Antibiotic Resistance in Aquaculture: Challenges, Trends Analysis, and Alternative Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Questions
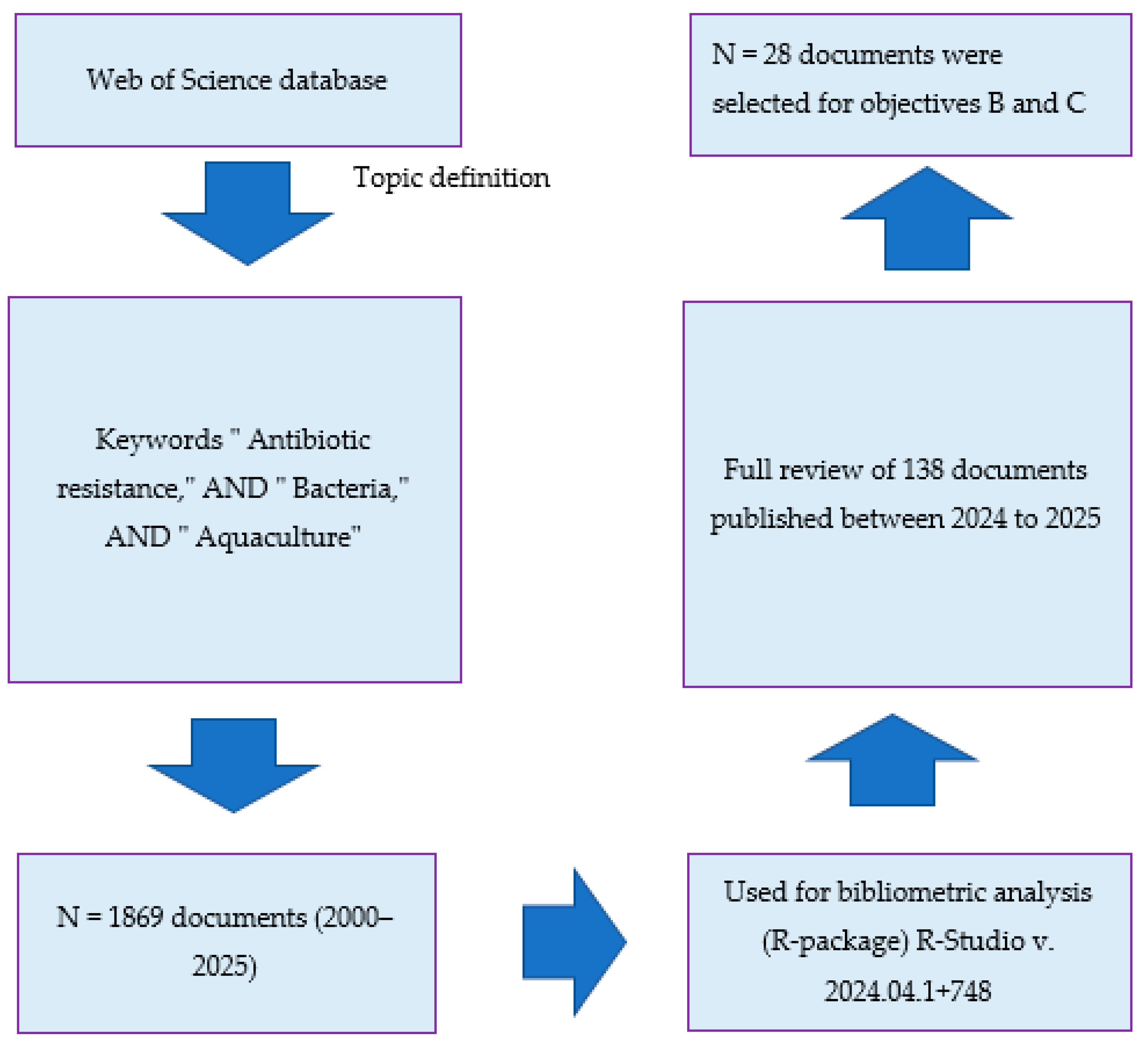
2.2. Framework and Searching Strategy
2.3. Bibliometric Analysis
2.4. Screening and Extraction
3. Results and Discussion
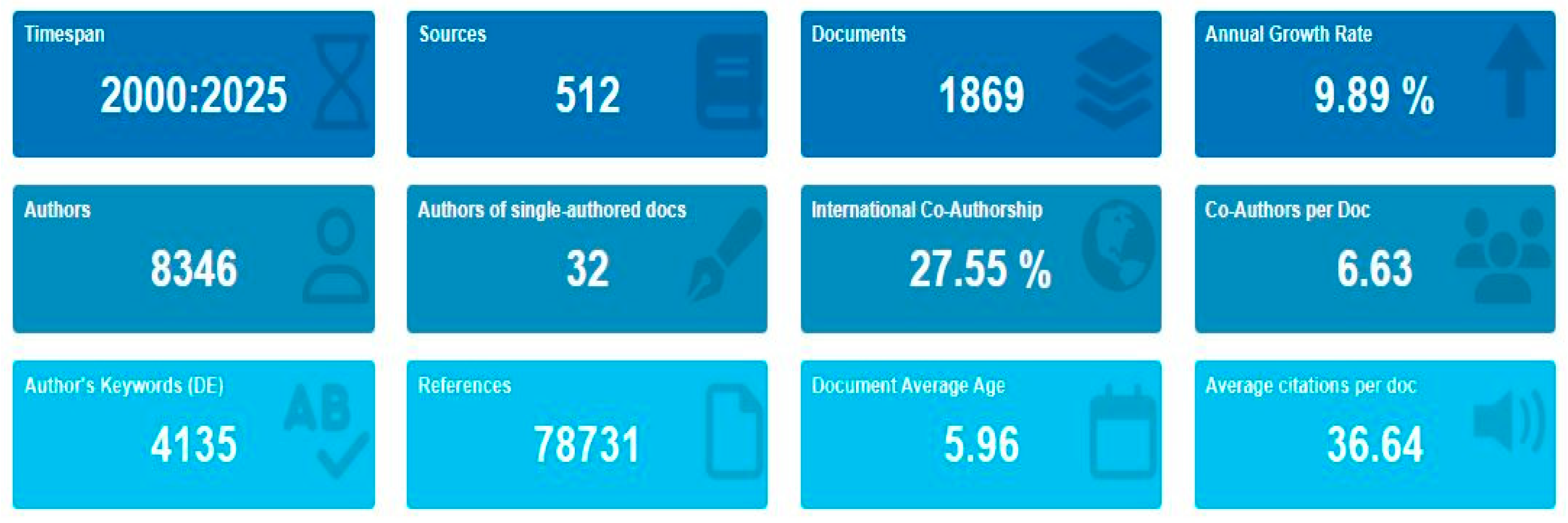
3.1. The Situation of the Scientific Publications Based on WoS Search on Antibiotic Resistance, Bacteria, and Aquaculture (ABA)
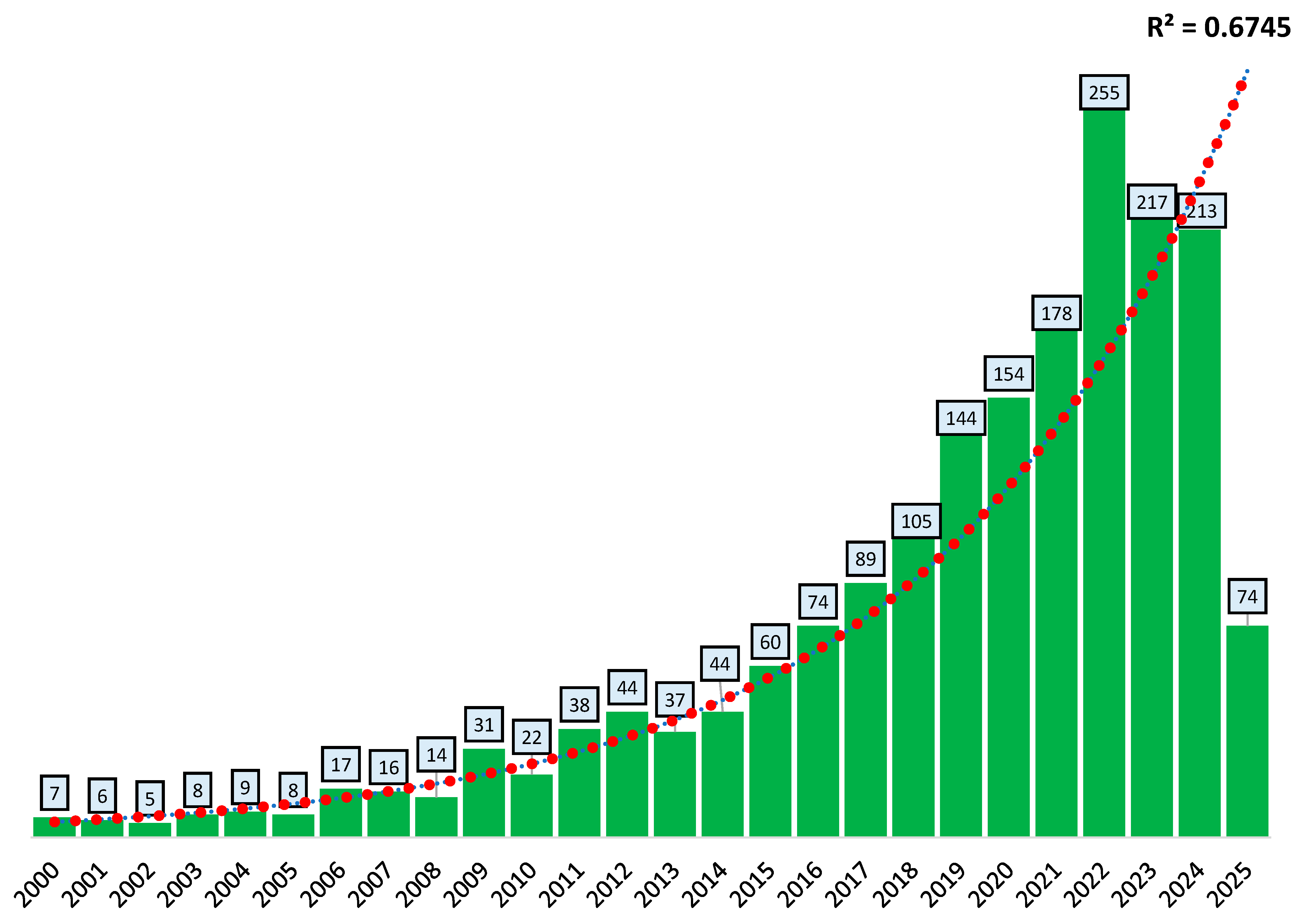
3.1.1. Growth of Scientific Publications of ABA Documents
3.1.2. Country-Level Scientific Collaboration for ABA Research
3.1.3. The Journals, Publishers, and Most Cited Publications on ABA Documents
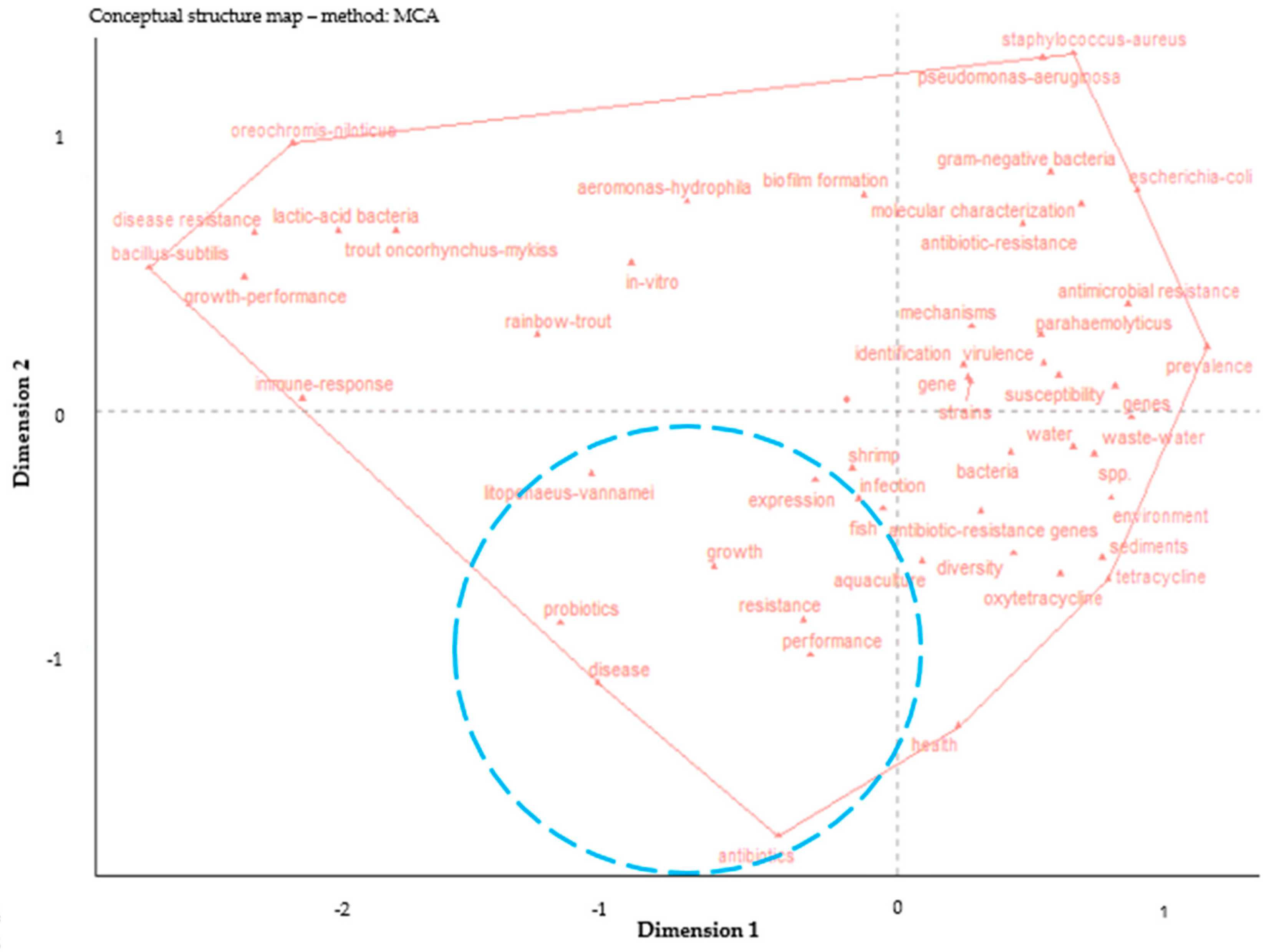
3.2. The Destructive Potential of Antibiotic-Resistant Bacteria and ARGs in Aquaculture Based on Keyword Analysis
3.2.1. Co-Occurrence Network of Keywords
3.2.2. The Most Frequent Keywords over Time
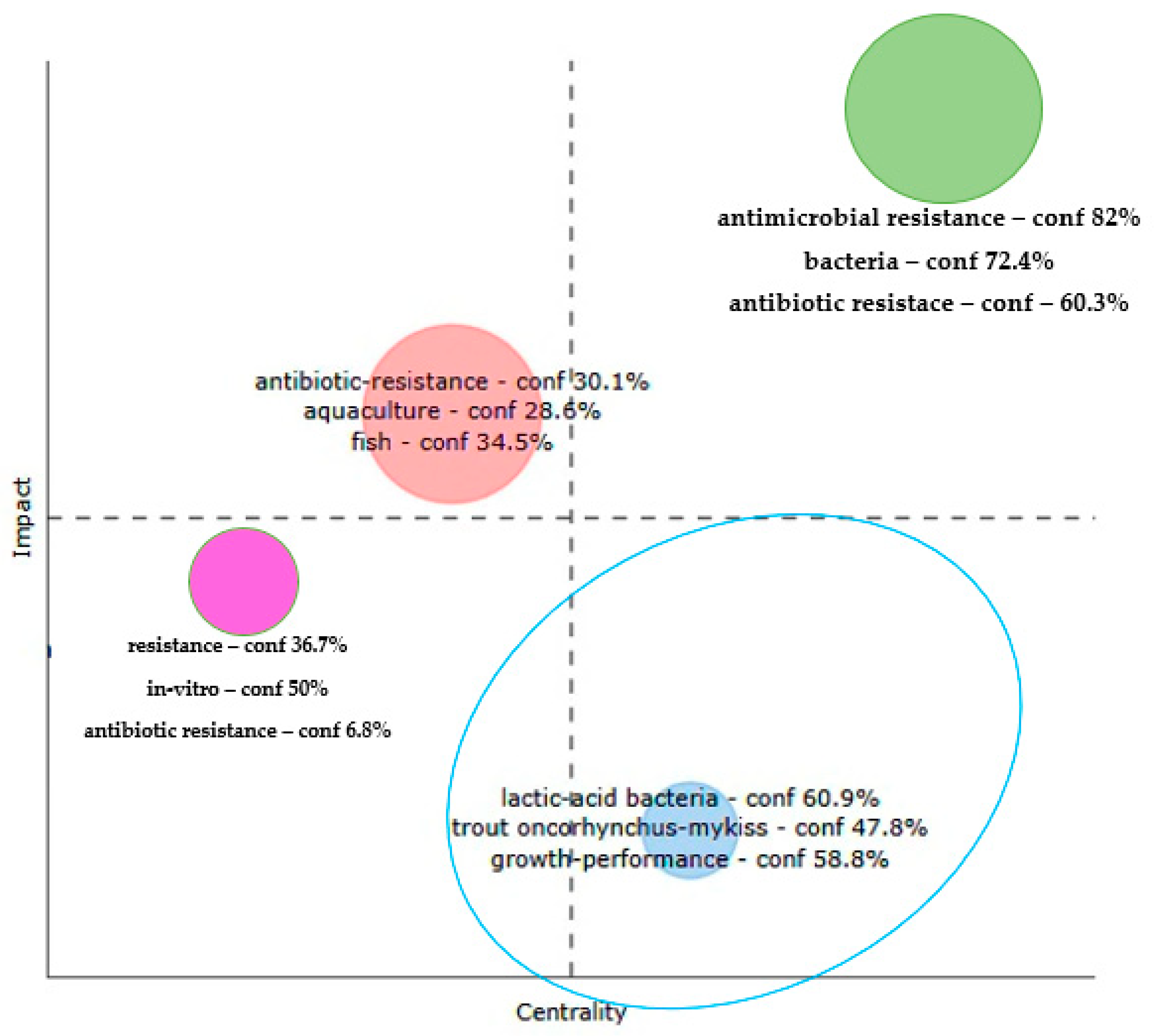
3.2.3. The Trend Topics
3.2.4. The Current Situation of Antibiotic Resistance in Aquaculture
3.3. The Alternative Approaches
| Country | Probiotic/Aquaculture Species | Main Results | Date | Ref. |
|---|---|---|---|---|
| China | Endozoicomonas spp. Clam (Meretrix petechialis) | Vibrio spp. loads ↓ Enhancing inflammatory response (NF-kappa B) ↑ Oxidative response (ROS metabolism) ↑ | March 2025 | [110] |
| China | Rhodopseudomonas palustris (3 × 108 CFU/mL) and composite probiotics (5 × 108 CFU/mL) with ratio of 1:1 Sea urchin, Strongylocentrotus intermedius | Immune indicators (ACP, AKP, LZM) ↑ Digestive enzyme ↑ Microbiota diversity ↑ Expression pf immune related genes ↑ | Feb. 2025 | [111] |
| China | Lacticaseibacillus rhamnosus FS3051, and L. reuteri FS3052 Grey mullet, Mugil cephalus | Resistance to Nocardia seriolae ↑ Secretion of hydrolytic enzymes ↑ weight gain, feed efficiency, and growth rate (%) ↑ Regulation of IL-8, TNF-alpha, IL-1 beta, IFN-gamma, and MHCI ↑ The abundance of Mycoplasma and Rhodobacter in the gut microbiome ↓ | Nov. 2024 | [112] |
| China | Bacillus velezensis BV1704-Y Zebrafish (Danio rerio) | Aeromonas hydrophila ↓ Mortality ↓ Expression IL-1 beta, TNF-alpha, IL6 ↓ Abundance of Cetobacterium ↓ | Sept. 2024 | [113] |
| Poland | Levilactobacillus brevis pikeperch (Sander lucioperca L.) | Inhibition of Aeromonas hydrophila, A. salmonicida, Acinetobacter junii, Pseudomonas fluorescens ↑ | Sept. 2024 | [114] |
| Malaysia | Probiotics tilapia (Oreochromis sp.) | Weight gain ↑ Feed conversion ratio ↓ Innate immunity ↑ pathogenic challenge ↑ | Feb. 2024 | [115] |
| China | Bacillus subtilis JSHY-K3 shrimp Penaeus vannamei | Resistance against Vibrio parahaemolyticus ↑ Acute hepatopancreatic necrosis ↓ | August 2024 | [116] |
| Thailand | Kratom leaf extract (Mitragyna speciose) Nile tilapia (Oreochromis niloticus) | Expression of IL6, IL8, NF-kB, IFN gamma, TNF alpha, CC-chemokine, MHC-II beta, CD4, TCR beta, IgT, IgM, IgD ↑ Resistance against Edwardsiella tarda ↑ | Sept. 2024 | [117] |
| USA | Lactococcus lactis (MA5) catfish (Ictalurus punctatus × I. furcatus) | Inhibition of catfish bacterial pathogens ↑ Antibiotic susceptibility ↑ | Oct. 2024 | [118] |
| Spain | Lactic acid bacteria (LAB) LAB and pathogens were obtained from mucus of Oncorhynchusmykiss and Salmo trutta | Susceptibility to oxytetracycline ↑ Antagonistic against Aeromonas salmonicida subsp. salmonicida, Carnobacterium maltaromaticum, Vagococcus salmoninarum, Yersinia ruckeri, Lactococcus garvieae, and Vibrio jasicida ↑ | Jan. 2024 | [119] |
| Malaysia | Lactobacillus plantarum L20 and Sargassum polycystum-supplemented diet Black tiger shrimp (Penaeus monodon) | Resistant against Acute hepatopancreatic necrosis caused by Vibrio parahaemolyticus ↑ Immune response ↑ | Feb. 2024 | [120] |
| Colombia | Cetobacterium sp. nov. C33 Nile Tilapia (Oreochromis niloticus) | Gastrointestinal adaptability ↑ Ability to adhere to intestinal epithelial cells ↑ Producing antimicrobial substances ↑ | Dec. 2023 | [121] |
| Indonesia | Serratia marcescens Van80 UB3 and Shewanella algae A3 White Shrimp (Litopenaeus vannamei) | Digestive enzyme ↑ Growth-related genes ↑ Improved immunity ↑ | May 2025 | [122] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2024: Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar]
- Shen, X.; Jin, G.; Zhao, Y.; Shao, X. Prevalence and Distribution Analysis of Antibiotic Resistance Genes in a Large-Scale Aquaculture Environment. Sci. Total Environ. 2020, 711, 134626. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture. Int. J. Fish. Aquac 2018, 10, 1–7. [Google Scholar]
- Boyd, C.E.; McNevin, A.A.; Davis, R.P. The Contribution of Fisheries and Aquaculture to the Global Protein Supply. Food Sec. 2022, 14, 805–827. [Google Scholar] [CrossRef]
- Lewbart, G.A. Bacteria and Ornamental Fish. Semin. Avian Exot. Pet Med. 2001, 10, 48–56. [Google Scholar] [CrossRef]
- Dawood, M.A.; Koshio, S.; Esteban, M.Á. Beneficial Roles of Feed Additives as Immunostimulants in Aquaculture: A Review. Rev. Aquac. 2017, 10, 950–974. [Google Scholar] [CrossRef]
- Chen, J.; Sun, R.; Pan, C.; Sun, Y.; Mai, B.; Li, Q.X. Antibiotics and Food Safety in Aquaculture. J. Agric. Food Chem. 2020, 68, 11908–11919. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Rheman, S.; Debnath, N.; Delamare-Deboutteville, J.; Akhtar, Z.; Ghosh, S.; Parveen, S.; Islam, K.; Islam, M.A.; Rashid, M.M.; et al. Antibiotics Usage Practices in Aquaculture in Bangladesh and Their Associated Factors. One Health 2022, 15, 100445. [Google Scholar] [CrossRef]
- Reda, R.M.; Ibrahim, R.E.; Ahmed, E.-N.G.; El-Bouhy, Z.M. Effect of Oxytetracycline and Florfenicol as Growth Promoters on the Health Status of Cultured Oreochromis niloticus. Egypt. J. Aquat. Res. 2013, 39, 241–248. [Google Scholar] [CrossRef]
- Shalaby, A.M.; Khattab, Y.A.; Abdel Rahman, A.M. Effects of Garlic (Alliumsativum) and Chloramphenicol on Growth Performance, Physiological Parameters and Survival of Nile Tilapia (Oreochromis niloticus). J. Venom. Anim. Toxins Incl. Trop. Dis. 2006, 12, 172–201. [Google Scholar] [CrossRef]
- Gao, P.; Mao, D.; Luo, Y.; Wang, L.; Xu, B.; Xu, L. Occurrence of Sulfonamide and Tetracycline-Resistant Bacteria and Resistance Genes in Aquaculture Environment. Water Res. 2012, 46, 2355–2364. [Google Scholar] [CrossRef]
- Elsabagh, M.; Mohamed, R.; Moustafa, E.M.; Hamza, A.; Farrag, F.; Decamp, O.; Dawood, M.A.O.; Eltholth, M. Assessing the Impact of Bacillus Strains Mixture Probiotic on Water Quality, Growth Performance, Blood Profile and Intestinal Morphology of Nile Tilapia, Oreochromis niloticus. Aquacult. Nutr. 2018, 24, 1613–1622. [Google Scholar] [CrossRef]
- Watts, J.; Schreier, H.; Lanska, L.; Hale, M. The Rising Tide of Antimicrobial Resistance in Aquaculture: Sources, Sinks and Solutions. Mar. Drugs 2017, 15, 158. [Google Scholar] [CrossRef] [PubMed]
- Won, S.; Hamidoghli, A.; Choi, W.; Bae, J.; Jang, W.J.; Lee, S.; Bai, S.C. Evaluation of Potential Probiotics Bacillus Subtilis WB60, Pediococcus Pentosaceus, and Lactococcus Lactis on Growth Performance, Immune Response, Gut Histology and Immune-Related Genes in Whiteleg Shrimp, Litopenaeus Vannamei. Microorganisms 2020, 8, 281. [Google Scholar] [CrossRef]
- Huss, H.H.; Reilly, A.; Karim Ben Embarek, P. Prevention and Control of Hazards in Seafood. Food Control 2000, 11, 149–156. [Google Scholar] [CrossRef]
- Liu, X.; Steele, J.C.; Meng, X.-Z. Usage, Residue, and Human Health Risk of Antibiotics in Chinese Aquaculture: A Review. Environ. Pollut. 2017, 223, 161–169. [Google Scholar] [CrossRef]
- Huang, L.; Xu, Y.-B.; Xu, J.-X.; Ling, J.-Y.; Chen, J.-L.; Zhou, J.-L.; Zheng, L.; Du, Q.-P. Antibiotic Resistance Genes (ARGs) in Duck and Fish Production Ponds with Integrated or Non-Integrated Mode. Chemosphere 2017, 168, 1107–1114. [Google Scholar] [CrossRef]
- Chen, X.; He, Z.; Zhao, J.; Liao, M.; Xue, Y.; Zhou, J.; Sun, C. Metagenomic Analysis of Bacterial Communities and Antibiotic Resistance Genes in Penaeus monodon Biofloc-Based Aquaculture Environments. Front. Mar. Sci. 2022, 8, 762345. [Google Scholar] [CrossRef]
- Sun, S.; Korheina, D.K.A.; Fu, H.; Ge, X. Chronic Exposure to Dietary Antibiotics Affects Intestinal Health and Antibiotic Resistance Gene Abundance in Oriental River Prawn (Macrobrachium Nipponense), and Provokes Human Health Risk. Sci. Total Environ. 2020, 720, 137478. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Lajqi Berisha, N.; Poceva Panovska, A.; Hajrulai-Musliu, Z. Antibiotic Resistance and Aquatic Systems: Importance in Public Health. Water 2024, 16, 2362. [Google Scholar] [CrossRef]
- Neela, F.A.; Nonaka, L.; Rahman, M.H.; Suzuki, S. Transfer of the Chromosomally Encoded Tetracycline Resistance Gene Tet(M) from Marine Bacteria to Escherichia Coli and Enterococcus Faecalis. World J. Microbiol. Biotechnol. 2009, 25, 1095–1101. [Google Scholar] [CrossRef]
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human Health Risk Assessment of Antibiotic Resistance Associated with Antibiotic Residues in the Environment: A Review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef]
- Wallin, J.A. Bibliometric Methods: Pitfalls and Possibilities. Basic Clin. Pharma. Tox. 2005, 97, 261–275. [Google Scholar] [CrossRef]
- Van, R.A. Advances in Bibliometric Analysis: Research Performance Assessment and Science Mapping. Bibliometr. Use Abus. Rev. Res. Perform. 2014, 87, 17–28. [Google Scholar]
- Lawani, S.M. Bibliometrics: Its Theoretical Foundations, Methods and Applications. Libri 1981, 31, 294–315. [Google Scholar] [CrossRef]
- Pritchard, A. Statistical Bibliography or Bibliometrics. J. Doc. 1969, 25, 348–349. [Google Scholar]
- Mohammed, E.A.H.; Ahmed, A.E.M.; Kovács, B.; Pál, K. The Significance of Probiotics in Aquaculture: A Review of Research Trend and Latest Scientific Findings. Antibiotics 2025, 14, 242. [Google Scholar] [CrossRef]
- Su, Y.; Yu, Y.; Zhang, N. Carbon Emissions and Environmental Management Based on Big Data and Streaming Data: A Bibliometric Analysis. Sci. Total Environ. 2020, 733, 138984. [Google Scholar] [CrossRef] [PubMed]
- Traça, A.B.; Campos, S.; Dionisio, A.; Jesus, M.; Santos, J.; Mata, F. Sustainability of Meat Value Chain: Bibliometric Review of Main Trends and Theoretical Connections. Sustainability 2025, 17, 1773. [Google Scholar] [CrossRef]
- Villagran, E.; Espitia, J.J.; Rodriguez, J.; Gomez, L.; Amado, G.; Baeza, E.; Aguilar-Rodríguez, C.E.; Flores-Velazquez, J.; Akrami, M.; Gil, R.; et al. Use of Lighting Technology in Controlled and Semi-Controlled Agriculture in Greenhouses and Protected Agriculture Systems—Part 1: Scientific and Bibliometric Analysis. Sustainability 2025, 17, 1712. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, H.; Wang, C.; Gao, H.; Wang, Y.; Xu, J. Trends in and Future Research Direction of Antimicrobial Resistance in Global Aquaculture Systems: A Review. Sustainability 2023, 15, 9012. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y. Research Trends and Areas of Focus on the Chinese Loess Plateau: A Bibliometric Analysis during 1991–2018. CATENA 2020, 194, 104798. [Google Scholar] [CrossRef]
- Şahin, S.; Sivri, N.; Akpinar, I.; Çinçin, Z.B.; Sönmez, V.Z. A Comprehensive Bibliometric Overview: Antibiotic Resistance and Escherichia Coli in Natural Water. Environ. Sci. Pollut. Res. 2021, 28, 32256–32263. [Google Scholar] [CrossRef]
- Zheng, C.; Liao, H.; Tu, C. An Improved Bibliometric Analysis on Antibiotics in Soil Research. Bull. Environ. Contam. Toxicol. 2022, 108, 276–283. [Google Scholar] [CrossRef]
- Castronovo, C.; Agozzino, V.; Schirò, G.; Mira, F.; Di Bella, S.; Lastra, A.; Antoci, F.; Pennisi, M.; Giudice, E.; Guercio, A. Evaluation of the Antimicrobial Resistance of Different Serotypes of Salmonella Enterica from Livestock Farms in Southern Italy. Appl. Sci. 2022, 13, 442. [Google Scholar] [CrossRef]
- Santos, L.; Ramos, F. Antimicrobial Resistance in Aquaculture: Current Knowledge and Alternatives to Tackle the Problem. Int. J. Antimicrob. Agents 2018, 52, 135–143. [Google Scholar] [CrossRef]
- Agersø, Y.; Guardabassi, L. Identification of Tet 39, a Novel Class of Tetracycline Resistance Determinant in Acinetobacter Spp. of Environmental and Clinical Origin. J. Antimicrob. Chemother. 2005, 55, 566–569. [Google Scholar] [CrossRef]
- Thornber, K.; Verner-Jeffreys, D.; Hinchliffe, S.; Rahman, M.M.; Bass, D.; Tyler, C.R. Evaluating Antimicrobial Resistance in the Global Shrimp Industry. Rev. Aquac. 2020, 12, 966–986. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre-Tudó, J.L.; Castelló-Cogollos, L.; Aleixandre, J.L.; Aleixandre-Benavent, R. Emerging Topics in Scientific Research on Global Water-Use Efficiency. J. Agric. Sci. 2019, 157, 480–492. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. Bibliometrix: An R-Tool for Comprehensive Science Mapping Analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Choruma, D.J.; Balkovic, J.; Odume, O.N. Calibration and Validation of the EPIC Model for Maize Production in the Eastern Cape, South Africa. Agronomy 2019, 9, 494. [Google Scholar] [CrossRef]
- Lulijwa, R.; Rupia, E.J.; Alfaro, A.C. Antibiotic Use in Aquaculture, Policies and Regulation, Health and Environmental Risks: A Review of the Top 15 Major Producers. Rev. Aquac. 2020, 12, 640–663. [Google Scholar] [CrossRef]
- Li, J.-Y.; Wen, J.; Chen, Y.; Wang, Q.; Yin, J. Antibiotics in Cultured Freshwater Products in Eastern China: Occurrence, Human Health Risks, Sources, and Bioaccumulation Potential. Chemosphere 2021, 264, 128441. [Google Scholar] [CrossRef]
- Schar, D.; Klein, E.Y.; Laxminarayan, R.; Gilbert, M.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Aquaculture. Sci. Rep. 2020, 10, 21878. [Google Scholar] [CrossRef]
- Hyland, K. Enter the Dragon: China and Global Academic Publishing. Learn. Publ. 2023, 36, 394–403. [Google Scholar] [CrossRef]
- Cabello, F.C. Heavy Use of Prophylactic Antibiotics in Aquaculture: A Growing Problem for Human and Animal Health and for the Environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef]
- Verschuere, L.; Rombaut, G.; Sorgeloos, P.; Verstraete, W. Probiotic Bacteria as Biological Control Agents in Aquaculture. Microbiol. Mol. Biol. Rev. 2000, 64, 655–671. [Google Scholar] [CrossRef]
- Martinez, J.L. Environmental Pollution by Antibiotics and by Antibiotic Resistance Determinants. Environ. Pollut. 2009, 157, 2893–2902. [Google Scholar] [CrossRef]
- Nikaido, H. Multidrug Resistance in Bacteria. Annu. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef]
- Sapkota, A.; Sapkota, A.R.; Kucharski, M.; Burke, J.; McKenzie, S.; Walker, P.; Lawrence, R. Aquaculture Practices and Potential Human Health Risks: Current Knowledge and Future Priorities. Environ. Int. 2008, 34, 1215–1226. [Google Scholar] [CrossRef]
- Zainab, S.M.; Junaid, M.; Xu, N.; Malik, R.N. Antibiotics and Antibiotic Resistant Genes (ARGs) in Groundwater: A Global Review on Dissemination, Sources, Interactions, Environmental and Human Health Risks. Water Res. 2020, 187, 116455. [Google Scholar] [CrossRef] [PubMed]
- Cabello, F.C.; Godfrey, H.P.; Tomova, A.; Ivanova, L.; Dölz, H.; Millanao, A.; Buschmann, A.H. Antimicrobial Use in Aquaculture Re-examined: Its Relevance to Antimicrobial Resistance and to Animal and Human Health. Environ. Microbiol. 2013, 15, 1917–1942. [Google Scholar] [CrossRef]
- Pruden, A.; Larsson, D.G.J.; Amézquita, A.; Collignon, P.; Brandt, K.K.; Graham, D.W.; Lazorchak, J.M.; Suzuki, S.; Silley, P.; Snape, J.R.; et al. Management Options for Reducing the Release of Antibiotics and Antibiotic Resistance Genes to the Environment. Env. Health Perspect. 2013, 121, 878–885. [Google Scholar] [CrossRef]
- Gothwal, R.; Shashidhar, T. Antibiotic Pollution in the Environment: A Review. CLEAN Soil Air Water 2015, 43, 479–489. [Google Scholar] [CrossRef]
- Defoirdt, T.; Sorgeloos, P.; Bossier, P. Alternatives to Antibiotics for the Control of Bacterial Disease in Aquaculture. Curr. Opin. Microbiol. 2011, 14, 251–258. [Google Scholar] [CrossRef]
- Zou, S.; Xu, W.; Zhang, R.; Tang, J.; Chen, Y.; Zhang, G. Occurrence and Distribution of Antibiotics in Coastal Water of the Bohai Bay, China: Impacts of River Discharge and Aquaculture Activities. Environ. Pollut. 2011, 159, 2913–2920. [Google Scholar] [CrossRef] [PubMed]
- Vieco-Saiz, N.; Belguesmia, Y.; Raspoet, R.; Auclair, E.; Gancel, F.; Kempf, I.; Drider, D. Benefits and Inputs From Lactic Acid Bacteria and Their Bacteriocins as Alternatives to Antibiotic Growth Promoters During Food-Animal Production. Front. Microbiol. 2019, 10, 57. [Google Scholar] [CrossRef]
- Heuer, O.E.; Kruse, H.; Grave, K.; Collignon, P.; Karunasagar, I.; Angulo, F.J. Human Health Consequences of Use of Antimicrobial Agents in Aquaculture. Clin. Infect. Dis. 2009, 49, 1248–1253. [Google Scholar] [CrossRef]
- Song, S.K.; Beck, B.R.; Kim, D.; Park, J.; Kim, J.; Kim, H.D.; Ringø, E. Prebiotics as Immunostimulants in Aquaculture: A Review. Fish Shellfish. Immunol. 2014, 40, 40–48. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Alagawany, M.; Patra, A.K.; Kar, I.; Tiwari, R.; Dawood, M.A.O.; Dhama, K.; Abdel-Latif, H.M.R. The Functionality of Probiotics in Aquaculture: An Overview. Fish Shellfish Immunol. 2021, 117, 36–52. [Google Scholar] [CrossRef]
- Romero, J.; Gloria, C.; Navarrete, P. Antibiotics in Aquaculture–Use, Abuse and Alternatives. In Health and Environment in Aquaculture; Carvalho, E., Ed.; InTech: Hong Kong, China, 2012; ISBN 978-953-51-0497-1. [Google Scholar]
- Akinbowale, O.L.; Peng, H.; Barton, M.D. Antimicrobial Resistance in Bacteria Isolated from Aquaculture Sources in Australia. J. Appl. Microbiol. 2006, 100, 1103–1113. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015; ISBN 978-92-4-150976-3. [Google Scholar]
- Hemamalini, N.; Shanmugam, S.A.; Kathirvelpandian, A.; Deepak, A.; Kaliyamurthi, V.; Suresh, E. A Critical Review on the Antimicrobial Resistance, Antibiotic Residue and Metagenomics—Assisted Antimicrobial Resistance Gene Detection in Freshwater Aquaculture Environment. Aquac. Res. 2022, 53, 344–366. [Google Scholar] [CrossRef]
- Nogueira, T.; Botelho, A. Metagenomics and Other Omics Approaches to Bacterial Communities and Antimicrobial Resistance Assessment in Aquacultures. Antibiotics 2021, 10, 787. [Google Scholar] [CrossRef]
- Mullany, P. Functional Metagenomics for the Investigation of Antibiotic Resistance. Virulence 2014, 5, 443–447. [Google Scholar] [CrossRef]
- Wang, J.-H.; Lu, J.; Zhang, Y.-X.; Wu, J.; Luo, Y.; Liu, H. Metagenomic Analysis of Antibiotic Resistance Genes in Coastal Industrial Mariculture Systems. Bioresour. Technol. 2018, 253, 235–243. [Google Scholar] [CrossRef]
- Zeng, Q.; Tian, X.; Wang, L. Genetic Adaptation of Microbial Populations Present in High-Intensity Catfish Production Systems with Therapeutic Oxytetracycline Treatment. Sci. Rep. 2017, 7, 17491. [Google Scholar] [CrossRef]
- Lu, T.-H.; Chen, C.-Y.; Wang, W.-M.; Liao, C.-M. A Risk-Based Approach for Managing Aquaculture Used Oxytetracycline-Induced TetR in Surface Water Across Taiwan Regions. Front. Pharmacol. 2021, 12, 803499. [Google Scholar] [CrossRef]
- Caputo, A.; Bondad-Reantaso, M.G.; Karunasagar, I.; Hao, B.; Gaunt, P.; Verner-Jeffreys, D.; Fridman, S.; Dorado-Garcia, A. Antimicrobial Resistance in Aquaculture: A Global Analysis of Literature and National Action Plans. Rev. Aquac. 2023, 15, 568–578. [Google Scholar] [CrossRef]
- Preena, P.G.; Swaminathan, T.R.; Kumar, V.J.R.; Singh, I.S.B. Antimicrobial Resistance in Aquaculture: A Crisis for Concern. Biologia 2020, 75, 1497–1517. [Google Scholar] [CrossRef]
- Fossen, J.D.; Campbell, J.R.; Gow, S.P.; Erickson, N.; Waldner, C.L. Antimicrobial Resistance in Enterococcus Isolated from Western Canadian Cow-Calf Herds. BMC Vet. Res. 2024, 20, 6. [Google Scholar] [CrossRef]
- Petersen, A.; Dalsgaard, A. Antimicrobial Resistance of Intestinal Aeromonas spp. and Enterococcus spp. in Fish Cultured in Integrated Broiler-Fish Farms in Thailand. Aquaculture 2003, 219, 71–82. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Aerts, M.; Baron, S.; Bortolaia, V.; Hendriksen, R.; Guerra, B.; Stoicescu, A.; Beloeil, P. Technical Specifications for a EU-wide Baseline Survey of Antimicrobial Resistance in Bacteria from Aquaculture Animals. EFS2 2024, 22, e8928. [Google Scholar] [CrossRef]
- Mestrovic, T.; Robles Aguilar, G.; Swetschinski, L.R.; Ikuta, K.S.; Gray, A.P.; Davis Weaver, N.; Han, C.; Wool, E.E.; Gershberg Hayoon, A.; Hay, S.I.; et al. The Burden of Bacterial Antimicrobial Resistance in the WHO European Region in 2019: A Cross-Country Systematic Analysis. Lancet Public Health 2022, 7, e897–e913. [Google Scholar] [CrossRef]
- Saticioglu, I.B.; Ajmi, N.; Coskuner-Weber, O.; Alpsoy, S.; Ay, H.; Aydin, F.; Abay, S.; Karakaya, E.; Kayman, T.; Dalyan, C.; et al. Three New Microbacterium Species Isolated from the Marmara Sea Mucilage Event: Microbacterium Istanbulense sp. nov., Microbacterium Bandirmense sp. nov., Microbacterium Marmarense sp. nov. Syst. Appl. Microbiol. 2025, 48, 126600. [Google Scholar] [CrossRef]
- Ortiz-Severín, J.; Hojas, I.; Redin, F.; Serón, E.; Santana, J.; Maass, A.; Cambiazo, V. From Metagenomes to Functional Expression of Resistance: FloR Gene Diversity in Bacteria from Salmon Farms. Antibiotics 2025, 14, 122. [Google Scholar] [CrossRef]
- Islam, M.M.M.; Islam, A.; Neela, F.A.; Hasanuzzaman, A.F. Occurrence of Antibiotic-Resistant Bacteria in Urban Surface Water Sources in Bangladesh. Curr. Microbiol. 2025, 82, 96. [Google Scholar] [CrossRef]
- Wu, C.; Ye, H.; Xu, M.; Zhao, X.; Zhao, X.; Li, L.; Li, M.; Wei, Y.; Li, Y.; Hu, B. Occurrence of Antibiotics and Antibiotic Resistance Genes at Various Stages of Different Aquaculture Modes Surrounding Tai Lake, China. Front. Microbiol. 2025, 16, 1543387. [Google Scholar] [CrossRef]
- Yu, Z.; Yin, X.; Lu, B.; Zhang, L.; Ma, Y.; Chen, Y.; Feng, Y.; Han, C.; Shu, H. Identification and Characteristics of Plesiomonas Shigelloides Isolated from Mastacembelus Armatus. Aquac. Rep. 2024, 39, 102414. [Google Scholar] [CrossRef]
- Ben Natan, M.; Masasa, M.; Shashar, N.; Guttman, L. Antibiotic Resistance in Vibrio Bacteria Associated with Red Spotting Disease in Sea Urchin Tripneustes Gratilla (Echinodermata). Microorganisms 2024, 12, 2460. [Google Scholar] [CrossRef]
- Huang, W.; Lin, C.; Wen, C.; Jiang, B.; Su, Y. Analysis of the Prevalence of Bacterial Pathogens and Antimicrobial Resistance Patterns of Edwardsiella Piscicida in Largemouth Bass (Micropterus salmoides) from Guangdong, China. Pathogens 2024, 13, 987. [Google Scholar] [CrossRef]
- Tsai, M.-A.; Chen, I.-C.; Chen, Z.-W.; Li, T.-H. Further Evidence of Anthropogenic Impact: High Levels of Multiple-Antimicrobial-Resistant Bacteria Found in Neritic-Stage Sea Turtles. Antibiotics 2024, 13, 998. [Google Scholar] [CrossRef]
- Nadella, R.K.; Panda, S.K.; Uchoi, D.; Kishore, P.; Chintada, B.; Madhu, V.R.; Minimol, V.A.; Badireddy, M.R.; Kuricheti, P.P.; Raman, R.P.; et al. Categorization of Antibiotic Resistant Bacterial Populations from Shrimp and Its Culture Environment of Andhra Pradesh, India. Aquaculture 2025, 595, 741702. [Google Scholar] [CrossRef]
- Zhihong, H.; Cuiyu, L.; Min, G.; Dikuang, P.; Chuchu, L.; Kecheng, Z.; Xueming, D.; Min, Y.; Xuezhu, L. Identification, Genome-Wide Sequencing and Analysis of Drug Resistance Genes in a Novel Vibrio Harveyi Strain Isolated from Yellowfin Seabream. Aquaculture 2025, 595, 741527. [Google Scholar] [CrossRef]
- Lu, J.; Wu, J.; Zhang, C.; Wang, J.; He, X. Occurrence and Possible Sources of Antibiotic Resistance Genes in Seawater of the South China Sea. Front. Environ. Sci. Eng. 2024, 18, 108. [Google Scholar] [CrossRef]
- Vishnupriya, V.; Swaminathan, T.R.; Dharmarathnam, A.; Sharma, S.R.K.; Preena, P.G. Virulent and Multi-Drug-Resistant Edwardsiella Tarda Infection in Oscar Fish: Unveiling the Threat of Mass Mortality and AMR Dissemination. Curr. Microbiol. 2024, 81, 174. [Google Scholar] [CrossRef]
- Cui, K.; Wang, S.; Pei, Y.; Zhou, B. Occurrence and Distribution of Antibiotic Pollution and Antibiotic Resistance Genes in Seagrass Meadow Sediments Based on Metagenomics. Sci. Total Environ. 2024, 935, 173438. [Google Scholar] [CrossRef]
- Amin, M.B.; Talukdar, P.K.; Sraboni, A.S.; Islam, M.R.; Mahmud, Z.H.; Berendes, D.; Narrod, C.; Parveen, S.; Islam, M.A. Prevalence and Antimicrobial Resistance of Major Foodborne Pathogens Isolated from Pangas and Tilapia Fish Sold in Retail Markets of Dhaka City, Bangladesh. Int. J. Food Microbiol. 2024, 418, 110717. [Google Scholar] [CrossRef]
- Ismail, E.T.; El-Son, M.A.M.; El-Gohary, F.A.; Zahran, E. Prevalence, Genetic Diversity, and Antimicrobial Susceptibility of Vibrio Spp. Infected Gilthead Sea Breams from Coastal Farms at Damietta, Egypt. BMC Vet. Res. 2024, 20, 129. [Google Scholar] [CrossRef]
- Torres-Maravilla, E.; Parra, M.; Maisey, K.; Vargas, R.A.; Cabezas-Cruz, A.; Gonzalez, A.; Tello, M.; Bermúdez-Humarán, L.G. Importance of Probiotics in Fish Aquaculture: Towards the Identification and Design of Novel Probiotics. Microorganisms 2024, 12, 626. [Google Scholar] [CrossRef]
- Nayak, S. Probiotics and Immunity: A Fish Perspective. Fish Shellfish Immunol. 2010, 29, 2–14. [Google Scholar] [CrossRef]
- Mohammed, E.A.H.; Fehér, M.; Bársony, P.; Pál, K. Alteration in Gut Microbiome of Common Carp (Cyprinus carpio L., 1758) Mediated by Probiotics and Yeast Prebiotic. Biol. Life Sci. Forum 2025, 45, 1. [Google Scholar] [CrossRef]
- Han, C.; Shi, H.; Cui, C.; Wang, J.; Li, L.; Bei, W.; Cai, Y.; Wang, S. Strain-Specific Benefits of Bacillus on Growth, Intestinal Health, Immune Modulation, and Ammonia-Nitrogen Stress Resilience in Hybrid Grouper. Antioxidants 2024, 13, 317. [Google Scholar] [CrossRef]
- Putra, A.N.; Mustahal, M.; Syamsunarno, M.B.; Hermawan, D.; Fatimah, D.G.; Putri, P.B.; Sevia, S.; Isnaeni, R.; Herjayanto, M. Dietary Bacillus NP5 Supplement Impacts on Growth, Nutrient Digestibility, Immune Response, and Resistance to Aeromonas Hydrophila Infection of African Catfish, Clarias Gariepinus. Biodiversitas 2021, 22, 131. [Google Scholar] [CrossRef]
- Zhou, X.; Tian, Z.; Wang, Y.; Li, W. Effect of Treatment with Probiotics as Water Additives on Tilapia (Oreochromis niloticus) Growth Performance and Immune Response. Fish Physiol. Biochem 2010, 36, 501–509. [Google Scholar] [CrossRef]
- Waiyamitra, P.; Zoral, M.A.; Saengtienchai, A.; Luengnaruemitchai, A.; Decamp, O.; Gorgoglione, B.; Surachetpong, W. Probiotics Modulate Tilapia Resistance and Immune Response against Tilapia Lake Virus Infection. Pathogens 2020, 9, 919. [Google Scholar] [CrossRef]
- Costa, L.; Lima, J.; Siqueira, A.; Da Silva, E.; Lima, V.; Da Silva, L.; Da Silva, S.; Santos, T.; Fugimura, M.; Vaz, L.; et al. Effect of Multi-species Probiotic Administration in Colossoma Macropomum Juvenile Rearing: Supplementation and Bioremediation. Aquacult. Nutr. 2021, 27, 1721–1729. [Google Scholar] [CrossRef]
- Ramesh, D.; Souissi, S. Effects of Potential Probiotic Bacillus Subtilis KADR1 and Its Subcellular Components on Immune Responses and Disease Resistance in Labeo Rohita. Aquac. Res. 2018, 49, 367–377. [Google Scholar] [CrossRef]
- Aguirre-Guzmán, G. The Use of Probiotics in Aquatic Organisms: A Review. Afr. J. Microbiol. Res. 2012, 6, 4845–4857. [Google Scholar] [CrossRef]
- Jahangiri, L.; Esteban, M.Á. Administration of Probiotics in the Water in Finfish Aquaculture Systems: A Review. Fishes 2018, 3, 33. [Google Scholar] [CrossRef]
- Pérez-Sánchez, T.; Ruiz-Zarzuela, I.; De Blas, I.; Balcázar, J.L. Probiotics in Aquaculture: A Current Assessment. Rev. Aquac. 2014, 6, 133–146. [Google Scholar] [CrossRef]
- Wuertz, S.; Schroeder, A.; Wanka, K.M. Probiotics in Fish Nutrition—Long-Standing Household Remedy or Native Nutraceuticals? Water 2021, 13, 1348. [Google Scholar] [CrossRef]
- Jiang, N.; Hong, B.; Luo, K.; Li, Y.; Fu, H.; Wang, J. Isolation of Bacillus Subtilis and Bacillus Pumilus with Anti-Vibrio Parahaemolyticus Activity and Identification of the Anti-Vibrio Parahaemolyticus Substance. Microorganisms 2023, 11, 1667. [Google Scholar] [CrossRef]
- Aly, S.M.; Mohamed, M.F.; John, G. Effect of Probiotics on the Survival, Growth and Challenge Infection in Tilapia Nilotica (Oreochromis niloticus). Aquac. Res. 2008, 39, 647–656. [Google Scholar] [CrossRef]
- Nayak, S.K.; Mukherjee, S.C. Screening of Gastrointestinal Bacteria of Indian Major Carps for a Candidate Probiotic Species for Aquaculture Practices: Screening of Gastrointestinal Bacteria of Indian Major Carps. Aquac. Res. 2011, 42, 1034–1041. [Google Scholar] [CrossRef]
- Zapata, A.A.; Lara-Flores, M. Antimicrobial Activities of Lactic Acid Bacteria Strains Isolated from Nile Tilapia Intestine (Oreochromis niloticus). JBLS 2012, 4. [Google Scholar] [CrossRef]
- Chizhayeva, A.; Amangeldi, A.; Oleinikova, Y.; Alybaeva, A.; Sadanov, A. Lactic Acid Bacteria as Probiotics in Sustainable Development of Aquaculture. Aquat. Living Resour. 2022, 35, 10. [Google Scholar] [CrossRef]
- Dong, L.; Wang, H.; Bo, H.; Fang, J.; Liu, D.; Yue, X.; Liu, B. Probiotic Potential of Gill-Symbiotic Endozoicomonas sp. in Enhancing Vibrio Resistance in the Clam Meretrix Petechialis: A Novel Approach to Sustainable Aquaculture. Aquaculture 2025, 598, 742041. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, R.; Guo, Z.; Chen, Y.; Meng, X.; Han, Y.; Zhao, X.; Ren, T. Effects of Rhodopseudomonas Palustris and Composite Probiotics on Growth Performance, Intestinal Health, and Non-Specific Immunity of Sea Urchin (Strongylocentrotus Intermedius). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2025, 276, 111058. [Google Scholar] [CrossRef]
- Chan, C.-H.; Chen, L.-H.; Chen, K.-Y.; Chen, I.-H.; Lee, K.-T.; Lai, L.-C.; Tsai, M.-H.; Chuang, E.Y.; Lin, M.-T.; Yan, T.-R. Single-Strain Probiotics Enhance Growth, Anti-Pathogen Immunity, and Resistance to Nocardia Seriolae in Grey Mullet (Mugil Cephalus) via Gut Microbiota Modulation. Anim. Microbiome 2024, 6, 67. [Google Scholar] [CrossRef]
- Qi, X.; Luo, F.; Zhang, Y.; Wang, G.; Ling, F. Exploring the Protective Role of Bacillus Velezensis BV1704–Y in Zebrafish Health and Disease Resistance against Aeromonas Hydrophila Infection. Fish Shellfish Immunol. 2024, 152, 109789. [Google Scholar] [CrossRef]
- Małaczewska, J.; Kazuń, B.; Żylińska-Urban, J.; Kazuń, K.; Rożyński, M.; Zakęś, Z. Characterization of Two Potential Probiotic Strains of Levilactobacillus Brevis Isolated from Carp (Cyprinus Carpio L.) with Strong Immunostimulatory Activity on Pikeperch (Sander Lucioperca L.) Head Kidney Cells. Aquac. Rep. 2024, 39, 102384. [Google Scholar] [CrossRef]
- Saba, A.O.; Yasin, I.S.M.; Azmai, M.N.A. Meta-Analyses Indicate That Dietary Probiotics Significantly Improve Growth, Immune Response, and Disease Resistance in Tilapia. Aquacult. Int. 2024, 32, 4841–4867. [Google Scholar] [CrossRef]
- Xiang, L.; Zhou, Z.; Wen, M.; Jiang, G.; Cheng, J.; Hu, Y.; Qian, J.; Sun, X.; Shen, H. Study on the Effects and Mechanisms of the Antagonistic Bacterium Bacillus Subtilis JSHY-K3 against Vibrio Parahaemolyticus Causing Acute Hepatopancreatic Necrosis Disease in Shrimp Penaeus Vannamei. Aquac. Rep. 2024, 37, 102254. [Google Scholar] [CrossRef]
- Paankhao, N.; Sangsawang, A.; Kantha, P.; Paankhao, S.; Promsee, K.; Soontara, C.; Kongsriprapan, S.; Srisapoome, P.; Kumwan, B.; Meachasompop, P.; et al. Antioxidant and Antibacterial Efficiency of the Ethanolic Leaf Extract of Kratom (Mitragyna Speciosa (Korth.) Havil) and Its Effects on Growth, Health, and Disease Resistance against Edwardsiella Tarda Infection in Nile Tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2024, 152, 109771. [Google Scholar] [CrossRef]
- Huang, J.; Jordan, H.R.; Older, C.E.; Griffin, M.J.; Allen, P.J.; Wise, D.J.; Goodman, P.M.; Reifers, J.G.; Yamamoto, F.Y. Lactococcus Lactis MA5 Is a Potential Autochthonous Probiotic for Nutrient Digestibility Enhancement and Bacterial Pathogen Inhibition in Hybrid Catfish (Ictalurus Punctatus × I. Furcatus). J. Fish Dis. 2024, 47, e13997. [Google Scholar] [CrossRef]
- Vargas-González, A.; Barajas, M.; Pérez-Sánchez, T. Isolation of Lactic Acid Bacteria (LAB) from Salmonids for Potential Use as Probiotics: In Vitro Assays and Toxicity Assessment of Salmo Trutta Embryonated Eggs. Animals 2024, 14, 200. [Google Scholar] [CrossRef]
- Chin, Y.K.; Haifa-Haryani, W.O.; Nazarudin, M.F.; Ahmad, M.I.; Azzam-Sayuti, M.; Mohamad, A.; Ida-Muryany, M.Y.; Karim, M.; Salleh, A.; Amal, M.N.A.; et al. The Synergistic Lactobacillus Plantarum L20 and Sargassum Polycystum -Added Diet for Improvement of Black Tiger Shrimp, Penaeus Monodon ‘s Growth, Immune Responses, Bacterial Profiles, and Resistance against Vibrio Parahaemolyticus Associated Acute Hepatopancreatic Necrosis Disease (AHPND) Infection. Aquac. Rep. 2024, 34, 101903. [Google Scholar] [CrossRef]
- Colorado Gómez, M.A.; Melo-Bolívar, J.F.; Ruíz Pardo, R.Y.; Rodriguez, J.A.; Villamil, L.M. Unveiling the Probiotic Potential of the Anaerobic Bacterium Cetobacterium Sp. Nov. C33 for Enhancing Nile Tilapia (Oreochromis niloticus) Cultures. Microorganisms 2023, 11, 2922. [Google Scholar] [CrossRef]
- Sutanti, S.; Sukenda, S.; Widanarni, W.; Alimuddin, A.; Aliah, R.S.; Ardiansyah, A.R.; Romadhona, E.I. Indigenous Gram-Negative Bacteria as Probiotics for Pacific White Shrimp (Litopenaeus Vannamei). Curr. Microbiol. 2025, 82, 205. [Google Scholar] [CrossRef]
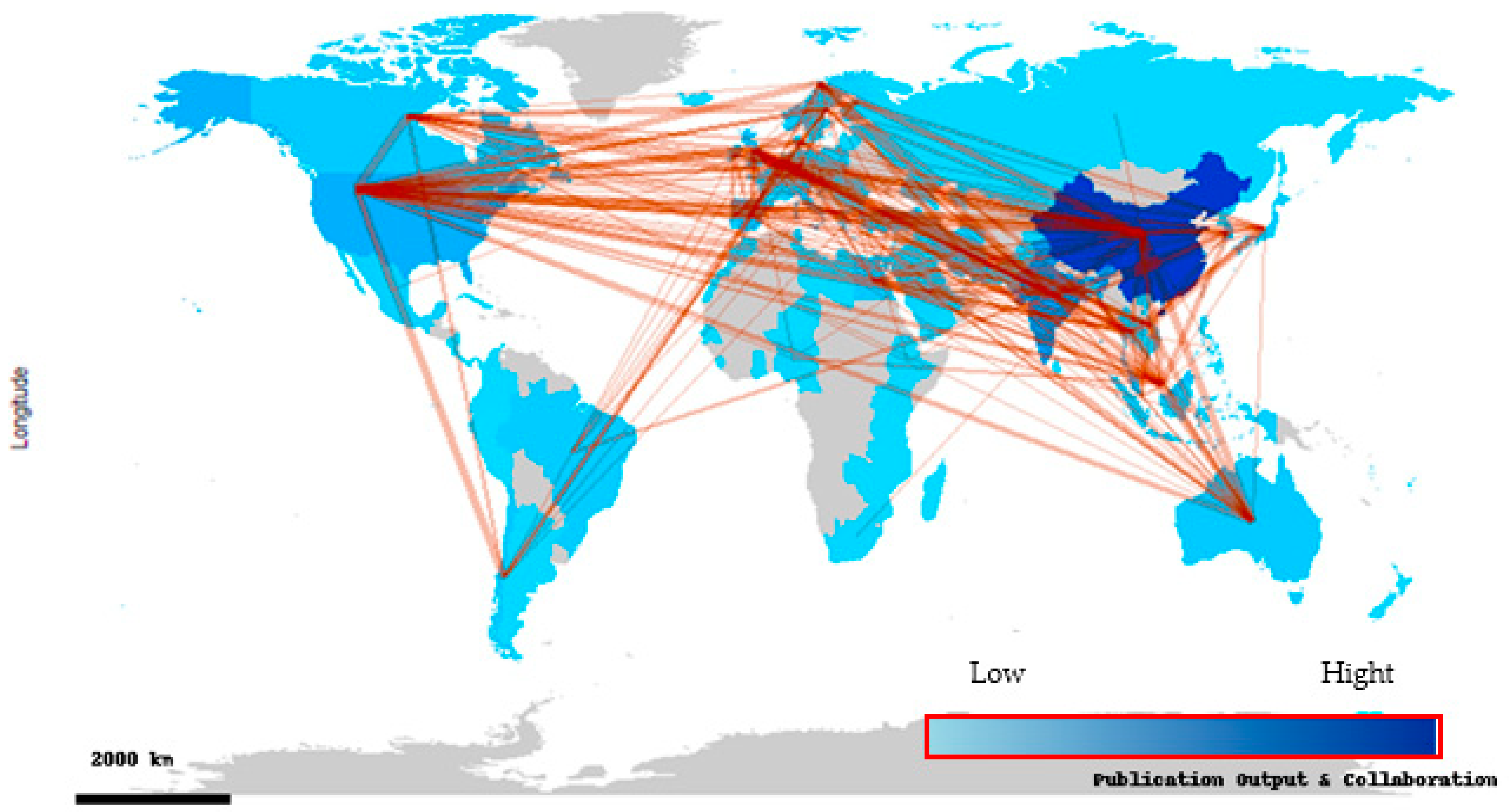
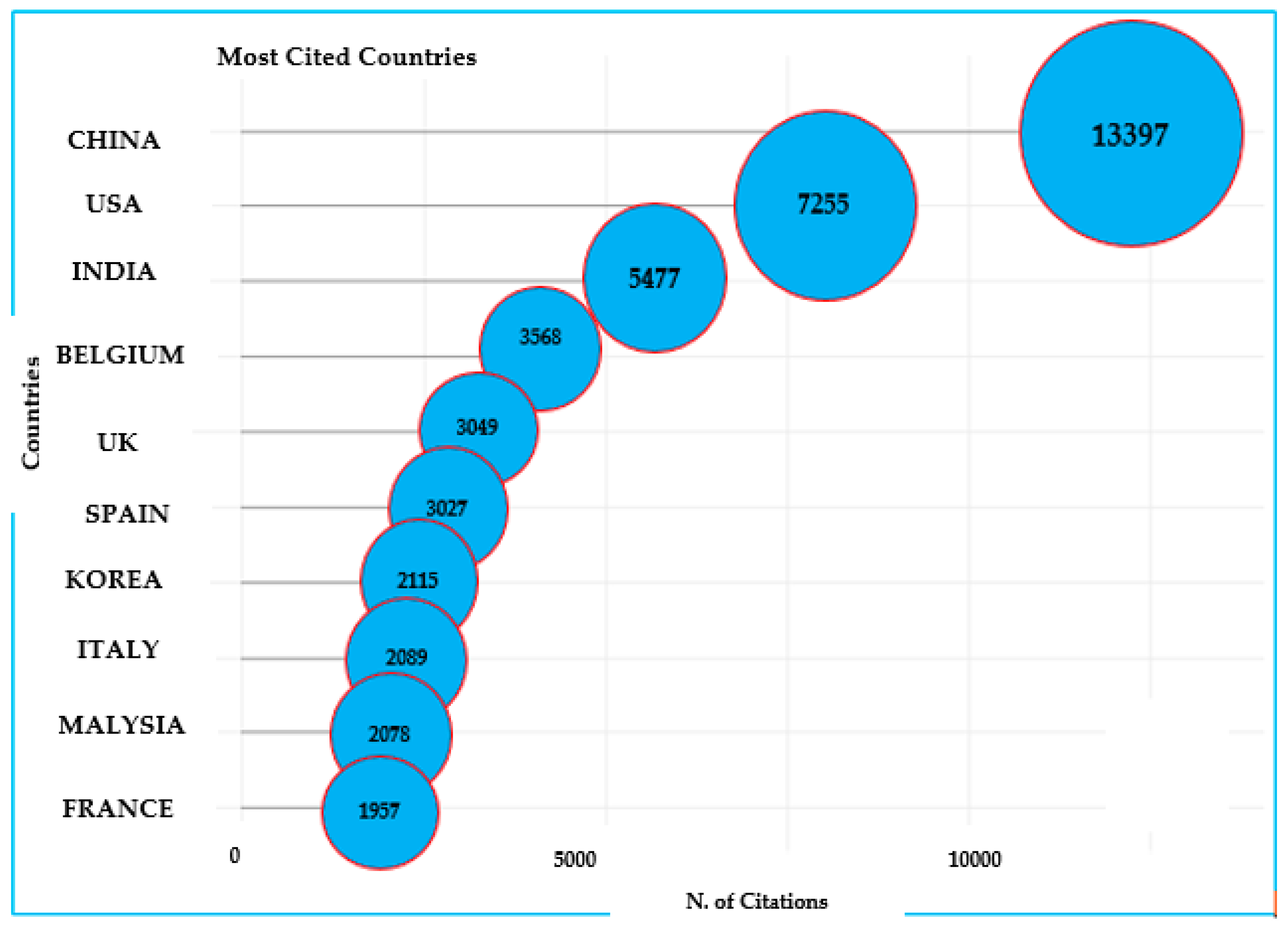
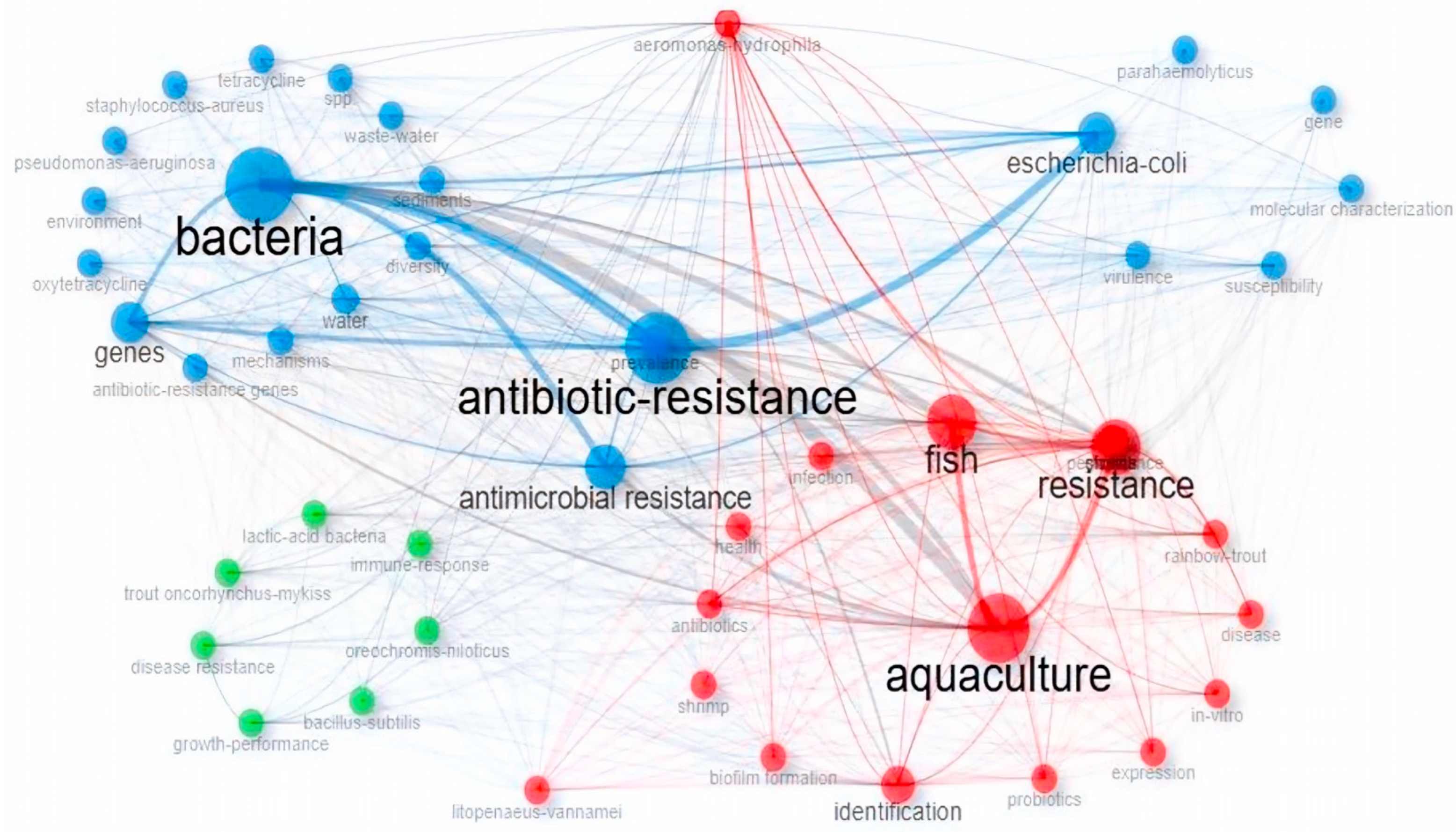
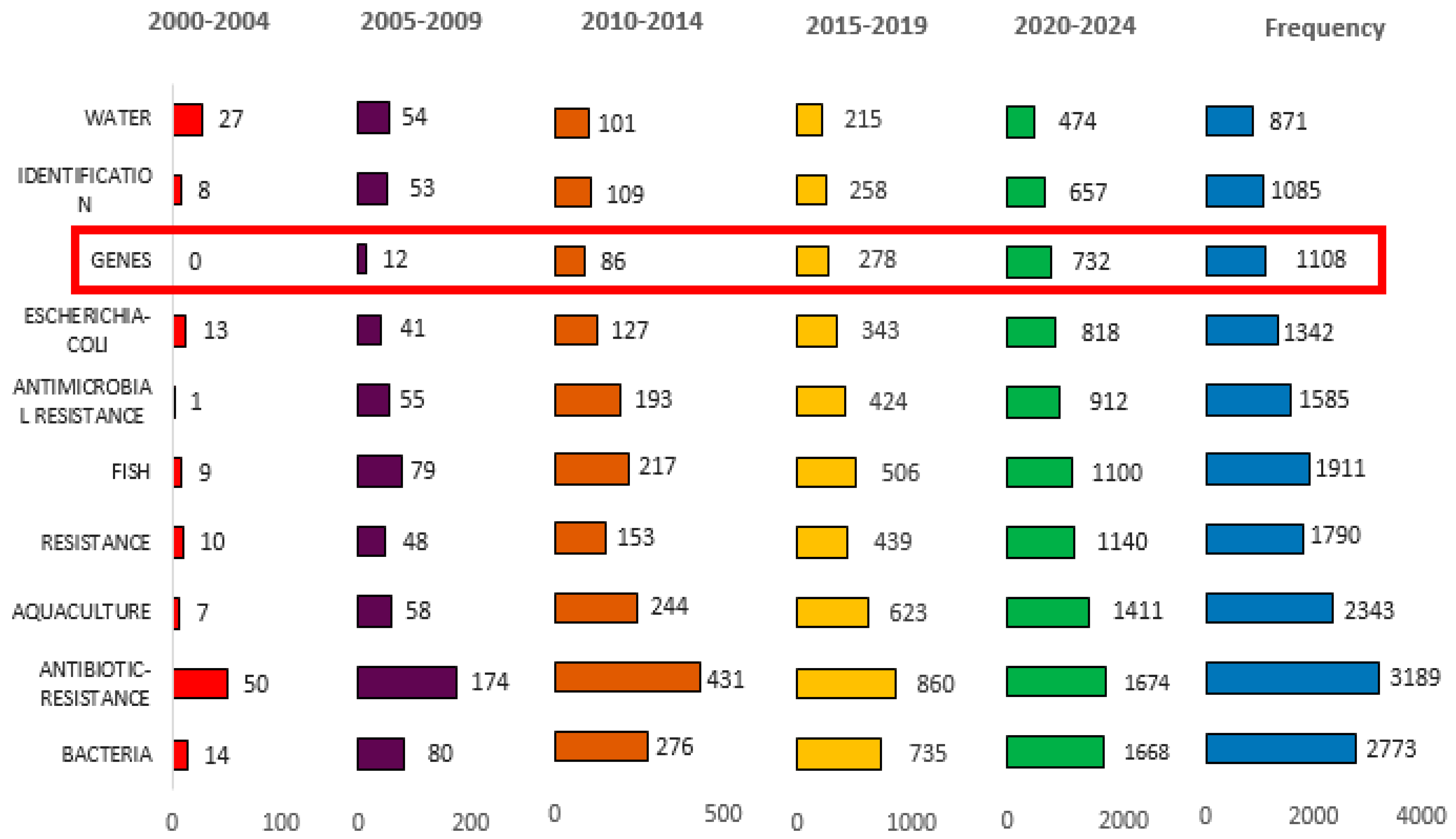
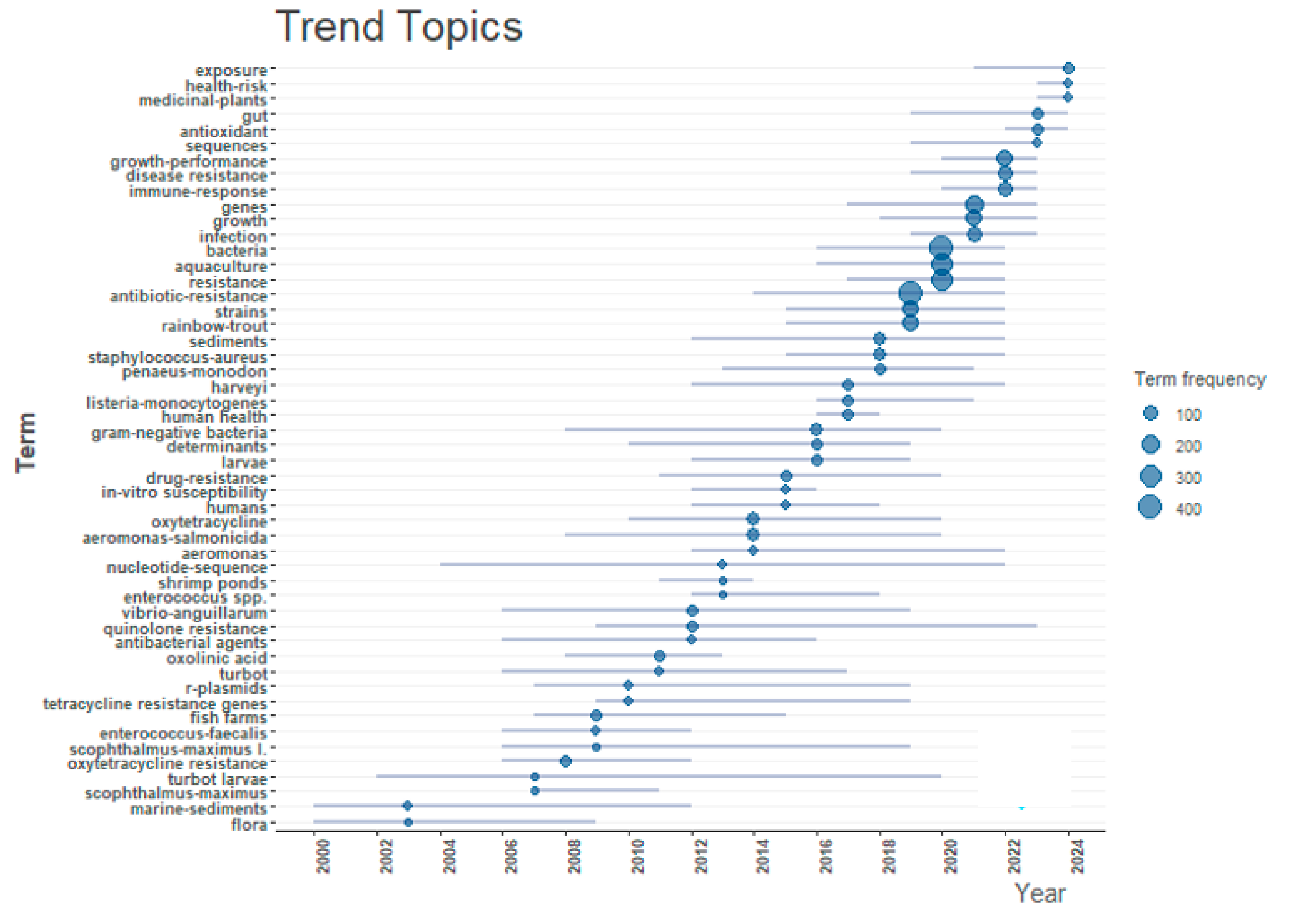
| Rank | Country | Papers | % of 1869 | SCP * | MCP | MCP Ratio |
|---|---|---|---|---|---|---|
| 1 | China | 520 | 27.82 | 442 | 78 | 15.00 |
| 2 | India | 203 | 10.86 | 170 | 33 | 16.26 |
| 3 | Malaysia | 77 | 4.12 | 46 | 31 | 40.26 |
| 4 | USA | 76 | 4.07 | 50 | 26 | 34.21 |
| 5 | Korea | 69 | 3.69 | 52 | 17 | 24.64 |
| 6 | Spain | 52 | 2.78 | 32 | 20 | 38.46 |
| 7 | United Kingdom | 46 | 2.46 | 24 | 22 | 47.83 |
| 8 | Brazil | 42 | 2.25 | 37 | 5 | 11.90 |
| 9 | Chile | 42 | 2.25 | 34 | 8 | 19.05 |
| 10 | Italy | 39 | 2.09 | 30 | 9 | 23.08 |
| 11 | Thailand | 37 | 1.98 | 14 | 23 | 62.16 |
| 12 | Egypt | 36 | 1.93 | 20 | 16 | 44.44 |
| 13 | Canada | 33 | 1.76 | 23 | 10 | 30.30 |
| 14 | Portugal | 33 | 1.76 | 24 | 9 | 27.27 |
| 15 | Turkey | 33 | 1.76 | 30 | 3 | 9.09 |
| 16 | France | 31 | 1.66 | 22 | 9 | 29.03 |
| 17 | Belgium | 29 | 1.55 | 14 | 15 | 51.72 |
| 18 | Mexico | 28 | 1.50 | 22 | 6 | 21.43 |
| 19 | Indonesia | 27 | 1.44 | 22 | 5 | 18.52 |
| 20 | Japan | 27 | 1.44 | 14 | 13 | 48.15 |
| Rank | Paper | * TC | TC per Year | Publisher | Ref. |
|---|---|---|---|---|---|
| 1 | CABELLO FC, 2006, ENVIRON MICROBIOL | 1607 | 80.35 | Wiley | [47] |
| 2 | VERSCHUERE L, 2000, MICROBIOL MOL BIOL REV | 1468 | 56.46 | American Society for Microbiology | [48] |
| 3 | LUIS MARTINEZ J, 2009, ENVIRON POLLUT | 1365 | 80.29 | Elsevier | [49] |
| 4 | NIKAIDO H, 2009, ANNU REV BIOCHEM | 1100 | 64.70 | Elsevier | [50] |
| 5 | LIU X, 2017, ENVIRON POLLUT | 709 | 78.78 | Elsevier | [16] |
| 6 | SAPKOTA A, 2008, ENVIRON INT | 625 | 34.72 | Elsevier | [51] |
| 7 | ZAINAB SM, 2020, WATER RES | 624 | 104.00 | Elsevier | [52] |
| 8 | CABELLO FC, 2013, ENVIRON MICROBIOL | 590 | 45.38 | Wiley | [53] |
| 9 | PRUDEN A, 2013, ENVIRON HEALTH PERSPECT | 579 | 44.54 | National Institute of Env. Health Sciences | [54] |
| 10 | GOTHWAL R, 2015, CLEAN-SOIL AIR WATER | 562 | 51.09 | Wiley | [55] |
| 11 | GAO P, 2012, WATER RES | 532 | 38.00 | Wiley | [11] |
| 12 | DEFOIRDT T, 2011, CURR OPIN MICROBIOL | 519 | 34.60 | Elsevier | [56] |
| 13 | WATTS JEM, 2017, MAR DRUGS | 438 | 48.67 | MDPI | [13] |
| 14 | ZOU S, 2011, ENVIRON POLLUT | 413 | 27.53 | Elsevier | [57] |
| 15 | VIECO-SAIZ N, 2019, FRONT MICROBIOL | 366 | 52.28 | Frontiers Media S.A. | [58] |
| 16 | HEUER OE, 2009, CLIN INFECT DIS | 365 | 21.47 | Oxford University Press | [59] |
| 17 | KYU-SONG S, 2014, FISH SHELLFISH IMMUNOL | 338 | 28.17 | Elsevier | [60] |
| 18 | EL-SAADONY MT, 2021, FISH SHELLFISH IMMUNOL | 336 | 67.20 | Elsevier | [61] |
| 19 | ROMERO J, 2012, HEALTH AND ENVIRONMENT IN AQUACULTURE | 335 | 23.93 | IntechOpen | [62] |
| 20 | SANTOS L, 2018, INT J ANTIMICROB AGENTS | 326 | 40.75 | Elsevier | [37] |
| Country | Aquatic Environment/Species | Antibiotic-Resistant Bacteria | Antibiotic | Resistant Genes/Toxic Proteins | Date | Ref. |
|---|---|---|---|---|---|---|
| Turkey | Marmara Sea | Microbacterium istanbulense sp. nov., M. bandirmense sp. nov., M. marmarense sp. nov | Macrolide | Erm23S_rRNA methyltransferase | March 2025 | [77] |
| Chile | Salmon Farms (2 fish farms) | Gammaproteobacteria | Phenicol | floR | Jan. 2025 | [78] |
| Bangladesh | Two fish lakes and three rivers | Shigella flexneri Escherichia ferguso Proteus mirabilis Enterobacter quasiroggenkampii | Ciprofloxacin Ceftriaxone Ampicillin | - | Jan. 2025 | [79] |
| China | Three traditional fish ponds, Tai Lake, Zhejiang | Proteobacteria Cyanobacteria Actinobacteriota Bacteroidota Verrucomicrobiota | Quinolone Chloramphenicol | tetA, tetB, tetC, tetG, tetM, sul1, qnrB, ermF, cat1 and intI1 | Jan. 2025 | [80] |
| China | Mastacembelus armatus Strain YY001 | Plesiomonas shigelloides | Oxacillin Norfloxacin Tetracycline | emrD, macB, catB, ksgA, tolC, MdfA, sul1, bacA and DegP | Dec. 2024 | [81] |
| Israel | Sea urchin (Tripneustes gratilla) | Vibrio spp. (Red-Spotting Diseases) | Tetracycline Penams | adeF CRP | Nov. 2024 | [82] |
| China | Internal organs of Largemouth Bass, Micropterus salmoides | Edwardsiella piscicida | Ciprofloxacin (96.25%) Sulfonamides (60–63%) Thiamphenicol (56.2%) Florfenicol (43.75%) Enrofloxacin (32.50%) Doxycycline (16.25%) Flumequine (1.25%) | - | Nov. 2024 | [83] |
| China | Sea turtles | Nine Vibrio spp. | Nitrofurans Aminoglycosides | merA | Nov. 2024 | [84] |
| India | Shrimp ponds | Vibrio spp. Exiguobacterium spp. Microbacterium spp. | Oxytetracycline Ciprofloxacin Co-trimoxazole Chloramphenicol Erythromycin | tetA (44.0%) sul1 (11.8%) | Jan. 2025 | [85] |
| China | Diseased yellowfin seabream, Acanthopagrus latus (VH-AQ-SCAU-GZ23) | Vibrio harveyi (VH-AQ-SCAU-GZ23) | Thermolysin Tetracyclines Aminoglycosides Quinolones Macrolides Other antibiotics. | TLH | Jan. 2025 | [86] |
| China | Seawater (South China Sea) | Proteobacteria Cyanobacteria | Aminoglycoside Tetracycline fluoroquinolone antibiotics accounted for | 77.3–88.6% of the total ARGs in seawater were resistant to these antibiotics. | Sept. 2024 | [87] |
| India | Oscar fish (Astronotus ocellatus) | Edwardsiella tarda | Multidrug | E. tarda caused 100% mortality within 240 h with 6.99 × 106 CFU/fish | Jul. 2024 | [88] |
| China | Coastal ecosystems (Sediments from seagrass meadow) | Halioglobus spp. Zeaxanthinibacter spp. Aureitalea spp. | 30 antibiotics were detected with total of 99.35–478.02 μg/kg Tetracyclines were the most common. | 22 ARG were identified. Multidru resistance genes and ranA were the most common. | Jul. 2024 | [89] |
| Bangladesh | Tilapia fish (Oreochromis niloticus) for sale at retail markets in Dhaka city | E. coli (92%) V. cholerae (62%) E. coli ESBL (48%) Salmonella spp. (24%) Salmonella spp. Shigella spp. Cryptosporidium spp. | β-lactamase concentration was 2.3 ± 0.8 log10 CFU/g in E. coli | 40% of Salmonella (stn, bcfC, avrA, sodC1, ssaQ) | April 2024 | [90] |
| Egypt | Fish farm in an earthen pond, Damietta. | V. alginolyticus (205 isolates) V. fluvialis (87 isolates) | Amoxicillin Erythromycin | - | April 2024 | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, E.A.H.; Kovács, B.; Kuunya, R.; Mustafa, E.O.A.; Abbo, A.S.H.; Pál, K. Antibiotic Resistance in Aquaculture: Challenges, Trends Analysis, and Alternative Approaches. Antibiotics 2025, 14, 598. https://doi.org/10.3390/antibiotics14060598
Mohammed EAH, Kovács B, Kuunya R, Mustafa EOA, Abbo ASH, Pál K. Antibiotic Resistance in Aquaculture: Challenges, Trends Analysis, and Alternative Approaches. Antibiotics. 2025; 14(6):598. https://doi.org/10.3390/antibiotics14060598
Chicago/Turabian StyleMohammed, Elshafia Ali Hamid, Béla Kovács, Ronald Kuunya, Eltayeb Omaima Awad Mustafa, Azza Siddig Hussien Abbo, and Károly Pál. 2025. "Antibiotic Resistance in Aquaculture: Challenges, Trends Analysis, and Alternative Approaches" Antibiotics 14, no. 6: 598. https://doi.org/10.3390/antibiotics14060598
APA StyleMohammed, E. A. H., Kovács, B., Kuunya, R., Mustafa, E. O. A., Abbo, A. S. H., & Pál, K. (2025). Antibiotic Resistance in Aquaculture: Challenges, Trends Analysis, and Alternative Approaches. Antibiotics, 14(6), 598. https://doi.org/10.3390/antibiotics14060598










