Effective Control of Salmonella Enteritidis in Poultry by Dietary Supplementation with Microencapsulated Essential Oils
Abstract
1. Introduction
2. Results
2.1. In Vitro Results
2.2. Encapsulation and Quantification of Active Compounds
2.3. Chick In Vivo Results
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents
4.2. Essential Oils
4.3. In Vitro Studies
4.3.1. Bacterial Strain
4.3.2. Antimicrobial Activity
4.3.3. Synergism Assessment of Essential Oils
4.4. Encapsulation Strategy and Analytical Assessment
4.5. Analytical Performance
4.6. Ethical Approval
4.6.1. Bacterial Inoculum
4.6.2. Experimental Design
4.6.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SE | Salmonella enterica subsp. enterica serotype Enteritidis |
| EOs | Essential oils |
| MIC | Minimum inhibitory concentration |
| MBC | Minimum bactericidal concentration |
| FICI | Fractional Inhibitory Concentration Index |
| CEUA | Ethics Committee on Animal Experimentation |
| MHB | Mueller–Hinton Broth |
| LBB | Luria–Bertani Broth |
| BGA | Brilliant Green Agar |
| Nal/Spc | Nalidixic acid/Spectinomycin |
| CFU | Colony-Forming Unit |
| v/v | Volume by volume |
| HPLC | High-Performance Liquid Chromatography |
References
- Li, S.; He, Y.; Mann, D.A.; Deng, X. Global Spread of Salmonella Enteritidis via Centralized Sourcing and International Trade of Poultry Breeding Stocks. Nat. Commun. 2021, 12, 5109. [Google Scholar] [CrossRef] [PubMed]
- Velge, P.; Menanteau, P.; Chaumeil, T.; Barilleau, E.; Trotereau, J.; Virlogeux-Payant, I. Two In Vivo Models to Study Salmonella Asymptomatic State in Chicks. In Bacterial Virulence; Methods in Molecular Biology; Humana: New York, NY, USA, 2022; ISBN 9781071619704. [Google Scholar]
- de Mesquita Souza Saraiva, M.; Lim, K.; do Monte, D.F.M.; Givisiez, P.E.N.; Alves, L.B.R.; de Freitas Neto, O.C.; Kariuki, S.; Júnior, A.B.; de Oliveira, C.J.B.; Gebreyes, W.A. Antimicrobial Resistance in the Globalized Food Chain: A One Health Perspective Applied to the Poultry Industry. Braz. J. Microbiol. 2022, 53, 465–486. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.M.S.; Moreira Filho, A.L.B.; Freitas Neto, O.C.; Silva, N.M.V.; Givisiez, P.E.N.; Gebreyes, W.A.; Oliveira, C.J.B. Off-Label Use of Ceftiofur in One-Day Chicks Triggers a Short-Term Increase of ESBL-Producing E. Coli in the Gut. PLoS ONE 2018, 13, e0203158. [Google Scholar] [CrossRef]
- Roque-Borda, C.A.; Primo, L.M.D.G.; Medina-Alarcón, K.P.; Campos, I.C.; Nascimento, C.F.; Saraiva, M.M.S.; Berchieri Junior, A.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S.; Perdigão, J.; et al. Antimicrobial Peptides: A Promising Alternative to Conventional Antimicrobials for Combating Polymicrobial Biofilms. Adv. Sci. 2024, 12, e2410893. [Google Scholar] [CrossRef] [PubMed]
- Puvača, N.; Milenković, J.; Galonja Coghill, T.; Bursić, V.; Petrović, A.; Tanasković, S.; Pelić, M.; Ljubojević Pelić, D.; Miljković, T. Antimicrobial Activity of Selected Essential Oils against Selected Pathogenic Bacteria: In Vitro Study. Antibiotics 2021, 10, 546. [Google Scholar] [CrossRef]
- Huerta Lorenzo, B.; Galán-Relaño, Á.; Barba-Sánchez, E.; Romero-Salmoral, A.; Solarte Portilla, A.L.; Gómez-Gascón, L.; Astorga Márquez, R.J. Potentiation of the Antimicrobial Effect of Oxytetracycline Combined with Cinnamon, Clove, Oregano, and Red Thyme Essential Oils against MDR Salmonella enterica Strains. Animals 2024, 14, 1347. [Google Scholar] [CrossRef]
- Robinson, K.; Assumpcao, A.L.F.V.; Arsi, K.; Donoghue, A.; Jesudhasan, P.R.R. Ability of Garlic and Ginger Oil to Reduce Salmonella in Post-Harvest Poultry. Animals 2022, 12, 2974. [Google Scholar] [CrossRef]
- Islam, Z.; Sultan, A.; Khan, S.; Khan, K.; Jan, A.U.; Aziz, T.; Alharbi, M.; Alshammari, A.; Alasmari, A.F. Effects of an Organic Acids Blend and Coated Essential Oils on Broiler Growth Performance, Blood Biochemical Profile, Gut Health, and Nutrient Digestibility. Ital. J. Anim. Sci. 2024, 23, 152–163. [Google Scholar] [CrossRef]
- Galgano, M.; Pellegrini, F.; Fracchiolla, G.; Mrenoshki, D.; Zarea, A.A.K.; Bianco, A.; Del Sambro, L.; Capozzi, L.; Schiavone, A.; Saleh, M.S.; et al. Pilot Study on the Action of Thymus Vulgaris Essential Oil in Treating the Most Common Bacterial Contaminants and Salmonella enterica Subsp. enterica Serovar Derby in Poultry Litter. Antibiotics 2023, 12, 436. [Google Scholar] [CrossRef]
- Oliveira, G.D.S.; McManus, C.; de Araújo, M.V.; de Sousa, D.E.R.; de Macêdo, I.L.; Castro, M.B.; Santos, V.M.D. Sanitizing Hatching Eggs with Essential Oils: Avian and Microbiological Safety. Microorganisms 2023, 11, 1890. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, L.; Guo, F.; Huang, J.; Qiao, J.; Bi, R.; Huang, J.; Zhang, K.; Guo, Y.; Wang, Z. Dietary Supplemental Coated Essential Oils and Organic Acids Mixture Improves Growth Performance and Gut Health along with Reduces Salmonella Load of Broiler Chickens Infected with Salmonella Enteritidis. J. Anim. Sci. Biotechnol. 2023, 14, 95. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, T.; Tang, J.; Huang, L.; Ding, Y.; Zeng, Z.; Liu, J. Antibacterial Activity of Surfactin and Synergistic Effect with Conventional Antibiotics Against Methicillin-Resistant Staphylococcus aureus Isolated from Patients with Diabetic Foot Ulcers. Diabetes Metab. Syndr. Obes. 2023, 16, 3727–3737. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fang, J.; Wang, H.; Zhang, B.; Wang, N.; Yao, X.; Li, H.; Qiu, J.; Deng, X.; Leng, B.; et al. Natural Medicine Can Substitute Antibiotics in Animal Husbandry: Protective Effects and Mechanisms of Rosewood Essential Oil against Salmonella Infection. Chin. J. Nat. Med. 2024, 22, 785–796. [Google Scholar] [CrossRef]
- Maggio, F.; Lauteri, C.; Rossi, C.; Ferri, G.; Serio, A.; Vergara, A.; Paparella, A. Combined Effect of Tetracycline Compounds and Essential Oils on Antimicrobial Resistant Salmonella enterica Isolated from the Swine Food Chain. Front. Microbiol. 2024, 15, 1439286. [Google Scholar] [CrossRef] [PubMed]
- de Souza Rodrigues, H.L.; Kolososki, I.M.M.; Benevides, V.P.; Saraiva, M.M.S.; Berchieri, A.; Junior, A.B. Essential Oils Used in the Poultry Industry: Would It Be an Effective Green Alternative against Salmonella Spp. Dissemination and Antimicrobial Resistance? Microbe 2025, 6, 100248. [Google Scholar] [CrossRef]
- Mo, K.; Li, J.; Liu, F.; Xu, Y.; Huang, X.; Ni, H. Superiority of Microencapsulated Essential Oils Compared With Common Essential Oils and Antibiotics: Effects on the Intestinal Health and Gut Microbiota of Weaning Piglet. Front. Nutr. 2022, 8, 808106. [Google Scholar] [CrossRef]
- Roque-Borda, C.A.; Chávez-Morán, M.R.; Primo, L.M.D.G.; Márquez Montesinos, J.C.E.; Borges Cardoso, V.M.; Saraiva, M.M.S.; Marcos, C.M.; Chorilli, M.; Albericio, F.; de la Torre, B.G.; et al. Alginate-Pectin Microparticles Embedding Self-Assembling Antimicrobial Peptides and Resveratrol for Antimicrobial and Anti-Inflammatory Applications. Food Hydrocoll. 2025, 167, 111454. [Google Scholar] [CrossRef]
- Saporito, F.; Sandri, G.; Bonferoni, M.C.; Rossi, S.; Boselli, C.; Icaro Cornaglia, A.; Mannucci, B.; Grisoli, P.; Vigani, B.; Ferrari, F. Essential Oil-Loaded Lipid Nanoparticles for Wound Healing. Int. J. Nanomed. 2017, 13, 175–186. [Google Scholar] [CrossRef]
- Silva, E.F.D.; Santos, F.A.L.D.; Pires, H.M.; Bastos, L.M.; Ribeiro, L.N.M. Lipid Nanoparticles Carrying Essential Oils for Multiple Applications as Antimicrobials. Pharmaceutics 2025, 17, 178. [Google Scholar] [CrossRef]
- Lai, F.; Wissing, S.A.; Müller, R.H.; Fadda, A.M. Artemisia Arborescens L Essential Oil-Loaded Solid Lipid Nanoparticles for Potential Agricultural Application: Preparation and Characterization. AAPS PharmSciTech 2006, 7, E10–E18. [Google Scholar] [CrossRef]
- Katopodi, A.; Detsi, A. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers of Natural Products as Promising Systems for Their Bioactivity Enhancement: The Case of Essential Oils and Flavonoids. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127529. [Google Scholar] [CrossRef]
- Shetta, A.; Kegere, J.; Mamdouh, W. Comparative Study of Encapsulated Peppermint and Green Tea Essential Oils in Chitosan Nanoparticles: Encapsulation, Thermal Stability, in-Vitro Release, Antioxidant and Antibacterial Activities. Int. J. Biol. Macromol. 2019, 126, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Malandrino, G.; Cafiso, V.; Stefani, S.; Geraci, C. Oregano and Thyme Essential Oils Encapsulated in Chitosan Nanoparticles as Effective Antimicrobial Agents against Foodborne Pathogens. Molecules 2021, 26, 4055. [Google Scholar] [CrossRef]
- de Carvalho, S.Y.B.; Almeida, R.R.; Pinto, N.A.R.; de Mayrinck, C.; Vieira, S.S.; Haddad, J.F.; Leitão, A.A.; de L. Guimarães, L.G. Encapsulation of Essential Oils Using Cinnamic Acid Grafted Chitosan Nanogel: Preparation, Characterization and Antifungal Activity. Int. J. Biol. Macromol. 2021, 166, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-x.; Erhunmwunsee, F.; Liu, M.; Yang, K.; Zheng, W.; Tian, J. Antimicrobial Mechanisms of Spice Essential Oils and Application in Food Industry. Food Chem. 2022, 382, 132312. [Google Scholar] [CrossRef]
- Omonijo, F.A.; Ni, L.; Gong, J.; Wang, Q.; Lahaye, L.; Yang, C. Essential Oils as Alternatives to Antibiotics in Swine Production. Anim. Nutr. 2018, 4, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Bona, T.D.M.M.; Pickler, L.; Miglino, L.B.; Kuritza, L.K.; Vasconcelos, S.P.; Santin, E. Óleo Essencial de Orégano, Alecrim, Canela e Extrato de Pimenta No Controle de Salmonella, Eimeria e Clostridium Em Frangos de Corte. Pesqui. Veterinária Bras. 2012, 32, 411–418. [Google Scholar] [CrossRef]
- Guedes Frazzon, A.P.; Saldanha, M.; Junior, L.C.; Frazzon, J. Modulation of Gene Expression by Essential Oils in Bacteria. Adv. Biotechnol. Microbiol. 2018, 8, 555731. [Google Scholar] [CrossRef]
- Spisni, E.; Petrocelli, G.; Imbesi, V.; Spigarelli, R.; Azzinnari, D.; Donati Sarti, M.; Campieri, M.; Valerii, M.C. Antioxidant, Anti-Inflammatory, and Microbial-Modulating Activities of Essential Oils: Implications in Colonic Pathophysiology. Int. J. Mol. Sci. 2020, 21, 4152. [Google Scholar] [CrossRef]
- Nikolić, M.; Stojković, D.; Glamočlija, J.; Ćirić, A.; Marković, T.; Smiljković, M.; Soković, M. Could Essential Oils of Green. and Black Pepper Be Used as Food Preservatives? J. Food Sci. Technol. 2015, 52, 6565–6573. [Google Scholar] [CrossRef]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A Comprehensive Review of the Antibacterial, Antifungal and Antiviral Potential of Essential Oils and Their Chemical Constituents against Drug-Resistant Microbial Pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- Alibi, S.; Selma, W.B.; Mansour, H.B.; Navas, J. Activity of Essential Oils Against Multidrug-Resistant Salmonella Enteritidis. Curr. Microbiol. 2022, 79, 273. [Google Scholar] [CrossRef]
- Somrani, M.; Huertas, J.P.; Iguaz, A.; Debbabi, H.; Palop, A. Biofilm Busters: Exploring the Antimicrobial and Antibiofilm Properties of Essential Oils against Salmonella Enteritidis. Food Sci. Technol. Int. 2024. [Google Scholar] [CrossRef]
- Purkait, S.; Bhattacharya, A.; Bag, A.; Chattopadhyay, R.R. Synergistic Antibacterial, Antifungal and Antioxidant Efficacy of Cinnamon and Clove Essential Oils in Combination. Arch. Microbiol. 2020, 202, 1439–1448. [Google Scholar] [CrossRef]
- Goñi, P.; López, P.; Sánchez, C.; Gómez-Lus, R.; Becerril, R.; Nerín, C. Antimicrobial Activity in the Vapour Phase of a Combination of Cinnamon and Clove Essential Oils. Food Chem. 2009, 116, 982–989. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Roque-Borda, C.A.; Souza Saraiva, M.M.; Monte, D.F.M.; Rodrigues Alves, L.B.; de Almeida, A.M.; Ferreira, T.S.; de Lima, T.S.; Benevides, V.P.; Cabrera, J.M.; Claire, S.; et al. HPMCAS-Coated Alginate Microparticles Loaded with Ctx(Ile 21)-Ha as a Promising Antimicrobial Agent against Salmonella Enteritidis in a Chicken Infection Model. ACS Infect. Dis. 2022, 8, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Roque-Borda, C.A.; Saraiva, M.M.S.; Macedo, W.D., Jr.; Márquez Montesinos, J.C.E.; Meneguin, A.B.; Toledo Borges, A.B.; Crusca, E., Jr.; Garrido, S.S.; de Almeida, A.M.; Marchetto, R.; et al. Chitosan and HPMCAS Double-Coating as Protective Systems for Alginate Microparticles Loaded with Ctx(Ile21)-Ha Antimicrobial Peptide to Prevent Intestinal Infections. Biomaterials 2023, 293, 121978. [Google Scholar] [CrossRef]
- Hasanzadeh Baboli, N.; Hosseini, S.F.; Gharsallaoui, A. Antibacterial and Anti-biofilm Properties of Cinnamaldehyde-loaded Nanoliposomes against Listeria monocytogenes and Salmonella enteritidis Adhered to Stainless Steel. Int. J. Food Sci. Technol. 2023, 58, 5275–5282. [Google Scholar] [CrossRef]
- Yucel, C.; Quagliariello, V.; Iaffaioli, R.V.; Ferrari, G.; Donsì, F. Submicron Complex Lipid Carriers for Curcumin Delivery to Intestinal Epithelial Cells: Effect of Different Emulsifiers on Bioaccessibility and Cell Uptake. Int. J. Pharm. 2015, 494, 357–369. [Google Scholar] [CrossRef]
- Ramalingam, S.; Sahu, B.; Rao, J.R. Hybrid Nanoparticles Emulsified Vegetable Oil as an Environmentally Friendly and Sustainable Leather Fatliquoring Agent. Process Saf. Environ. Prot. 2023, 179, 896–906. [Google Scholar] [CrossRef]
- Jurić, S.; Jurić, M.; Siddique, M.A.B.; Fathi, M. Vegetable Oils Rich in Polyunsaturated Fatty Acids: Nanoencapsulation Methods and Stability Enhancement. Food Rev. Int. 2022, 38, 32–69. [Google Scholar] [CrossRef]
- Moharreri, M.; Vakili, R.; Oskoueian, E.; Rajabzadeh, G. Effects of Microencapsulated Essential Oils on Growth Performance and Biomarkers of Inflammation in Broiler Chickens Challenged with Salmonella Enteritidis. J. Saudi Soc. Agric. Sci. 2022, 21, 349–357. [Google Scholar] [CrossRef]
- Salam, H.S.H.; Mohammed, A.N.; Hosni, A.R.; Shehata, A.A.E. Tracking of Resistant Salmonella Species in Poultry Farms: New Method of Control Using Essential Oils Nano-Emulsion Conjugated with Antimicrobial Agents. Trop. Anim. Sci. J. 2021, 44, 489–501. [Google Scholar] [CrossRef]
- Qu, J.; Zuo, X.; Xu, Q.; Li, M.; Zou, L.; Tao, R.; Liu, X.; Wang, X.; Wang, J.; Wen, L.; et al. Effect of Two Particle Sizes of Nano Zinc Oxide on Growth Performance, Immune Function, Digestive Tract Morphology, and Intestinal Microbiota Composition in Broilers. Animals 2023, 13, 1454. [Google Scholar] [CrossRef]
- El-Shall, N.A.; Shewita, R.S.; Abd El-Hack, M.E.; AlKahtane, A.; Alarifi, S.; Alkahtani, S.; Abdel-Daim, M.M.; Sedeik, M.E. Effect of Essential Oils on the Immune Response to Some Viral Vaccines in Broiler Chickens, with Special Reference to Newcastle Disease Virus. Poult. Sci. 2020, 99, 2944–2954. [Google Scholar] [CrossRef]
- Hesabi Nameghi, A.; Edalatian, O.; Bakhshalinejad, R. Effects of a Blend of Thyme, Peppermint and Eucalyptus Essential Oils on Growth Performance, Serum Lipid and Hepatic Enzyme Indices, Immune Response and Ileal Morphology and Microflora in Broilers. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1388–1398. [Google Scholar] [CrossRef]
- Cerisuelo, A.; Marín, C.; Sánchez-Vizcaíno, F.; Gómez, E.A.; De La Fuente, J.M.; Durán, R.; Fernández, C. The Impact of a Specific Blend of Essential Oil Components and Sodium Butyrate in Feed on Growth Performance and Salmonella Counts in Experimentally Challenged Broilers. Poult. Sci. 2014, 93, 599–606. [Google Scholar] [CrossRef]
- Olgun, O.; Yıldız, A.Ö. Effect of Dietary Supplementation of Essential Oils Mixture on Performance, Eggshell Quality, Hatchability, and Mineral Excretion in Quail Breeders. Environ. Sci. Pollut. Res. 2014, 21, 13434–13439. [Google Scholar] [CrossRef]
- Stingelin, G.M.; Scherer, R.S.; Machado, A.C.; Piva, A.; Grilli, E.; Penha Filho, R.C. The Use of Thymol, Carvacrol and Sorbic Acid in Microencapsules to Control Salmonella Heidelberg, S. Minnesota and S. Typhimurium in Broilers. Front. Vet. Sci. 2023, 9, 1046395. [Google Scholar] [CrossRef]
- Laptev, G.Y.; Yildirim, E.A.; Ilina, L.A.; Filippova, V.A.; Kochish, I.I.; Gorfunkel, E.P.; Dubrovin, A.V.; Brazhnik, E.A.; Narushin, V.G.; Novikova, N.I.; et al. Effects of Essential Oils-Based Supplement and Salmonella Infection on Gene Expression, Blood Parameters, Cecal Microbiome, and Egg Production in Laying Hens. Animals 2021, 11, 360. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Armas, J.P.; Arroyo-Acevedo, J.L.; Ortiz-Sánchez, J.M.; Palomino-Pacheco, M.; Hilario-Vargas, H.J.; Herrera-Calderón, O.; Hilario-Vargas, J. Potential Toxicity of the Essential Oil from Minthostachys Mollis: A Medicinal Plant Commonly Used in the Traditional Andean Medicine in Peru. J. Toxicol. 2019, 2019, 1987935. [Google Scholar] [CrossRef]
- Gebremickael, A.; Gebru, G.; Debella, A.; Addis, G. Acute and Sub-Chronic Oral Toxicity Evaluation of Eucalyptus Globulus Essential Oil-Water Emulsion in Mice. J. Cytol. Histol. 2017, 8. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A. Toxicity of Selected Monoterpenes and Essential Oils Rich in These Compounds. Molecules 2022, 27, 1716. [Google Scholar] [CrossRef]
- Hu, Y.; He, Z.; Zhang, J.; Zhang, C.; Wang, Y.; Zhang, W.; Zhang, F.; Zhang, W.; Gu, F.; Hu, W. Effect of Piper Nigrum Essential Oil in Dextran Sulfate Sodium (DSS)-Induced Colitis and Its Potential Mechanisms. Phytomedicine 2023, 119, 155024. [Google Scholar] [CrossRef]
- Dheyauldeen Salahdin, O.; Othman, H.; Hafsan, H.; Mohammed, F.; Ahmed Hamza, T.; Kadhim, M.M.; Aravindhan, S.; Prakaash, A.S.; Fakri Mustafa, Y. Effect of Licorice Essential Oil (Glycyrrhizaglabraglabra) on Performance and Some Biochemical Parameters of Broiler Chickens. Arch. Razi Inst. 2023, 78, 89–99. [Google Scholar] [CrossRef]
- Huang, J.; Guo, F.; Abbas, W.; Hu, Z.; Liu, L.; Qiao, J.; Bi, R.; Xu, T.; Zhang, K.; Huang, J.; et al. Effects of Microencapsulated Essential Oils and Organic Acids Preparation on Growth Performance, Slaughter Characteristics, Nutrient Digestibility and Intestinal Microenvironment of Broiler Chickens. Poult. Sci. 2024, 103, 103655. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. CLSI M100 Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; Volume 1, ISBN 156238838X. [Google Scholar]
- Munive Nuñez, K.V.; da S. Abreu, A.C.; de Almeida, J.M.; Gonçalves, J.L.; Bonsaglia, É.C.R.; dos Santos, M.V.; Silva, N.C.C. Antimicrobial Activity of Selected Essential Oils against Staphylococcus Aureus from Bovine Mastitis. Dairy 2024, 5, 54–65. [Google Scholar] [CrossRef]
- Wang, P.; Chi, J.; Guo, H.; Wang, S.-X.; Wang, J.; Xu, E.-P.; Dai, L.-P.; Wang, Z.-M. Identification of Differential Compositions of Aqueous Extracts of Cinnamomi Ramulus and Cinnamomi Cortex. Molecules 2023, 28, 2015. [Google Scholar] [CrossRef]
- Berchieri, A.J.; Wigley, P.; Page, K.; Murphy, C.K.; Barrow, P.A. Further Studies on Vertical Transmission and Persistence of Salmonella enterica Serovar Enteritidis Phage Type 4 in Chickens. Avian Pathol. 2001, 30, 297–310. [Google Scholar] [CrossRef]
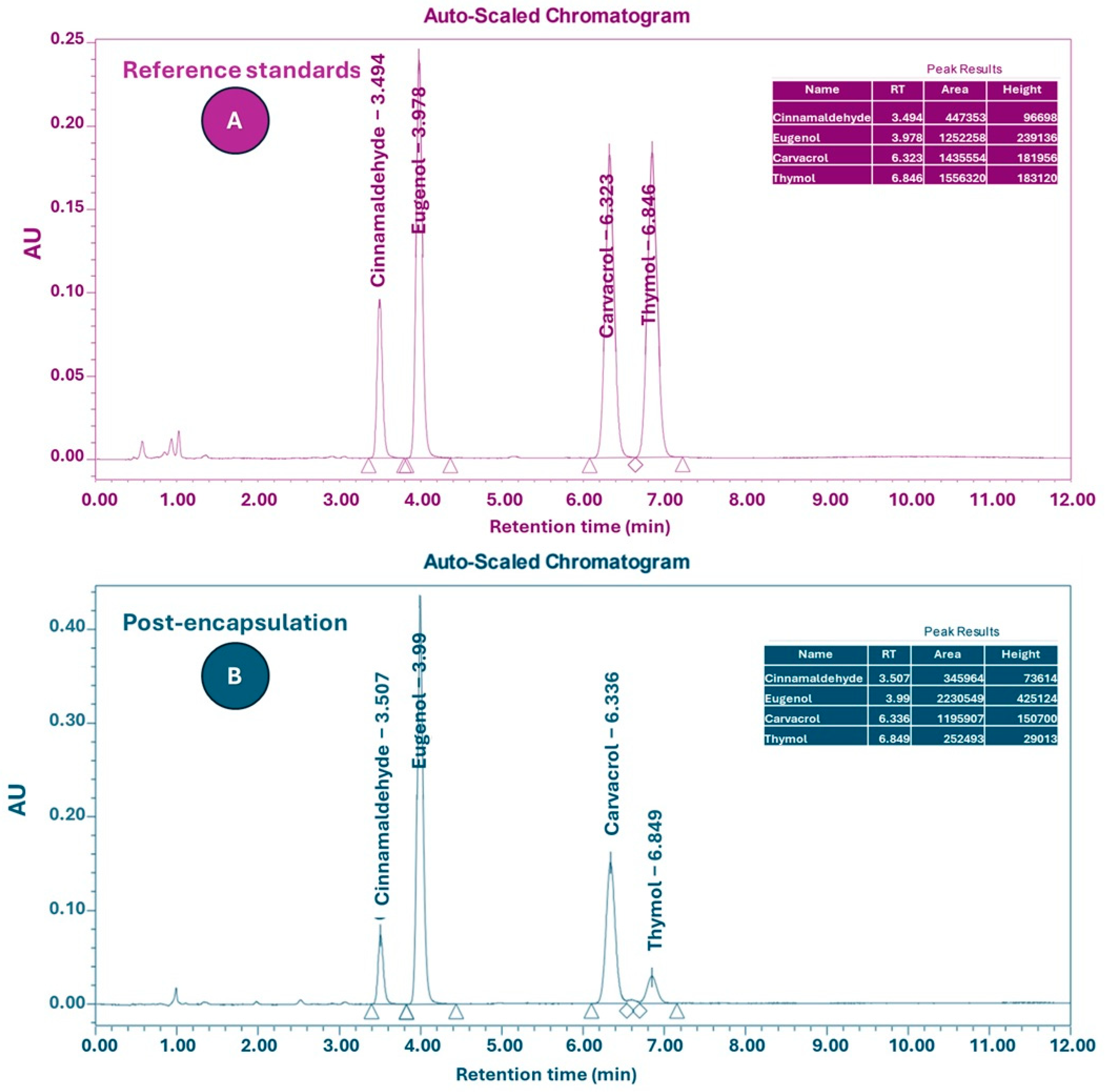
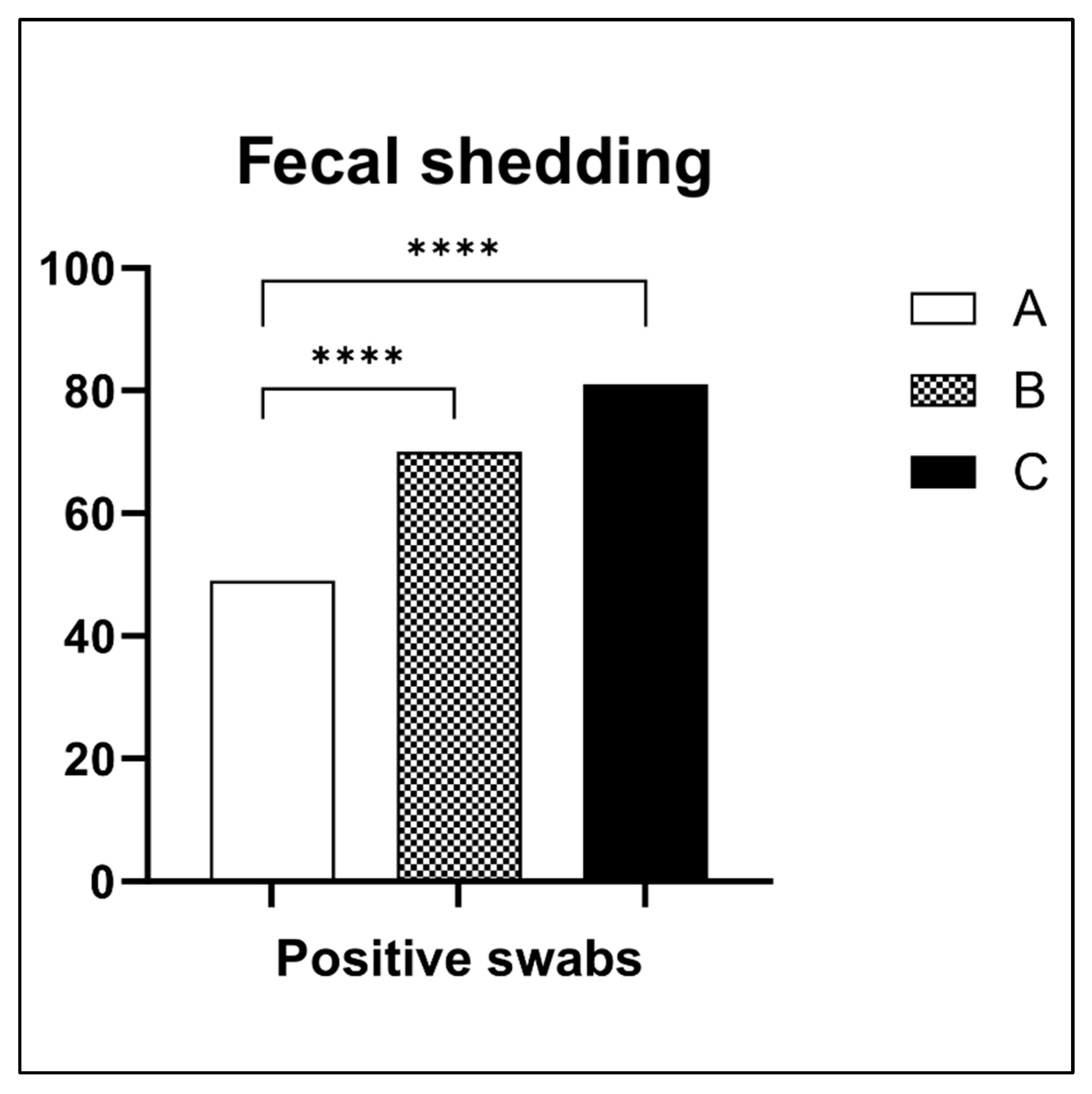
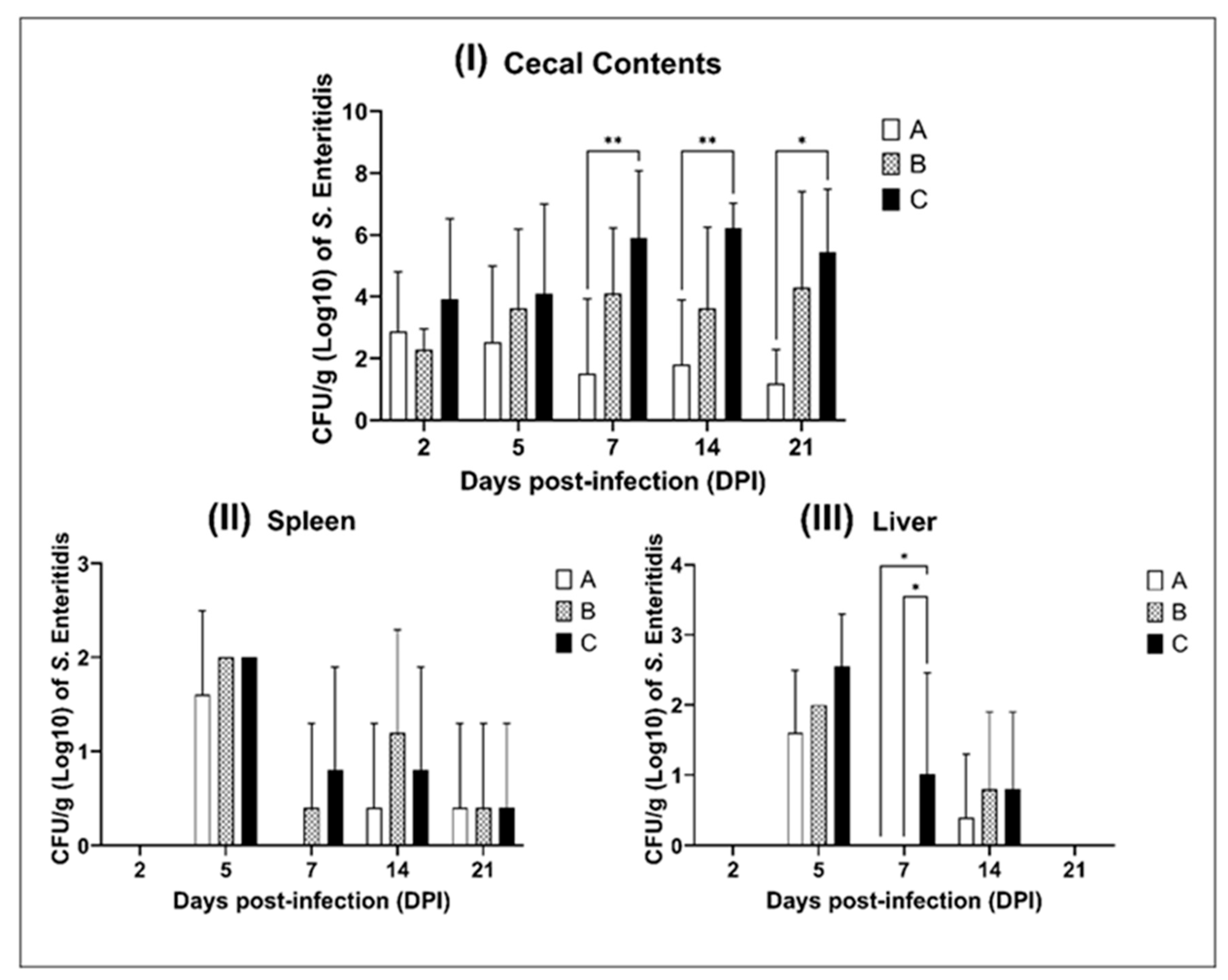

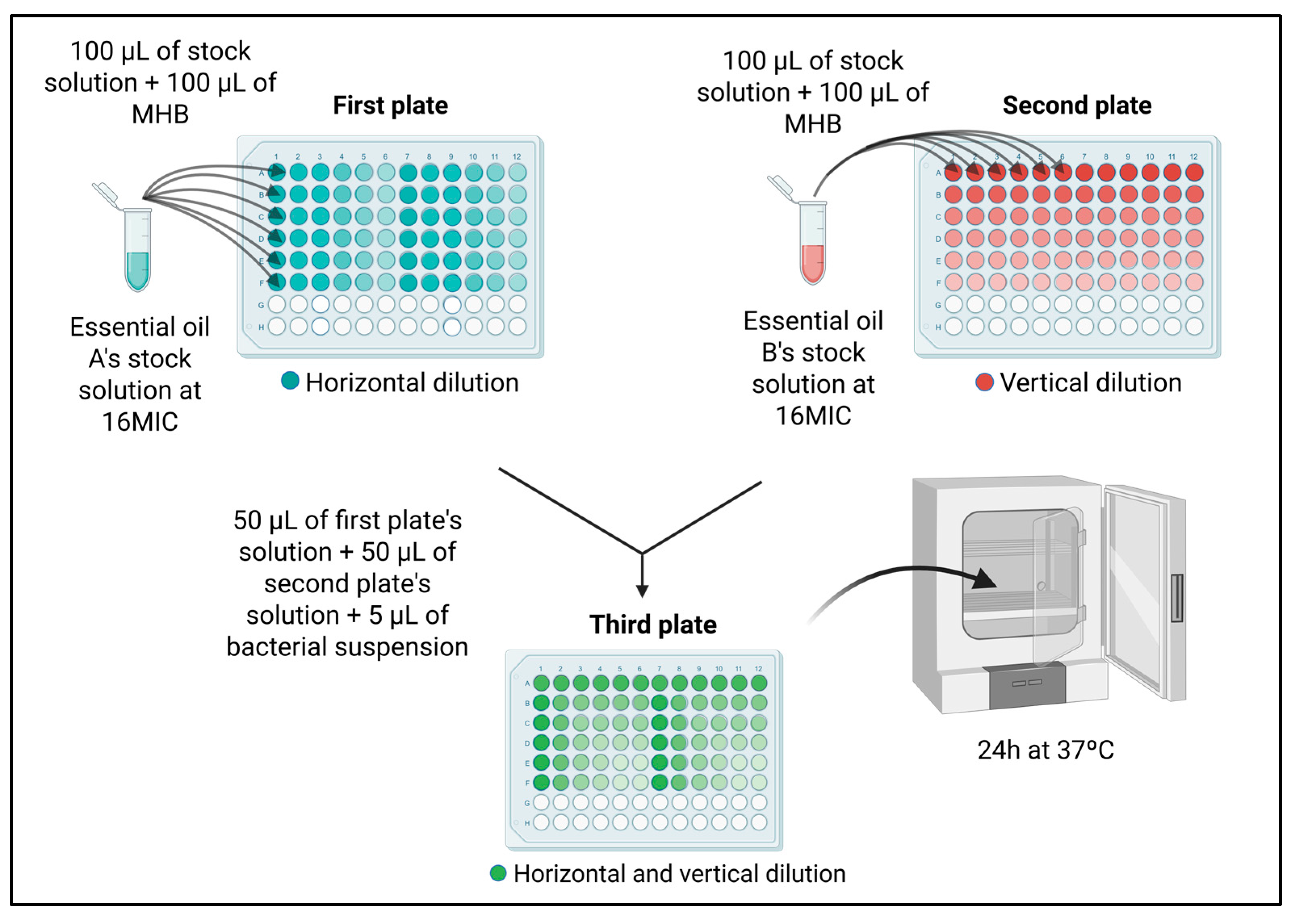
| EOs | MIC (mg/mL) | MBC (mg/mL) |
|---|---|---|
| Oregano | 0.49 | 0.99 |
| Cinnamon | 0.22 | 0.44 |
| Clove | 0.69 | 2.76 |
| Bay laurel | 4.56 | 4.56 |
| Citronella | 4.48 | 4.48 |
| White thyme | 0.57 | 2.28 |
| Lemongrass | 2.25 | 2.25 |
| Rosemary | 11.52 | 23.05 |
| Tea tree | 1.37 | 1.37 |
| Black pepper * | - | - |
| Eucalyptus | 5.83 | 23.32 |
| Ginger * | - | - |
| Basil | 5.79 | 23.17 |
| Copaiba * | - | - |
| Sweet pear orange * | - | - |
| Mentha | 0.69 | 2.77 |
| EOs Combinations | FICI | Classification |
|---|---|---|
| Oregano/Cinnamon | 2 | Commutative |
| Oregano/White thyme | 2 | Commutative |
| Cinnamon/White thyme | 4 | Antagonistic |
| Cinnamon/Clove | 2 | Commutative |
| Oregano/Clove | 1 | Indifferent |
| White thyme/Clove | 2 | Commutative |
| Mentha/White thyme | 8 | Antagonistic |
| Mentha/Clove | 2 | Commutative |
| Mentha/Cinnamon | 6 | Antagonistic |
| Mentha/Oregano | 0.75 | Synergistic |
| Compounds | Before Encapsulation (%) | Post-Encapsulation (%) |
|---|---|---|
| Carvacrol | 20.7 | 2.02 |
| Thymol | 3.98 | 0.39 |
| Eugenol | 35.3 | 3.49 |
| Cinnamaldehyde | 19.9 | 1.99 |
| Common Name | Scientific Name | Botanical Source | Geographical Origin |
|---|---|---|---|
| Oregano | Origanum vulgare | Leaf | Spain |
| Cinnamon | Cinnamomum zeylanicum | Bark | Brazil |
| Clove | Eugenia caryophyllus | Flower | Brazil |
| Mentha | Mentha arvensis | Leaf | Brazil |
| White thyme | Thymus vulgaris | Leaf | India |
| Citronella | Cymbopogon nardus | Leaf | Brazil |
| Lemongrass | Cymbopogon flexuosos | Leaf | Brazil |
| Rosemary | Rosmarinus officinalis | Leaf | Spain |
| Tea tree | Melaleuca alternifolia | Leaf | India |
| Black pepper | Piper nigrum L. | Fruit | India |
| Eucalyptus | Eucalyptus globulus | Leaf | Brazil |
| Ginger | Zingiber officinale | Root | China |
| Basil | Ocimun basilicum L. | Leaf | India |
| Copaiba | Copaifera officinalis | Leaf | Brazil |
| Sweet pear orange | Citrus sinensis | Fruit bark | Brazil |
| Bay laurel | Laurus nobilis | Leaf | Brazil |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, H.L.d.S.; Benevides, V.P.; Kolososki, I.M.M.; Saraiva, M.M.S.; Souki, N.P.D.B.G.; Barros, T.A.; Rabelo, A.L.C.; Ferreira, V.A.; Dix, M.F.F.; Almeida, A.M.; et al. Effective Control of Salmonella Enteritidis in Poultry by Dietary Supplementation with Microencapsulated Essential Oils. Antibiotics 2025, 14, 552. https://doi.org/10.3390/antibiotics14060552
Rodrigues HLdS, Benevides VP, Kolososki IMM, Saraiva MMS, Souki NPDBG, Barros TA, Rabelo ALC, Ferreira VA, Dix MFF, Almeida AM, et al. Effective Control of Salmonella Enteritidis in Poultry by Dietary Supplementation with Microencapsulated Essential Oils. Antibiotics. 2025; 14(6):552. https://doi.org/10.3390/antibiotics14060552
Chicago/Turabian StyleRodrigues, Heitor Leocádio de Souza, Valdinete Pereira Benevides, Isis Mari Miyashiro Kolososki, Mauro M. S. Saraiva, Nayla Pádua Del Bianco Gontijo Souki, Tarley Araújo Barros, André Luis Costa Rabelo, Viviane Amorim Ferreira, Melissa Freitas Feitosa Dix, Adriana Maria Almeida, and et al. 2025. "Effective Control of Salmonella Enteritidis in Poultry by Dietary Supplementation with Microencapsulated Essential Oils" Antibiotics 14, no. 6: 552. https://doi.org/10.3390/antibiotics14060552
APA StyleRodrigues, H. L. d. S., Benevides, V. P., Kolososki, I. M. M., Saraiva, M. M. S., Souki, N. P. D. B. G., Barros, T. A., Rabelo, A. L. C., Ferreira, V. A., Dix, M. F. F., Almeida, A. M., Roque-Borda, C. A., & Berchieri Junior, A. (2025). Effective Control of Salmonella Enteritidis in Poultry by Dietary Supplementation with Microencapsulated Essential Oils. Antibiotics, 14(6), 552. https://doi.org/10.3390/antibiotics14060552











