Phenotypic and Genotypic Characterization of ESBL and AmpC β-Lactamase-Producing E. coli Isolates from Poultry in Northwestern Romania
Abstract
1. Introduction
2. Results
2.1. Antimicrobial Susceptibility Testing (AST) Results
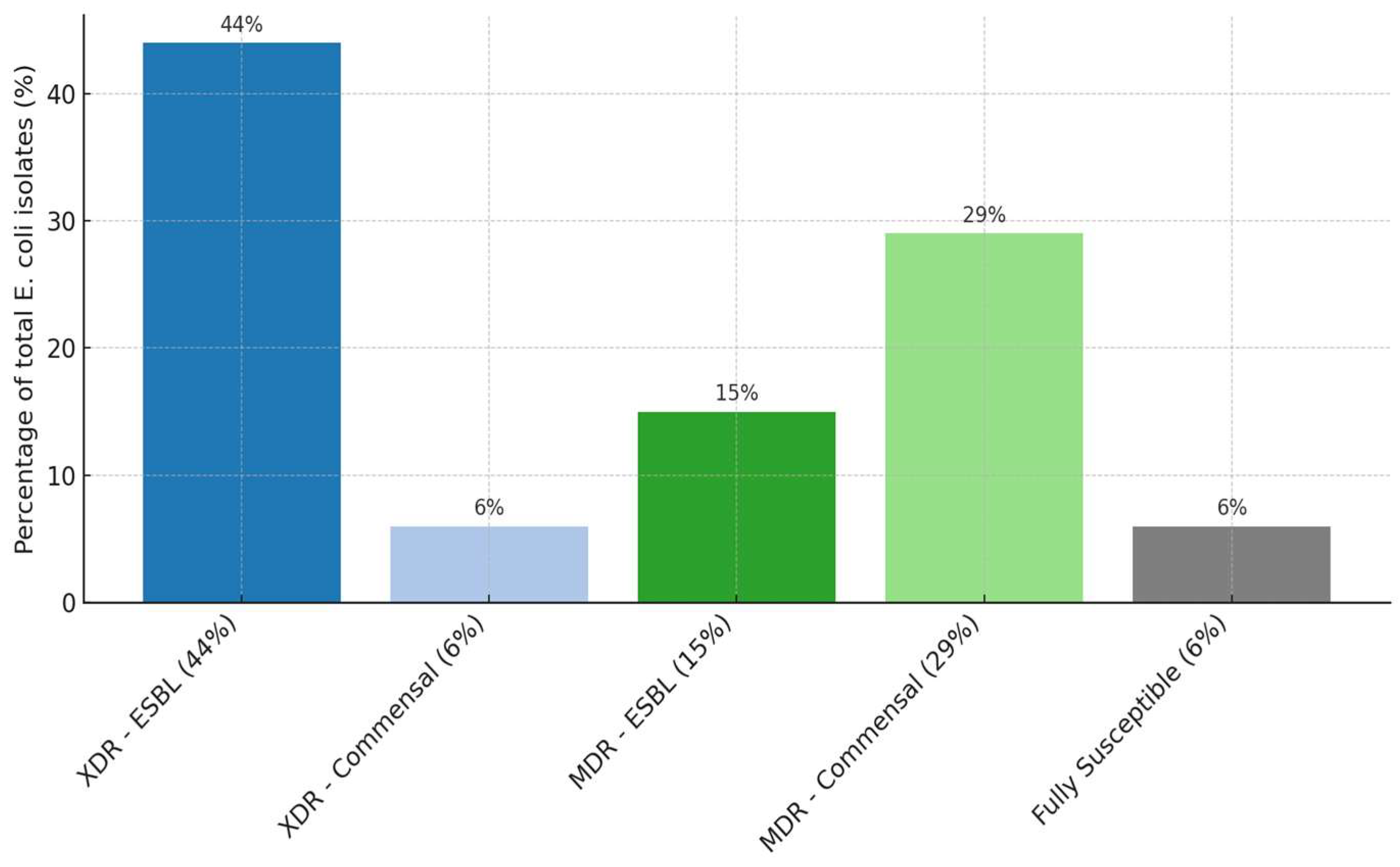
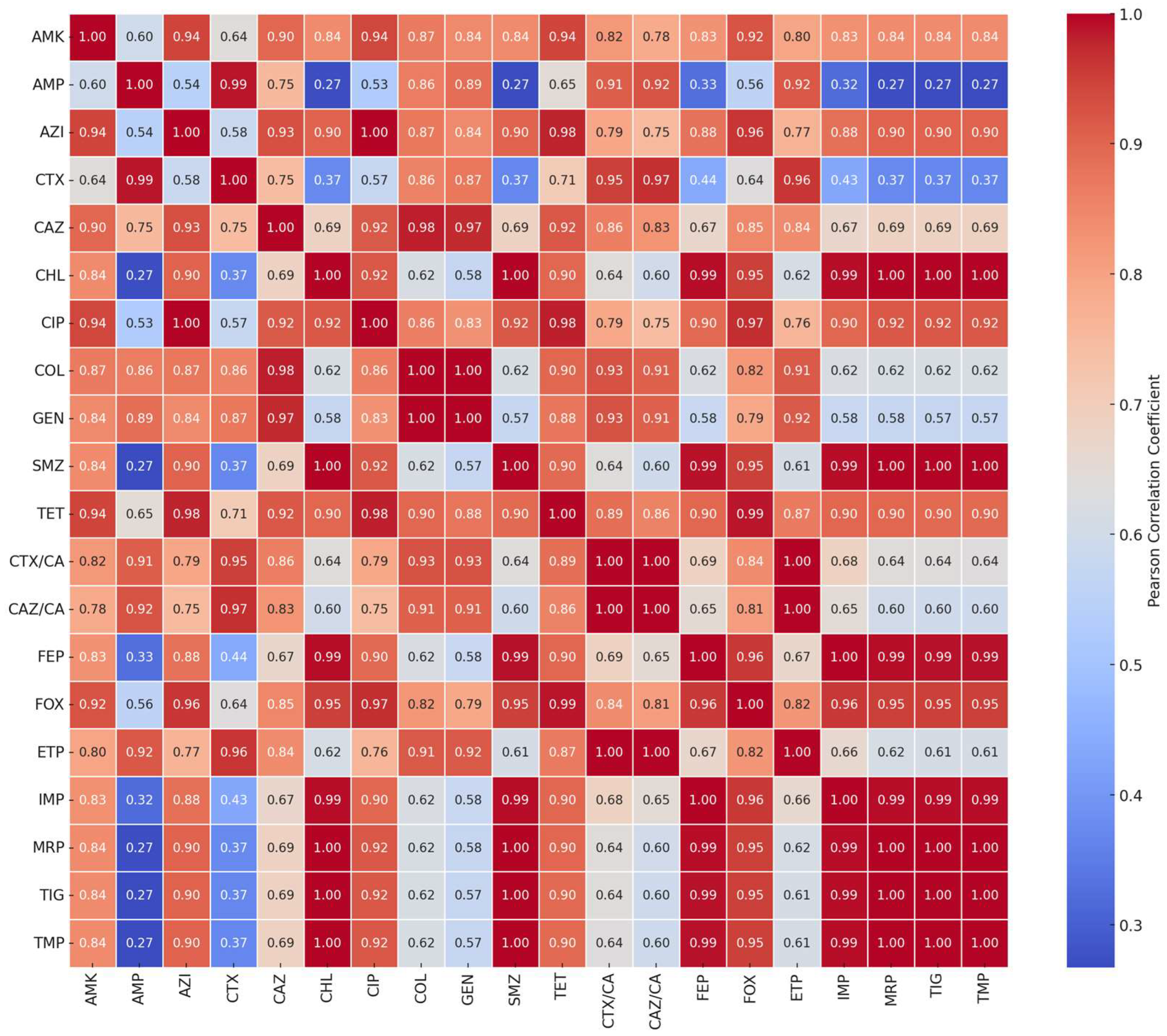
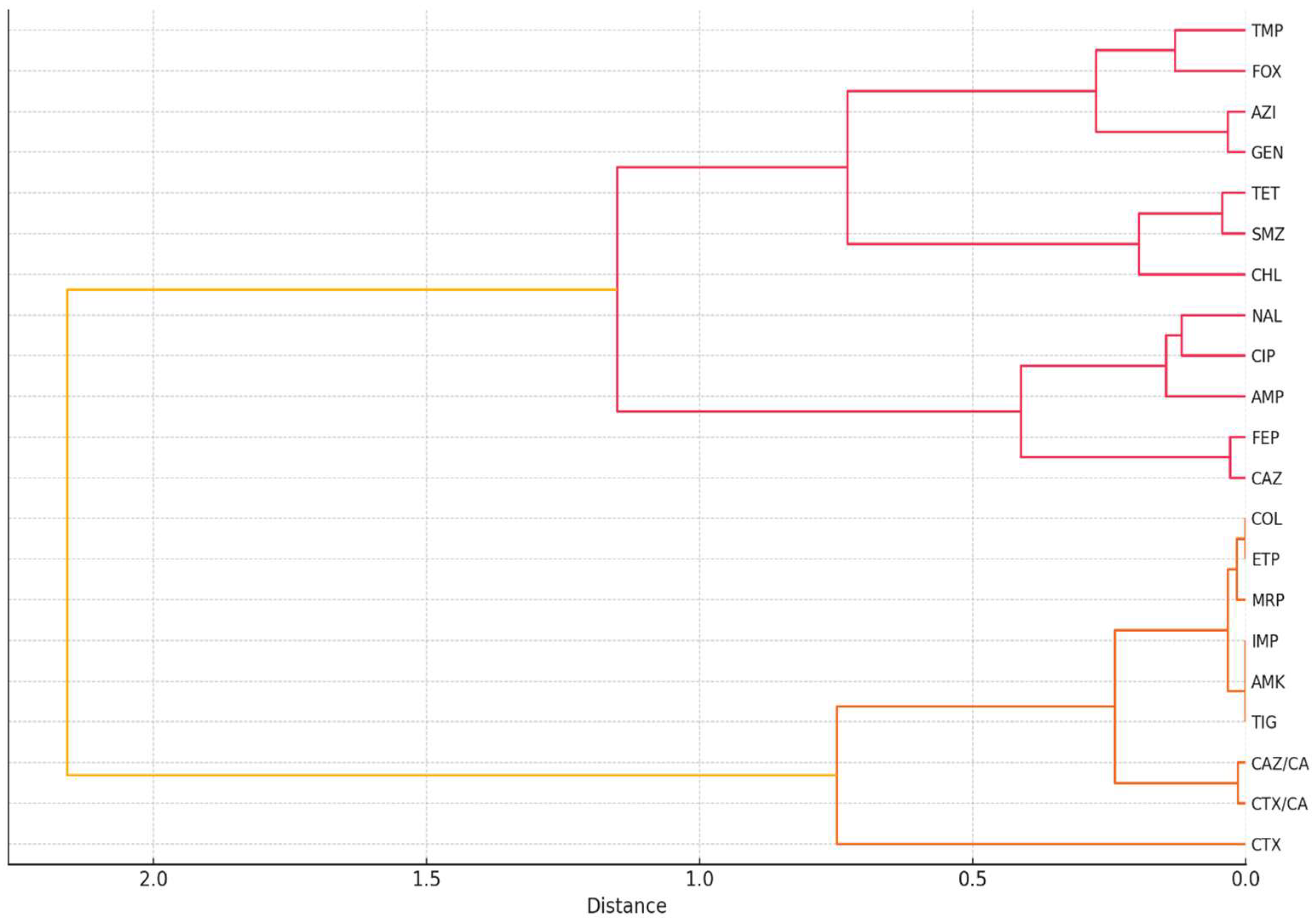
2.2. Correlation and Clustering Analysis of Antibiotic Resistance Patterns
2.3. Prevalence and Penetrance of Resistance Genes, Diagnostic Odds Ratio
2.3.1. Penetrance
2.3.2. Diagnostic Odds Ratio
3. Discussion
4. Materials and Methods
4.1. Material Selection
4.2. Isolation of E. coli Strains
4.3. Antimicrobial Susceptibility Testing (AST) Using the Microdilution Plate Method
4.4. DNA Extraction and Gene Detection by PCR
4.5. Statistical Analysis of Phenotypic and Genotypic Antibiotic Resistance
- Multidrug resistance;
- Non-susceptible to >1 agent from ≥3 classes of antibiotics.
- Extended antibiotic resistance;
- Non-susceptible to >1 agent from all categories of antibiotics except for ≤2, or susceptible only to ≤2 of the antibiotic categories.
- RG+ = phenotypically resistant strains that carry antibiotic resistance genes;
- RG− = phenotypically resistant strains that do not carry antibiotic resistance genes;
- SG+ = phenotypically susceptible strains that carry the genes;
- SG− = phenotypically susceptible strains that do not carry the genes.
5. Conclusions
6. Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Sun, S.; Sun, Y.; Li, X.; Gu, W.; Luo, Y.; Wang, N.; Wang, Q. Antibiotic Resistance of Escherichia coli Isolated from Food and Clinical Environment in China from 2001 to 2020. Sci. Total Environ. 2024, 939, 173498. [Google Scholar] [CrossRef]
- Dimaapi, L.K.A.; Dela Cruz, A.L.G.; Francisco, R.A.D.; Noble, R.G.D.; Sabangan, H.E.G.; Gavino-Lacuna, A.R.; Lota, M.M.M. Antimicrobial Resistance Profile of Escherichia coli Isolated from Raw Chicken Meat in a Selected Wet Market in Manila City, Philippines. Acta Med. Philipp. 2024, 58, 1–16. [Google Scholar] [CrossRef]
- Nasrollahian, S.; Graham, J.P.; Halaji, M. A Review of the Mechanisms That Confer Antibiotic Resistance in Pathotypes of Escherichia coli. Front. Cell. Infect. Microbiol. 2024, 14, 1387497. [Google Scholar] [CrossRef]
- Crăciun, S.; Novac, C.Ş.; Fiţ, N.I.; Bouari, C.M.; Bel, L.V.; Nadăş, G.C. Bacterial Diversity in Pet Rabbits: Implications for Public Health, Zoonotic Risks, and Antimicrobial Resistance. Microorganisms 2025, 13, 653. [Google Scholar] [CrossRef]
- Kim, D.; Kim, S.; Kwon, Y.; Kim, Y.; Park, H.; Kwak, K.; Lee, H.; Lee, J.; Jang, K.-M.; Kim, D.; et al. Structural Insights for β-Lactam Antibiotics. Biomol. Ther. 2023, 31, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Cascella, M. Beta-Lactam Antibiotics; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Javed, M.U.; Hayat, M.T.; Mukhtar, H.; Imre, K. CRISPR-Cas9 System: A Prospective Pathway toward Combatting Antibiotic Resistance. Antibiotics 2023, 12, 1075. [Google Scholar] [CrossRef] [PubMed]
- Nadăș, G.C.; Novac, C.Ș.; Matei, I.A.; Bouari, C.M.; Gal, Z.M.; Tamas-Krumpe, O.M.; Macri, A.M.; Fiț, N.I. Prevalence of Antimicrobial Resistant Bacteria from Conjunctival Flora in an Eye Infection Prone Breed (Saint Bernard). Molecules 2021, 26, 2219. [Google Scholar] [CrossRef]
- Kuan, N.-L.; Chen, Y.-P.; Shien, J.-H.; Yeh, K.-S. Characteristics of the Extended-Spectrum-β-Lactamase-Producing Escherichia coli Isolated from Diseased Livestock and Poultry in Taiwan. Sci. Rep. 2024, 14, 29459. [Google Scholar] [CrossRef]
- Leverstein-van Hall, M.A.; Dierikx, C.M.; Cohen Stuart, J.; Voets, G.M.; van den Munckhof, M.P.; van Essen-Zandbergen, A.; Platteel, T.; Fluit, A.C.; van de Sande-Bruinsma, N.; Scharinga, J.; et al. Dutch Patients, Retail Chicken Meat and Poultry Share the Same ESBL Genes, Plasmids and Strains. Clin. Microbiol. Infect. 2011, 17, 873–880. [Google Scholar] [CrossRef]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.A.; Takebayashi, Y.; Spencer, J. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J. Mol. Biol. 2019, 431, 3472–3500. [Google Scholar] [CrossRef]
- UniProt. Available online: https://www.uniprot.org/uniprotkb/P62593/entry (accessed on 10 April 2025).
- Athanasakopoulou, Z.; Reinicke, M.; Diezel, C.; Sofia, M.; Chatzopoulos, D.C.; Braun, S.D.; Reissig, A.; Spyrou, V.; Monecke, S.; Ehricht, R.; et al. Antimicrobial Resistance Genes in ESBL-Producing Escherichia coli Isolates from Animals in Greece. Antibiotics 2021, 10, 389. [Google Scholar] [CrossRef] [PubMed]
- Pacholewicz, E.; Liakopoulos, A.; Swart, A.; Gortemaker, B.; Dierikx, C.; Havelaar, A.; Schmitt, H. Reduction of extended-spectrum-β-lactamase- and AmpC-β-lactamase-producing Escherichia coli through processing in two broiler chicken slaughterhouses. Int. J. Food Microbiol. 2015, 215, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ghodousi, A.; Bonura, C.; Di Noto, A.M.; Mammina, C. Extended-spectrum β-lactamase, AmpC-producing, and fluoroquinolone-resistant Escherichia coli in retail broiler chicken meat, Italy. Foodborne Pathog. Dis. 2015, 12, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Parzygnat, J.L.; Crespo, R.; Koci, M.D.; Dunn, R.R.; Harden, L.; Fosnaught, M.; Thakur, S. Widespread prevalence of plasmid-mediated blaCTX-M type extended-spectrum beta-lactamase Escherichia coli in backyard broiler production systems in the United States. PLoS ONE 2024, 19, e0304599. [Google Scholar] [CrossRef]
- Ayinla, A.O.; Mateus, A.L.P. Extended-Spectrum Beta-Lactamases in Poultry in Africa: A Systematic Review. Front. Antibiot. 2023, 2, 1140750. [Google Scholar] [CrossRef]
- Abo-Almagd, E.E.; Sabala, R.F.; Abd-Elghany, S.M.; Jackson, C.R.; Ramadan, H.; Imre, K.; Morar, A.; Herman, V.; Sallam, K.I. β-lactamase producing Escherichia coli encoding blaCTX-M and blaCMY genes in chicken carcasses from Egypt. Foods 2023, 12, 598. [Google Scholar] [CrossRef]
- Tofani, S.; Albini, E.; Blasi, F.; Cucco, L.; Lovito, C.; Maresca, C.; Pesciaroli, M.; Orsini, S.; Scoccia, E.; Pezzotti, G.; et al. Assessing the Load, Virulence and Antibiotic-Resistant Traits of ESBL/Ampc E. coli from Broilers Raised on Conventional, Antibiotic-Free, and Organic Farms. Antibiotics 2022, 11, 1484. [Google Scholar] [CrossRef]
- Zahra, R.L.A.; Prayudi, S.K.A.; Suprayogi, T.W.; Wardhana, D.K.; Effendi, M.H.; Khairullah, A.R.; Kurniawan, S.C. Identification of the genotypes of extended-spectrum β-lactamase producing Escherichia coli in the small intestine of broiler chickens at a traditional market in Surabaya, Indonesia. Asian J. Dairy Food Res. 2024, 10, 1–8. [Google Scholar] [CrossRef]
- Robé, C.; Blasse, A.; Merle, R.; Friese, A.; Roesler, U.; Guenther, S. Low dose colonization of broiler chickens with ESBL-/AmpC-producing Escherichia coli in a seeder-bird model independent of antimicrobial selection pressure. Front. Microbiol. 2019, 10, 2124. [Google Scholar] [CrossRef]
- Gaşpar, C.-M.; Cziszter, L.T.; Lăzărescu, C.F.; Ţibru, I.; Pentea, M.; Butnariu, M. Antibiotic Resistance among Escherichia coli Isolates from Hospital Wastewater Compared to Community Wastewater. Water 2021, 13, 3449. [Google Scholar] [CrossRef]
- WHO. Available online: https://www.who.int/groups/one-health-global-leaders-group-on-antimicrobial-resistance (accessed on 10 April 2025).
- Poirel, L.; Madec, J.-Y.; Lupo, A.; Schink, A.-K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial Resistance in Escherichia coli. Microbiol. Spectr. 2018, 6, ARBA-0026-2017. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.-J.; Balasubramanian, B.; Ming, Y.-Y.; Niu, J.-L.; Yi, C.-M.; Ma, Y.; Liu, W.-C. Identification of Antimicrobial Resistance Genes and Drug Resistance Analysis of Escherichia coli in the Animal Farm Environment. J. Infect. Public Health 2021, 14, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, M.K.; Jadhav, I.; Banjara, M.R. Molecular detection of plasmid mediated blaTEM, blaCTX−M, and blaSHV genes in extended spectrum β-lactamase (ESBL) Escherichia coli from clinical samples. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 33. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.A.; Hamed, E.A.; Abdelaty, M.F.; Sorour, H.K.; Badr, H.; Hassan, W.M.; Shalaby, A.G.; El Halem, A.A.; Soliman, M.A.; Roshdy, H. Distribution Pattern of Antibiotic Resistance Genes in Escherichia coli Isolated from Colibacillosis Cases in Broiler Farms of Egypt. Vet. World 2023, 16, 1–11. [Google Scholar] [CrossRef]
- EFSA; ECDC. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2021–2022. EFSA J. 2024, 22, e8583. [Google Scholar] [CrossRef]
- EURL-AR. Available online: https://www.food.dtu.dk/english/-/media/institutter/foedevareinstituttet/temaer/antibiotikaresistens/eurl-ar/protocols/esbl-ampc-and-camrbapenemase-producing-e-coli/esbl_ampc_cpeprotocol_version_caecal_v9_17122024.pdf (accessed on 10 April 2025).
- EURL-AR. Available online: https://www.food.dtu.dk/english/-/media/institutter/foedevareinstituttet/temaer/antibiotikaresistens/eurl-ar/protocols/esbl-ampc-and-camrbapenemase-producing-e-coli/esbl_ampc_cpeprotocol_version_meat_v9_17122024.pdf (accessed on 10 April 2025).
- European Union. Available online: https://eur-lex.europa.eu/legal-content/RO/TXT/PDF/?uri=CELEX:32020D172 (accessed on 10 April 2025).
- Wang, Y.; Xu, C.; Zhang, R.; Chen, Y.; Shen, Y.; Hu, F.; Liu, D.; Lu, J.; Guo, Y.; Xia, X.; et al. Changes in colistin resistance and mcr-1 abundance in Escherichia coli of animal and human origins following the ban of colistin-positive additives in China: An epidemiological comparative study. Lancet Infect. Dis. 2020, 20, 1161–1171. [Google Scholar] [CrossRef]
- Olaitan, A.O.; Dandachi, I.; Baron, S.A.; Daoud, Z.; Morand, S.; Rolain, J.M. Banning colistin in feed additives: A small step in the right direction. Lancet Infect. Dis. 2021, 21, 29–30. [Google Scholar] [CrossRef]
- Leinyuy, J.F.; Ali, I.M.; Ousenu, K.; Tume, C.B. Molecular Characterization of Antimicrobial Resistance Related Genes in E. coli, Salmonella, and Klebsiella Isolates from Broilers in the West Region of Cameroon. PLoS ONE 2023, 18, e0280150. [Google Scholar] [CrossRef]
- Almakrami, M.; Salmen, M.; Aldashel, Y.A.; Alyami, M.H.; Alquraishah, N.; AlZureea, M.; Almakrami, J. Prevalence of Multidrug-, Extensively Drug-, and Pandrug-Resistant Bacteria in Clinical Isolates from King Khaled Hospital, Najran, Saudi Arabia. Discov. Med. 2024, 1, 108. [Google Scholar] [CrossRef]
- Rubab, M.; Oh, D.H. Molecular Detection of Antibiotic Resistance Genes in Shiga Toxin-Producing Escherichia coli Isolated from Different Sources. Antibiotics 2021, 10, 344. [Google Scholar] [CrossRef]
- Müller, A.; Jansen, W.; Grabowski, N.T.; Monecke, S.; Ehricht, R.; Kehrenberg, C. ESBL- and AmpC-producing Escherichia coli from legally and illegally imported meat: Characterization of isolates brought into the EU from third countries. Int. J. Food Microbiol. 2018, 283, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Rojs, O.Z.; Zdovc, I.; Dovč, A.; Žgajnar, J.; Slavec, B.; Krapež, U.; Avguštin Ambrožič, J. Presence and distribution of extended-spectrum and AmpC beta-lactamases-producing Escherichia coli on poultry farms in Slovenia. J. Appl. Poult. Res. 2019, 28, 200–209. [Google Scholar] [CrossRef]
- Gazal, L.E.S.; Medeiros, L.P.; Dibo, M.; Nishio, E.K.; Koga, V.L.; Gonçalves, B.C.; Grassotti, T.T.; de Camargo, T.C.L.; Pinheiro, J.J.; Vespero, E.C.; et al. Detection of ESBL/AmpC-producing and fosfomycin-resistant Escherichia coli from different sources in poultry production in Southern Brazil. Front. Microbiol. 2021, 11, 604544. [Google Scholar] [CrossRef] [PubMed]
- Niero, G.; Bortolaia, V.; Vanni, M.; Intorre, L.; Guardabassi, L.; Piccirillo, A. High diversity of genes and plasmids encoding resistance to third-generation cephalosporins and quinolones in clinical Escherichia coli from commercial poultry flocks in Italy. Vet. Microbiol. 2018, 216, 93–98. [Google Scholar] [CrossRef]
- Gundran, R.S.; Cardenio, P.A.; Villanueva, M.A.; Sison, F.B.; Benigno, C.C.; Kreausukon, K.; Pichpol, D.; Punyapornwithaya, V. Prevalence and distribution of blaCTX-M, blaSHV, blaTEM genes in extended-spectrum β-lactamase-producing E. coli isolates from broiler farms in the Philippines. BMC Vet. Res. 2019, 15, 227. [Google Scholar] [CrossRef]
- Laube, H.; Friese, A.; von Salviati, C.; Guerra, B.; Käsbohrer, A.; Kreienbrock, L.; Roesler, U. Longitudinal monitoring of extended-spectrum-beta-lactamase/AmpC-producing Escherichia coli at German broiler chicken fattening farms. Appl. Environ. Microbiol. 2013, 79, 4815–4820. [Google Scholar] [CrossRef]
- von Tippelskirch, P.; Gölz, G.; Projahn, M.; Daehre, K.; Friese, A.; Roesler, U.; Alter, T.; Orquera, S. Prevalence and quantitative analysis of ESBL and AmpC beta-lactamase producing Enterobacteriaceae in broiler chicken during slaughter in Germany. Int. J. Food Microbiol. 2018, 281, 82–89. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Allende, A.; Álvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F.; et al. Role played by the environment in the emergence and spread of antimicrobial resistance (AMR) through the food chain. EFSA J. 2021, 19, e06651. [Google Scholar] [CrossRef]
- Pesciaroli, M.; Magistrali, C.F.; Filippini, G.; Epifanio, E.M.; Lovito, C.; Marchi, L.; Maresca, C.; Massacci, F.R.; Orsini, S.; Scoccia, E.; et al. Antibiotic-resistant commensal Escherichia coli are less frequently isolated from poultry raised using non-conventional management systems than from conventional broiler. Int. J. Food Microbiol. 2020, 314, 108391. [Google Scholar] [CrossRef]
- Messai, Y.; Benhassine, T.; Naim, M.; Paul, G.; Bakour, R. Prevalence of β-lactam resistance among Escherichia coli clinical isolates from a hospital in Algiers. Rev. Esp. Quimioter. 2006, 19, 144–151. [Google Scholar]
- Ng, L.-K.; Martin, I.; Alfa, M.; Mulvey, M. Multiplex PCR for the detection of tetracycline resistant genes. Mol. Cell. Probes 2001, 15, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Titilawo, Y.; Obi, L.; Okoh, A. Antimicrobial resistance determinants of Escherichia coli isolates recovered from some rivers in Osun State, South-Western Nigeria: Implications for public health. Sci. Total Environ. 2015, 523, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Singh, P.; Rajurkar, M. Multidrug resistant and extensively drug resistant bacteria: A study. J. Pathog. 2016, 2016, 4065603. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Samudio, V.; Pimentel-Peralta, G.; De La Cruz, A.; Landires, I. Multidrug-resistant phenotypes of genetically diverse Escherichia coli isolates from healthy domestic cats. Sci. Rep. 2024, 14, 11260. [Google Scholar] [CrossRef]
- Yuan, Y.; Hu, Y.; Zhang, X.; Zhong, W.; Pan, S.; Wang, L.; Zhou, Z.; Liu, H.; Zhang, S.; Peng, G. Characteristics of MDR E. coli strains isolated from pet dogs with clinic diarrhea: A pool of antibiotic resistance genes and virulence-associated genes. PLoS ONE 2024, 19, e0298053. [Google Scholar] [CrossRef]
| Species | Commensal Strains No. (%) | Presumptive ESBL/AmpC Strains No. (%) | Total | |
|---|---|---|---|---|
| Turkeys | 14 (45) | 17 (55) | 31 | X2 = 0.1 p = 0.75 |
| Broiler chickens | 28 (39) | 43 (61) | 71 | |
| Total | 42 (41) | 60 (59) | 102 |
| Antibiotic Class | Antibiotic | Cut-Off Value (µg/L) | MIC (µg/L) | Resistant (No./%) | Susceptibile (No./%) |
|---|---|---|---|---|---|
| Aminoglycosides | GEN | 8 | 0.5–≥16 | 13 (42) | 18 (58) |
| AMK | 8 | ≤4 | 0 (0) | 31 (100) | |
| Amphenicols | CHL | 16 | 0.8–≥64 | 23 (74) | 8 (26) |
| β-lactams (penicillins, cephalosporins, 1st, 2nd and 3rd generation cephalosporins) | AMP | 16 | 1–≥32 | 25 (81) | 6 (19) |
| CTX | 0.25 | 0.25–≥4 | 17 (54) | 14 (45) | |
| CAZ | 0.5 | 0.25–≥8 | 17 (54) | 14 (45) | |
| FOX | 8 | 0.5–≥64 | 9 (29) | 22 (71) | |
| FEP | 0.125 | 0.064–≥32 | 17 (55) | 14 (45) | |
| CTX/CA | 0.25 | 0.064–64 | 0 (0) | 31 (100) | |
| CAZ/CA | 0.5 | 0.125–128 | 0 (0) | 31 (100) | |
| Quinolones | CIP | 0.064 | 0.015–8 | 21 (68) | 10 (32) |
| NAL | 8 | 4–≥64 | 22 (71) | 9 (29) | |
| Folate inhibitors | SMZ | 64 | 8–512 | 23 (74) | 8 (26) |
| TMP | 2 | 0.5–≥16 | 13 (42) | 18 (58) | |
| Carbapenems | MRP | 0.125 | 0.03–0.125 | 0 (0) | 31 (100) |
| IMP | 0.5 | ≤0.125 | 0 (0) | 31 (100) | |
| ETP | 0.064 | 0.016–0.064 | 0 (0) | 31 (100) | |
| Polimyxins | COL | 2 | 1–2 | 0 (0) | 31 (100) |
| Tetracyclines | TET | 8 | 2–≥64 | 24 (77) | 7 (23) |
| TIG | 0.5 | ≤0.25 | 0 (0) | 31 (100) | |
| Macrolides | AZI | 16 | 2–≥64 | 14 (45) | 17 (55) |
| Antibiotic Class | Antibiotic | Cut-Off Value (µg/L) | MIC (µg/L) | Resistant (No./%) | Susceptibile (No./%) |
|---|---|---|---|---|---|
| Aminoglycosides | GEN | 8 | 0.5–≥16 | 5 (7) | 66 (93) |
| AMK | 8 | ≤4 | 0 (0) | 71 (100) | |
| Amphenicols | CHL | 16 | 0.8–≥64 | 15 (21) | 56 (79) |
| β-lactams (penicillins, cephalosporins, 1st, 2nd and 3rd generation cephalosporins) | AMP | 16 | 1–≥32 | 52 (73) | 19 (27) |
| CTX | 0.25 | 0.25–≥4 | 43 (61) | 28 (39) | |
| CAZ | 0.5 | 0.25–≥8 | 42 (59) | 29 (41) | |
| FOX | 8 | 0.5–≥64 | 17 (24) | 54 (76) | |
| FEP | 0.125 | 0.064–≥32 | 40 (56) | 31 (44) | |
| CTX/CA | 0.25 | 0.064–64 | 11 (15) | 60 (85) | |
| CAZ/CA | 0.5 | 0.125–128 | 10 (14) | 61 (86) | |
| Quinolones | CIP | 0.064 | 0.015–8 | 52 (73) | 19 (27) |
| NAL | 8 | 4–≥64 | 60 (85) | 11 (15) | |
| Folate inhibitors | SMZ | 64 | 8–512 | 26 (37) | 45 (63) |
| TMP | 2 | 0.5–≥16 | 18 (25) | 53 (75) | |
| Carbapenems | MRP | 0.125 | 0.03–0.125 | 2 (3) | 69 (97) |
| IMP | 0.5 | ≤0.125 | 0 (0) | 71 (100) | |
| ETP | 0.064 | 0.016–0.064 | 1 (1) | 70 (99) | |
| Polimyxins | COL | 2 | 1–2 | 1 (1) | 70 (99) |
| Tetracyclines | TET | 8 | 2–≥64 | 28 (39) | 43 (61) |
| TIG | 0.5 | ≤0.25 | 0 (0) | 71 (100) | |
| Macrolides | AZI | 16 | 2–≥64 | 5 (7) | 66 (93) |
| Crt. No. | Antibiotic | Broilers | Turkeys | X2 Value | p-Value | ||
|---|---|---|---|---|---|---|---|
| Resistant (No) | Susceptible (No) | Resistant (No) | Susceptible (No) | ||||
| 1. | AMP | 48 | 23 | 29 | 42 | 9.19 | 0.0024 |
| 2. | CTX | 33 | 38 | 27 | 44 | 0.72 | 0.3957 |
| 3. | CAZ | 32 | 39 | 27 | 44 | 0.46 | 0.4959 |
| 4. | FEP | 32 | 39 | 25 | 46 | 1.06 | 0.3043 |
| 5. | FOX | 13 | 58 | 13 | 55 | 0.01 | 0.9203 |
| 6. | CHL | 13 | 58 | 23 | 45 | 3.58 | 0.0522 |
| 7. | SMZ | 26 | 45 | 23 | 45 | 0.03 | 0.8624 |
| 8. | TET | 37 | 34 | 22 | 49 | 5.68 | 0.0171 |
| 9. | TMP | 31 | 40 | 19 | 52 | 3.74 | 0.0531 |
| 10 | NAL | 10 | 61 | 9 | 62 | 0 | 1 |
| 11. | AMK | 5 | 66 | 4 | 67 | * | 0.9999 |
| 12. | AZI | 18 | 53 | 11 | 60 | 1.56 | 0.2116 |
| 13. | CAZ/CA | 22 | 49 | 17 | 54 | 0.57 | 0.4502 |
| 14. | CTX/CA | 23 | 48 | 22 | 49 | 0 | 1 |
| 15. | GEN | 11 | 60 | 8 | 63 | 0.24 | 0.6242 |
| 16. | CIP | 27 | 44 | 18 | 53 | 2.08 | 0.1492 |
| 17. | ETP | 3 | 68 | 4 | 67 | * | 1 |
| 18. | IMP | 2 | 69 | 3 | 68 | * | 1 |
| 19. | MRP | 3 | 68 | 2 | 69 | * | 1 |
| 20. | TIG | 2 | 69 | 1 | 70 | * | 1 |
| 21. | COL | 6 | 65 | 3 | 68 | * | 0.4934 |
| Gene | Antibiotic | R | S | RG+ nr. (%) | RG− nr. (%) | SG+ nr. (%) | SG− nr. (%) | P (%) | DOR |
|---|---|---|---|---|---|---|---|---|---|
| ampC | AMP | 77 | 25 | 69 (90) | 8 (10) | 18 (72) | 7 (28) | 79 | 3.35 |
| CTX | 60 | 42 | 50 (83) | 10 (17) | 30 (71) | 12 (29) | 62.5 | 2.00 | |
| CAZ | 59 | 43 | 45 (76) | 14 (24) | 32 (74) | 11 (26) | 58 | 1.10 | |
| FEP | 57 | 45 | 40 (70) | 17 (30) | 35 (78) | 10 (22) | 53 | 0.67 | |
| FOX | 26 | 76 | 23 (88) | 3 (12) | 50 (66) | 26 (34) | 31.5 | 3.99 | |
| CTX AC | 11 | 91 | 10 (91) | 1 (9) | 75 (82) | 16 (17) | 12 | 2.13 | |
| CAZ AC | 10 | 92 | 9 (90) | 1 (10) | 75 (82) | 17 (18) | 11 | 2.04 | |
| Subtotal | 300 | 414 | 246 | 54 | 310 | 104 | |||
| blaZ | AMP | 77 | 25 | 40 (52) | 37 (48) | 15 (60) | 10 (40) | 72.7 | 0.72 |
| Subtotal | 77 | 25 | 40 | 37 | 15 | 10 | |||
| blaTEM | AMP | 77 | 25 | 40 (52) | 37 (48) | 10 (40) | 15 (60) | 80 | 1.62 |
| CTX | 60 | 42 | 30 (50) | 30 (50) | 22 (52) | 20 (48) | 58 | 0.91 | |
| CAZ | 59 | 43 | 35 (59) | 24 (41) | 29 (67) | 14 (33) | 55 | 0.70 | |
| FEP | 57 | 45 | 34 (60) | 23 (40) | 20 (44) | 25 (56) | 63 | 1.85 | |
| FOX | 26 | 76 | 14 (54) | 12 (46) | 20 (26) | 56 (74) | 41 | 3.26 | |
| CTX/AC | 11 | 91 | 10 (91) | 1 (9) | 10 (11) | 81 (89) | 50 | 81 | |
| CAZ/AC | 10 | 92 | 8 (80) | 2 (20) | 12 (13) | 80 (87) | 40 | 26.66 | |
| MRP | 2 | 100 | 1 (50) | 1 (50) | 0 | 100 (100) | - | - | |
| Subtotal | 302 | 514 | 172 | 130 | 123 | 391 | |||
| TOTAL | 679 | 953 | 468 | 211 | 448 | 505 |
| Gene | Primer Sequence | Elongation Temperature (°C) | Amplicons (pB) |
|---|---|---|---|
| AmpC | F: ATCAAAACTGGCAGCCG R: GAGCCCGTTTTATGCACCCA | 65 | 510 |
| blaZ | F: ACT TCA ACA CCT GCT GCT TTC R: TGA CCA CTT TTA TCA GCA ACC | 60 | 490 |
| blaTEM | F: GAGTATTCAACATTTCCGTGTC R: TAATCAGTGAGGCACCTATCTC | 42 | 850 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rus, A.; Bucur, I.-M.; Imre, K.; Tirziu, A.T.; Ivan, A.A.; Gros, R.V.; Moza, A.C.; Popa, S.A.; Ban-Cucerzan, A.; Tirziu, E. Phenotypic and Genotypic Characterization of ESBL and AmpC β-Lactamase-Producing E. coli Isolates from Poultry in Northwestern Romania. Antibiotics 2025, 14, 578. https://doi.org/10.3390/antibiotics14060578
Rus A, Bucur I-M, Imre K, Tirziu AT, Ivan AA, Gros RV, Moza AC, Popa SA, Ban-Cucerzan A, Tirziu E. Phenotypic and Genotypic Characterization of ESBL and AmpC β-Lactamase-Producing E. coli Isolates from Poultry in Northwestern Romania. Antibiotics. 2025; 14(6):578. https://doi.org/10.3390/antibiotics14060578
Chicago/Turabian StyleRus, Anca, Iulia-Maria Bucur, Kalman Imre, Andreea Talida Tirziu, Andrei Alexandru Ivan, Radu Valentin Gros, Alex Cristian Moza, Sebastian Alexandru Popa, Alexandra Ban-Cucerzan, and Emil Tirziu. 2025. "Phenotypic and Genotypic Characterization of ESBL and AmpC β-Lactamase-Producing E. coli Isolates from Poultry in Northwestern Romania" Antibiotics 14, no. 6: 578. https://doi.org/10.3390/antibiotics14060578
APA StyleRus, A., Bucur, I.-M., Imre, K., Tirziu, A. T., Ivan, A. A., Gros, R. V., Moza, A. C., Popa, S. A., Ban-Cucerzan, A., & Tirziu, E. (2025). Phenotypic and Genotypic Characterization of ESBL and AmpC β-Lactamase-Producing E. coli Isolates from Poultry in Northwestern Romania. Antibiotics, 14(6), 578. https://doi.org/10.3390/antibiotics14060578










