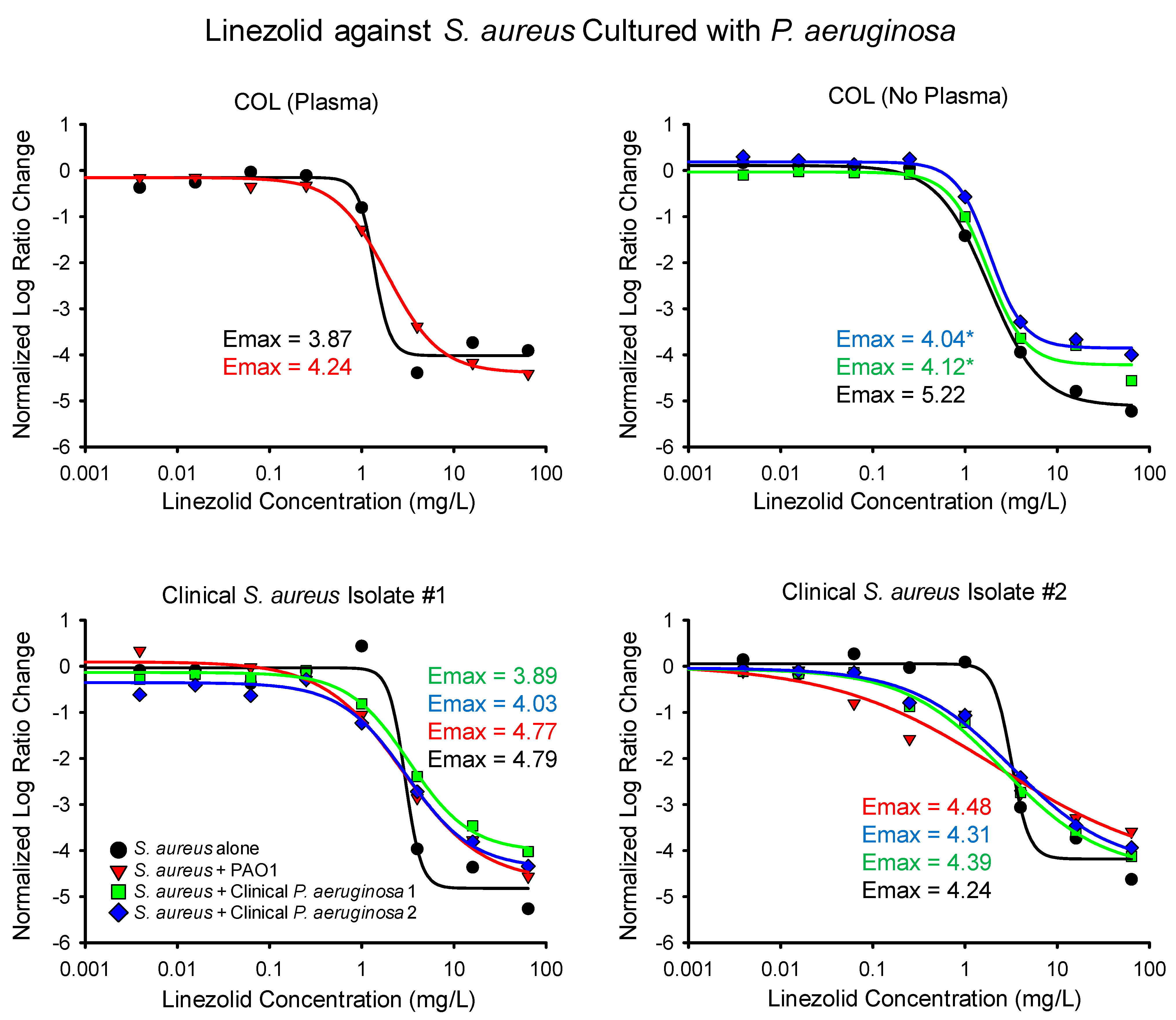
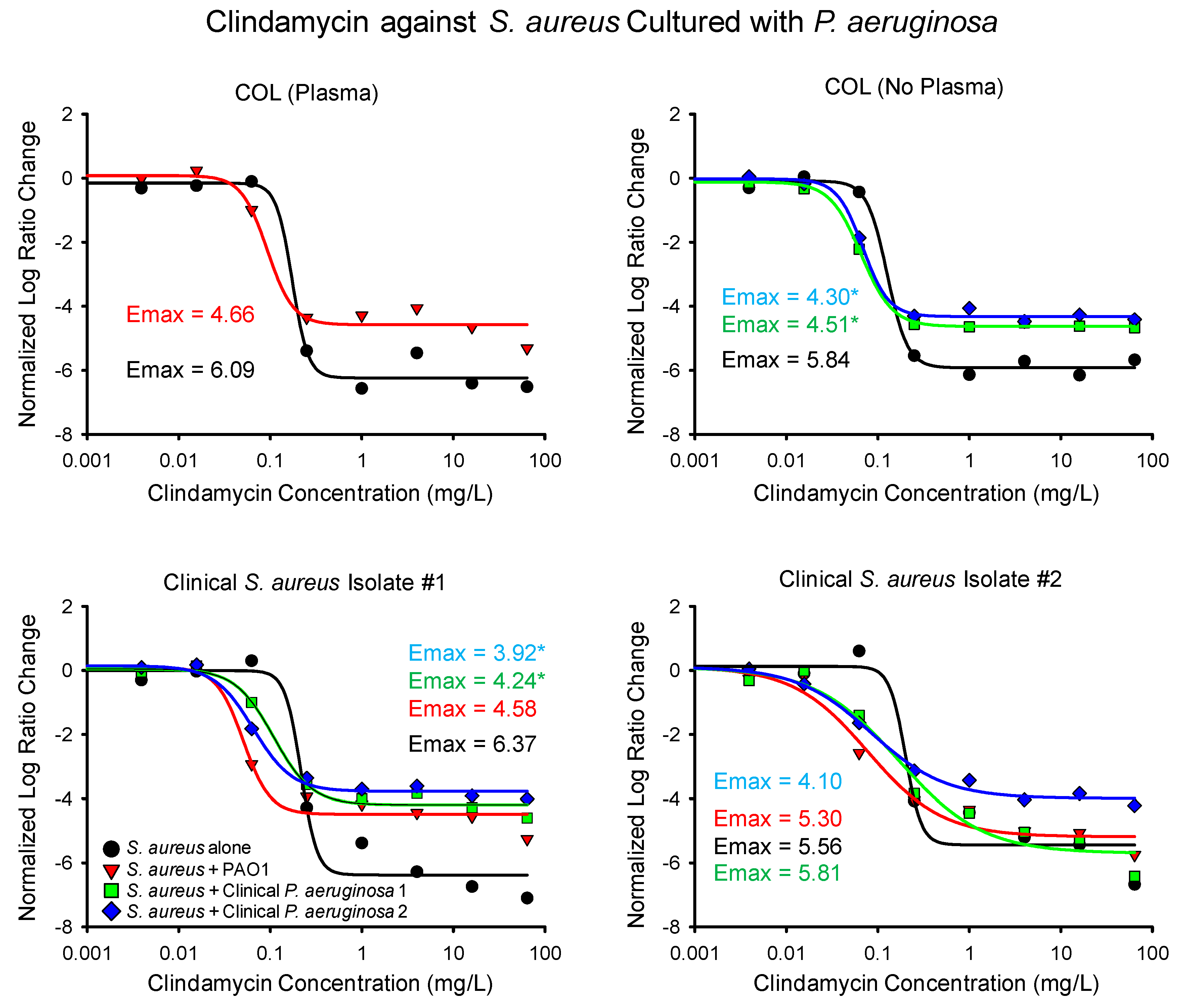
| S. aureus Isolate | P. aeruginosa Isolate and Number of Experimental Replicates (Linezolid and Clindamycin) | Linezolid Emax (95% CI) | Linezolid EC50 (95% CI) | Clindamycin Emax (95% CI) | Clindamycin EC50 (95% CI) |
|---|---|---|---|---|---|
| COL (plasma) | None (2, 2) | 3.87 (3.42–4.32) | 1.37 (1.04–1.70) | 6.09 (5.39–6.78) | 0.17 (0.12–0.22) |
| PAO1 (2, 2) | 4.24 (3.92–4.55) | 1.91 (1.49–2.33) | 4.66 (3.72–5.60) | 0.093 (0.025–0.16) | |
| COL | None (2, 2) | 5.22 (4.84–5.61) | 1.81 (1.42–2.21) | 5.84 (5.34–6.34) | 0.13 (0.076–0.18) |
| Clinical 1 (2, 2) | 4.12 (3.60–4.77) * | 1.75 (1.03–2.46) | 4.51 (4.28–4.74) * | 0.066 (0.059–0.072) * | |
| Clinical 2 (2, 2) | 4.04 (3.68–4.40) * | 1.87 (1.37–2.36) | 4.30 (3.93–4.66) * | 0.069 (0.056–0.081) | |
| Clinical S. aureus 1 | None (2, 2) | 4.79 (4.03–5.54) | 2.96 (2.07–3.85) | 6.37 (5.37–7.38) | 0.22 (0.17–0.27) |
| PAO1 (2, 2) | 4.77 (3.89–5.65) | 2.82 (1.27–4.37) | 4.58 (3.50–5.65) | 0.051 (0.022–0.079) * | |
| Clinical 1 (2, 3) | 3.89 (3.35–4.43) | 3.28 (2.04–4.51) | 4.24 (3.63–4.86) * | 0.11 (0.060–0.16) * | |
| Clinical 2 (2, 2) | 4.03 (3.02–5.04) | 3.07 (0.91–5.23) | 3.92 (3.40–4.44) * | 0.065 (0.044–0.086) * | |
| Clinical S. aureus 2 | None (3, 2) | 4.24 (3.48–5.00) | 3.19 (−1.77–8.14) | 5.56 (4.30–6.83) | 0.20 (0.13–0.27) |
| PAO1 (4, 2) | 4.48 (−0.22–9.18) | 2.29 (−8.18–12.76) | 5.30 (3.91–6.69) | 0.078 (0.0068–0.15) | |
| Clinical 1 (5, 2) | 4.39 (3.39–5.39) | 2.62 (0.70–4.53) | 5.81 (4.07–7.54) | 0.17 (−0.012–0.35) | |
| Clinical 2 (3, 2) | 4.31 (3.31–5.31) | 3.23 (0.84–5.62) | 4.10 (3.49–4.71) | 0.084 (0.041–0.13) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, S.A.; Luu, V.T.; Nsuga, T.C.O.; Burgos, S.E.; Kreys, E.; Arquiette, J.; Lenhard, J.R. Ability of Linezolid to Combat Staphylococcus aureus and Pseudomonas aeruginosa Isolated from Polymicrobial Wound Infections. Antibiotics 2025, 14, 597. https://doi.org/10.3390/antibiotics14060597
Ahmed SA, Luu VT, Nsuga TCO, Burgos SE, Kreys E, Arquiette J, Lenhard JR. Ability of Linezolid to Combat Staphylococcus aureus and Pseudomonas aeruginosa Isolated from Polymicrobial Wound Infections. Antibiotics. 2025; 14(6):597. https://doi.org/10.3390/antibiotics14060597
Chicago/Turabian StyleAhmed, Samar A., Vy T. Luu, Teresa C. Oyono Nsuga, Steven E. Burgos, Eugene Kreys, Jered Arquiette, and Justin R. Lenhard. 2025. "Ability of Linezolid to Combat Staphylococcus aureus and Pseudomonas aeruginosa Isolated from Polymicrobial Wound Infections" Antibiotics 14, no. 6: 597. https://doi.org/10.3390/antibiotics14060597
APA StyleAhmed, S. A., Luu, V. T., Nsuga, T. C. O., Burgos, S. E., Kreys, E., Arquiette, J., & Lenhard, J. R. (2025). Ability of Linezolid to Combat Staphylococcus aureus and Pseudomonas aeruginosa Isolated from Polymicrobial Wound Infections. Antibiotics, 14(6), 597. https://doi.org/10.3390/antibiotics14060597







