Know Your Enemy: Piscirickettsia salmonis and Phage Interactions Using an In Silico Perspective
Abstract
1. Introduction
2. Results
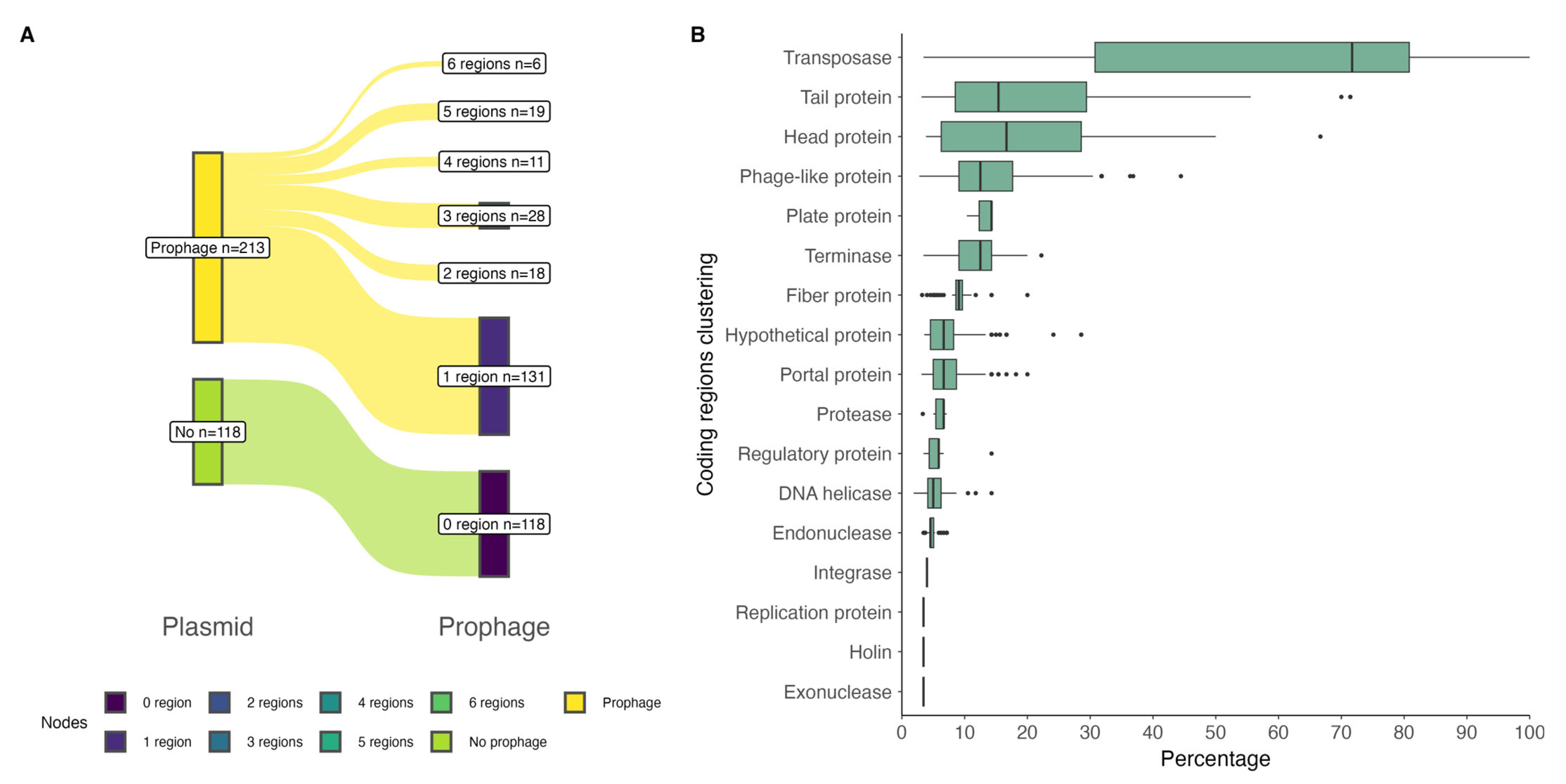
2.1. Prophage Regions in P. salmonis Strains
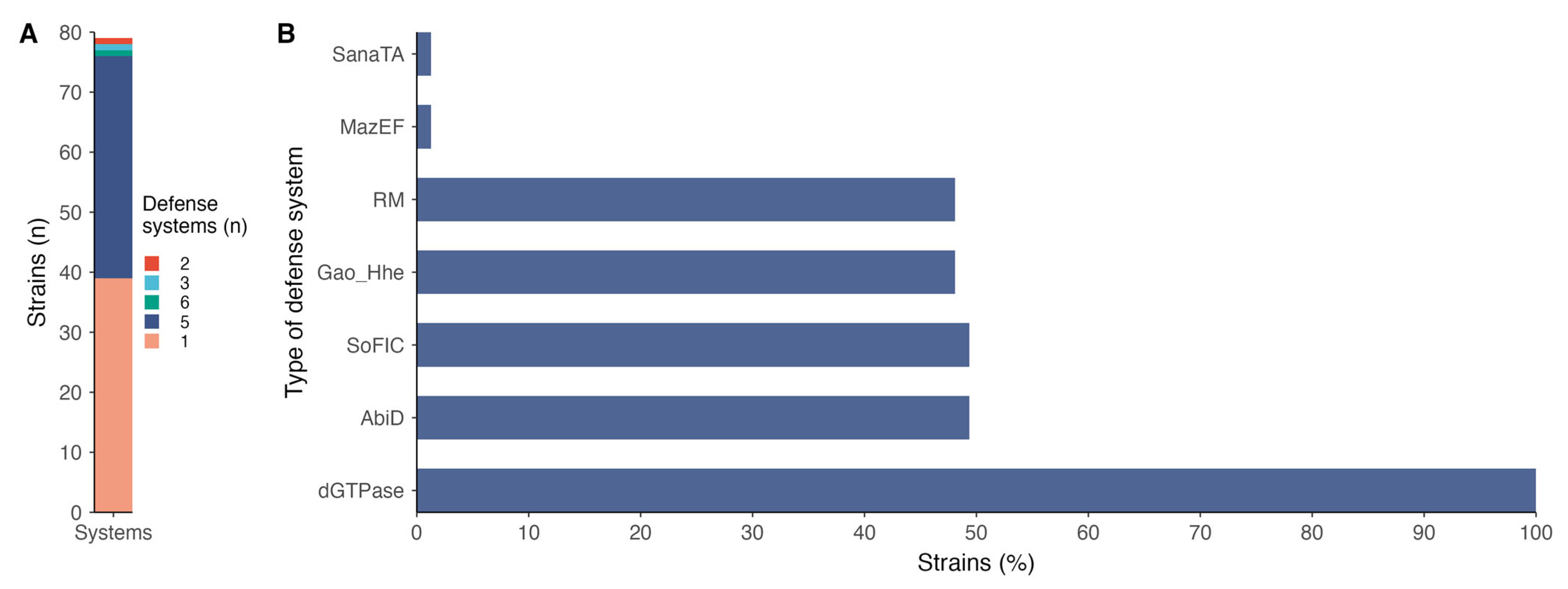
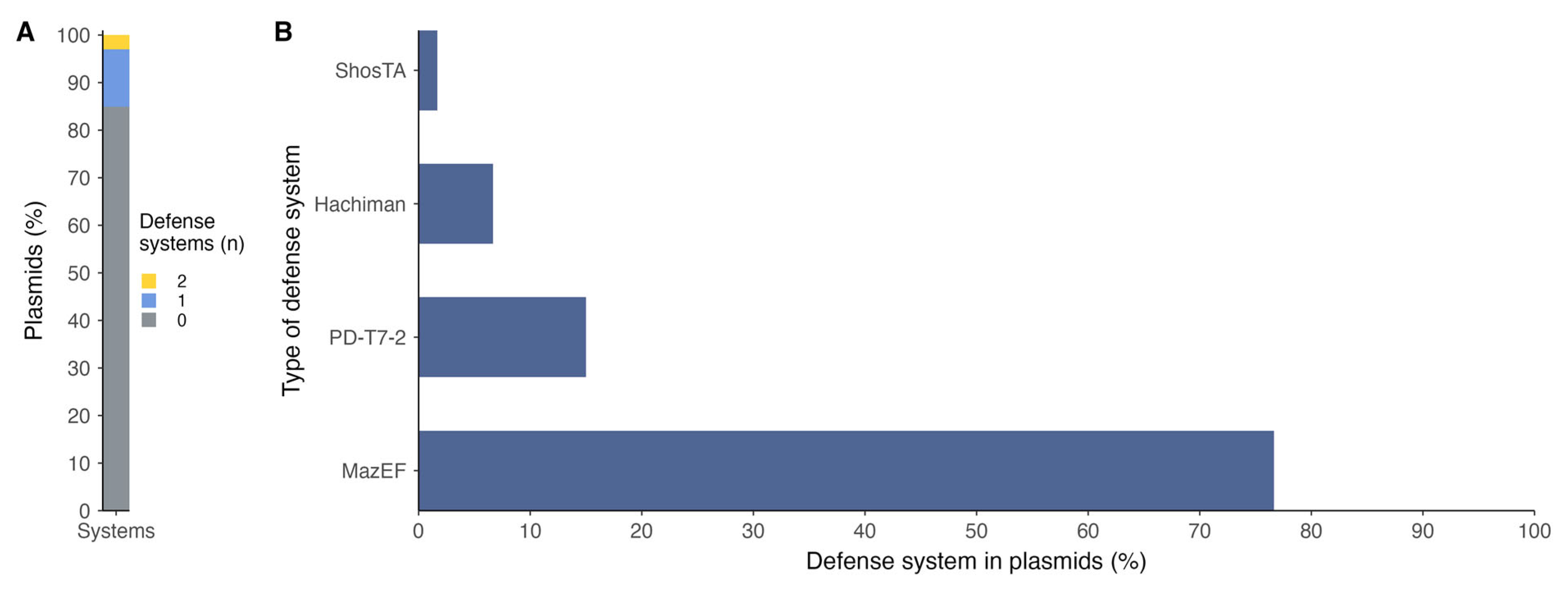
2.2. Defense System Diversities Across P. salmonis Strains
3. Discussion
4. Materials and Methods
Bioinformatic Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDS | Coding sequences |
| SRS | Salmonid Rickettsial Septicemia |
References
- Fryer, J.; Lannan, C.; Giovannoni, S.; Wood, N. Piscirickettsia salmonis gen. nov., sp. nov., the causative agent of an epizootic disease in salmonid fishes. Int. J. Syst. Bacteriol. 1992, 42, 120–126. [Google Scholar] [CrossRef] [PubMed]
- SERNAPESCA 2023. Available online: https://www.sernapesca.cl/app/uploads/2024/09/Informe-Sanitario-ANO-2023.pdf (accessed on 26 February 2025).
- Cabello, F.C.; Millanao, A.; Godfrey, H.P. Piscirickettsia salmonis Pathogenicity: Using the Damage–Response Framework to Look Beyond Smoke and Mirrors. mBio 2025, 16, e03821-24. [Google Scholar] [CrossRef] [PubMed]
- Isla, A.; Sánchez, P.; Ruiz, P.; Albornoz, R.; Pontigo, J.P.; Rauch, M.C.; Hawes, C.; Vargas-Chacoff, L.; Yáñez, A.J. Effect of low-dose Piscirickettsia salmonis infection on haematological-biochemical blood parameters in Atlantic salmon (Salmo salar). J. Fish Biol. 2022, 101, 1021–1032. [Google Scholar] [CrossRef]
- Maisey, K.; Montero, R.; Christodoulides, M. Vaccines for piscirickettsiosis (salmonid rickettsial septicaemia, SRS): The Chile perspective. Expert Rev. Vaccines 2017, 16, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Pontigo, J.P.; Espinoza, C.; Hernandez, M.; Nourdin, G.; Oliver, C.; Avendaño-Herrera, R.; Figueroa, J.; Rauch, C.; Troncoso, J.M.; Vargas-Chacoff, L.; et al. Protein-Based Vaccine Protects Against Piscirickettsia salmonis in Atlantic Salmon (Salmo salar). Front. Immunol. 2021, 12, 602689. [Google Scholar] [CrossRef]
- Valenzuela-Avilés, P.; Torrealba, D.; Figueroa, C.; Mercado, L.; Dixon, B.; Conejeros, P.; Gallardo-Matus, J. Why vaccines fail against piscirickettsiosis in farmed salmon and trout and how to avoid it: A review. Front. Immunol. 2022, 13, 1019404. [Google Scholar] [CrossRef]
- SERNAPESCA 2024. Available online: https://www.sernapesca.cl/app/uploads/2024/06/Informe-sobre-el-uso-de-antimicrobianos-y-antiparasitarios-en-la-salmonicultura-nacional-Ano-2023_v20240606.pdf (accessed on 26 February 2025).
- Cabello, F. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef]
- Miranda, C.D.; Godoy, F.A.; Lee, M.R. Current status of the use of antibiotics and the antimicrobial resistance in the Chilean salmon farms. Front. Microbiol. 2018, 9, 1284. [Google Scholar] [CrossRef]
- Higuera, G.; Bastías, R.; Tsertsvadze, G.; Romero, J.; Espejo, R.T. Recently discovered Vibrio anguillarum phages can protect against experimentally induced vibriosis in Atlantic salmon, Salmo salar. Aquaculture 2013, 392–395, 128–133. [Google Scholar] [CrossRef]
- Kalatzis, P.G.; Bastías, R.; Kokkari, C.; Katharios, P. Isolation and characterization of two lytic bacteriophages, St2 and Grn1; phage therapy application for biological control of Vibrio alginolyticus in aquaculture live feeds. PLoS ONE 2016, 11, e0151101. [Google Scholar] [CrossRef]
- Katharios, P.; Kalatzis, P.G.; Kokkari, C.; Sarropoulou, E.; Middelboe, M. Isolation and characterization of a N4-like lytic bacteriophage infecting Vibrio splendidus, a pathogen of fish and bivalves. PLoS ONE 2017, 12, e0190083. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://stim.uk/r-d/bacteriophages/ (accessed on 26 February 2025).
- Yuksel, S.A.; Thompson, K.D.; Ellis, A.E.; Adams, A. Purification of Piscirickettsia salmonis and associated phage particles. Dis. Aquat. Organ. 2001, 44, 231–235. [Google Scholar] [CrossRef] [PubMed]
- de Jonge, P.A.; Nobrega, F.L.; Brouns, S.J.J.; Dutilh, B.E. Molecular and evolutionary determinants of bacteriophage host range. Trends Microbiol. 2019, 27, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Murtazalieva, K.; Mu, A.; Petrovskaya, A.; Finn, R.D. The growing repertoire of phage anti-defence systems. Trends Microbiol. 2024, 32, 1212–1228. [Google Scholar] [CrossRef]
- Śliwka, P.; Ochocka, M.; Skaradzińska, A. Applications of Bacteriophages against Intracellular Bacteria. Crit. Rev. Microbiol. 2022, 48, 222–239. [Google Scholar] [CrossRef]
- Fajardo-Lubian, A.; Venturini, C. Use of Bacteriophages to Target Intracellular Pathogens. Clin. Infect. Dis. 2023, 77, S423–S432. [Google Scholar] [CrossRef]
- Nepal, R.; Houtak, G.; Wormald, P.J.; Psaltis, A.J.; Vreugde, S. Prophage: A crucial catalyst in infectious disease modulation. Lancet Microbe 2022, 3, e162–e163. [Google Scholar] [CrossRef]
- Bobay, L.M.; Touchon, M.; Rocha, E.P.C. Pervasive Domestication of Defective Prophages by Bacteria. Proc. Natl. Acad. Sci. USA 2014, 111, 12127–12132. [Google Scholar] [CrossRef]
- Jia, C.; Wang, Z.; Huang, C.; Teng, L.; Zhou, H.; An, H.; Liao, S.; Liu, Y.; Huang, L.; Tang, B.; et al. Mobilome-Driven Partitions of the Resistome in Salmonella. mSystems 2023, 8, e00883-23. [Google Scholar] [CrossRef]
- Casjens, S. Prophages and Bacterial Genomics: What Have We Learned So Far? Mol. Microbiol. 2003, 49, 277–300. [Google Scholar] [CrossRef]
- Bobay, L.M.; Rocha, E.P.; Touchon, M. The adaptation of temperate bacteriophages to their host genomes. Mol. Biol. Evol. 2013, 30, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.R.; Nguyen, A.; Liao, J.; Cheng, R.A. What’s on a Prophage: Analysis of Salmonella spp. Prophages Identifies a Diverse Range of Cargo with Multiple Virulence- and Metabolism-Associated Functions. mSphere 2024, 9, e00031-24. [Google Scholar] [CrossRef]
- Cook, G.M.; Robson, J.R.; Frampton, R.A.; McKenzie, J.; Przybilski, R.; Fineran, P.C.; Arcus, V.L. Ribonucleases in Bacterial Toxin-Antitoxin Systems. Biochim. Biophys. Acta 2013, 1829, 523–531. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Park, J.H.; Inouye, M. Toxin-Antitoxin Systems in Bacteria and Archaea. Annu. Rev. Genet. 2011, 45, 61–79. [Google Scholar] [CrossRef]
- Marshall, S.H.; Gómez, F.A.; Ramírez, R.; Nilo, L.; Henríquez, V. Biofilm generation by Piscirickettsia salmonis under growth stress conditions: A putative in vivo survival/persistence strategy in marine environments. Res. Microbiol. 2012, 163, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, P.; Iredell, J.R. Plasmid Carriage of type II toxin-antitoxin systems by the growing group of IncX plasmids. Plasmid 2017, 91, 19–27. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, K.; Wang, Y.; Xu, J.; Zheng, Q.; Luo, T.; Jiao, N. Genomic insights into the adaptation of Synechococcus to the coastal environment on Xiamen. Front. Microbiol. 2023, 14, 1292150. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.K.; Breitbart, M.; Edwards, R.A. Transposases Are the Most Abundant, Most Ubiquitous Genes in Nature. Nucleic Acids Res. 2010, 38, 4207–4217. [Google Scholar] [CrossRef]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile Genetic Elements Associated with Antimicrobial Resistance. Clin. Microbiol. Rev. 2018, 31, e00088-17. [Google Scholar] [CrossRef]
- Brüssow, H.; Canchaya, C.; Hardt, W.-D. Phages and the Evolution of Bacterial Pathogens: From Genomic Rearrangements to Lysogenic Conversion. Microbiol. Mol. Biol. Rev. 2004, 68, 560–602. [Google Scholar] [CrossRef]
- Touchon, M.; Bernheim, A.; Rocha, E.P.C. Genetic and Functional Diversity of Prophage DNA in Prokaryotic Genomes. Curr. Opin. Microbiol. 2016, 31, 171–178. [Google Scholar] [CrossRef]
- Bondy-Denomy, J.; Davidson, A.R. When a Virus Is Not a Parasite: The Beneficial Effects of Prophages on Bacterial Fitness. J. Microbiol. 2014, 52, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Tesson, F.; Hervé, A.; Mordret, E.; Touchon, M.; D’humières, C.; Cury, J.; Bernheim, A. Systematic and Quantitative View of the Antiviral Arsenal of Prokaryotes. Nat. Commun. 2022, 13, 2561. [Google Scholar] [CrossRef]
- Guegler, C.K.; Laub, M.T. Shutoff of Host Transcription Triggers a Toxin-Antitoxin System to Cleave Phage RNA and Abort Infection. Mol. Cell 2021, 81, 2361–2373.e9. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, B.Y.; Severin, G.B.; Elg, C.A.; Waldron, E.J.; Kant, A.; Wessel, A.J.; Dover, J.A.; Rhoades, C.R.; Ridenhour, B.J.; Parent, K.N.; et al. Phage Defence by Deaminase-Mediated Depletion of Deoxynucleotides in Bacteria. Nat. Microbiol. 2022, 7, 1210–1220. [Google Scholar] [CrossRef] [PubMed]
- Lopatina, A.; Tal, N.; Sorek, R. Abortive Infection: Bacterial Suicide as an Antiviral Immune Strategy. Annu. Rev. Virol. 2020, 7, 371–384. [Google Scholar] [CrossRef]
- Chopin, M.C.; Chopin, A.; Bidnenko, E. Phage Abortive Infection in Lactococci: Variations on a Theme. Curr. Opin. Microbiol. 2005, 8, 473–479. [Google Scholar] [CrossRef]
- Tal, N.; Millman, A.; Stokar-Avihail, A.; Fedorenko, T.; Leavitt, A.; Melamed, S.; Yirmiya, E.; Avraham, C.; Brandis, A.; Mehlman, T.; et al. Bacteria Deplete Deoxynucleotides to Defend against Bacteriophage Infection. Nat. Microbiol. 2022, 7, 1200–1209. [Google Scholar] [CrossRef]
- Gao, L.; Altae-Tran, H.; Benton, M.L.; Tourigny, M.; Mejia, L.; Fang, B.; Gozashti, L.; Gootenberg, J.S.; Abudayyeh, O.O. Diverse Enzymatic Activities Mediate Antiviral Immunity in Prokaryotes. Science 2020, 369, 1077–1084. [Google Scholar] [CrossRef]
- Millman, A.; Melamed, S.; Amitai, G.; Sorek, R. An Expanded Arsenal of Immune Systems That Protect Bacteria from Phages. Cell Host Microbe 2022, 30. [Google Scholar] [CrossRef]
- Labrie, S.J.; Samson, J.E.; Moineau, S. Bacteriophage Resistance Mechanisms. Nat. Rev. Microbiol. 2010, 8, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.H.; Touchon, M.; Rocha, E.P.C. The Interplay of Restriction–Modification Systems with Mobile Genetic Elements and Their Prokaryotic Hosts. Nucleic Acids Res. 2014, 42, 10618–10631. [Google Scholar] [CrossRef] [PubMed]
- Harms, A.; Brodersen, D.E.; Mitarai, N.; Gerdes, K. Toxins, Targets, and Triggers: An Overview of Toxin-Antitoxin Biology. Mol. Cell 2018, 70, 768–784. [Google Scholar] [CrossRef] [PubMed]
- Bernheim, A.; Sorek, R. The Pan-Immune System of Bacteria: Antiviral Defense as a Community Resource. Nat. Rev. Microbiol. 2020, 18, 113–119. [Google Scholar] [CrossRef]
- Touchon, M.; Moura de Sousa, J.A.; Rocha, E.P.C. Embracing the Enemy: The Diversification of Microbial Gene Repertoires by Phage-Mediated Horizontal Gene Transfer. Curr. Opin. Microbiol. 2017, 38, 66–73. [Google Scholar] [CrossRef]
- Wishart, D.S.; Han, S.; Saha, S.; Oler, E.; Peters, H.; Grant, J.; Stothard, P.; Gautam, V. PHASTEST: Faster than PHASTER, Better than PHAST. Nucleic Acids Res. 2023, 51, W443–W450. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 26 February 2025).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 26 February 2025).
- Sjoberg, D. ggsankey: Sankey, Alluvial and Sankey Bump Plots. 2022. Available online: https://github.com/davidsjoberg/ggsankey (accessed on 21 February 2025).
- Harrell, F., Jr. Hmisc: Harrell Miscellaneous; R Package Version 5.2-3. 2025. Available online: https://github.com/harrelfe/hmisc (accessed on 26 February 2025).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez, C.; Romero, J. Know Your Enemy: Piscirickettsia salmonis and Phage Interactions Using an In Silico Perspective. Antibiotics 2025, 14, 558. https://doi.org/10.3390/antibiotics14060558
Ramírez C, Romero J. Know Your Enemy: Piscirickettsia salmonis and Phage Interactions Using an In Silico Perspective. Antibiotics. 2025; 14(6):558. https://doi.org/10.3390/antibiotics14060558
Chicago/Turabian StyleRamírez, Carolina, and Jaime Romero. 2025. "Know Your Enemy: Piscirickettsia salmonis and Phage Interactions Using an In Silico Perspective" Antibiotics 14, no. 6: 558. https://doi.org/10.3390/antibiotics14060558
APA StyleRamírez, C., & Romero, J. (2025). Know Your Enemy: Piscirickettsia salmonis and Phage Interactions Using an In Silico Perspective. Antibiotics, 14(6), 558. https://doi.org/10.3390/antibiotics14060558





