Therapeutic Drug Monitoring-Based Population Pharmacokinetics of Amikacin in Patients at a Teaching Hospital
Abstract
1. Introduction
2. Results
2.1. Patients
2.2. Amikacin popPK Model
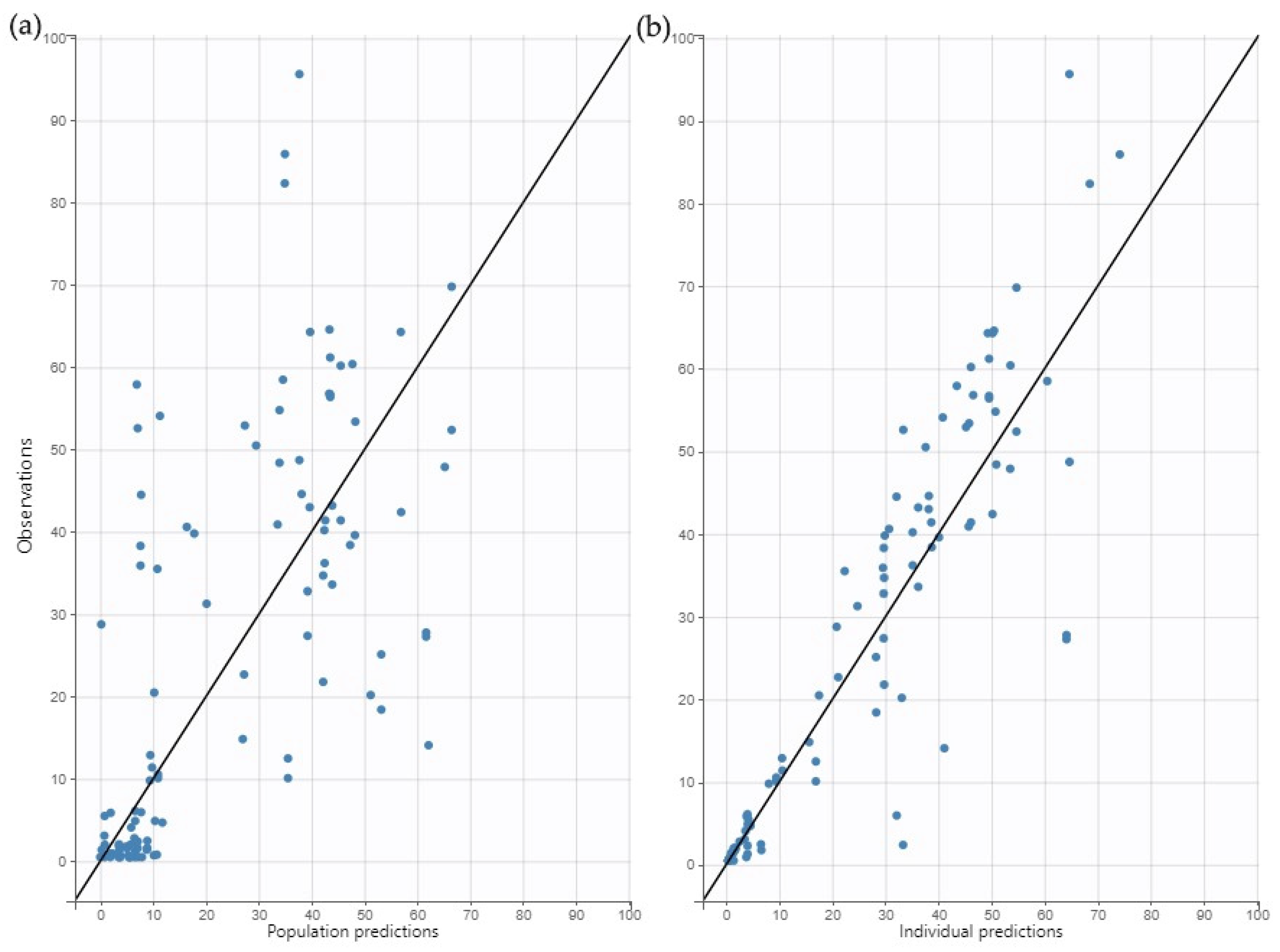
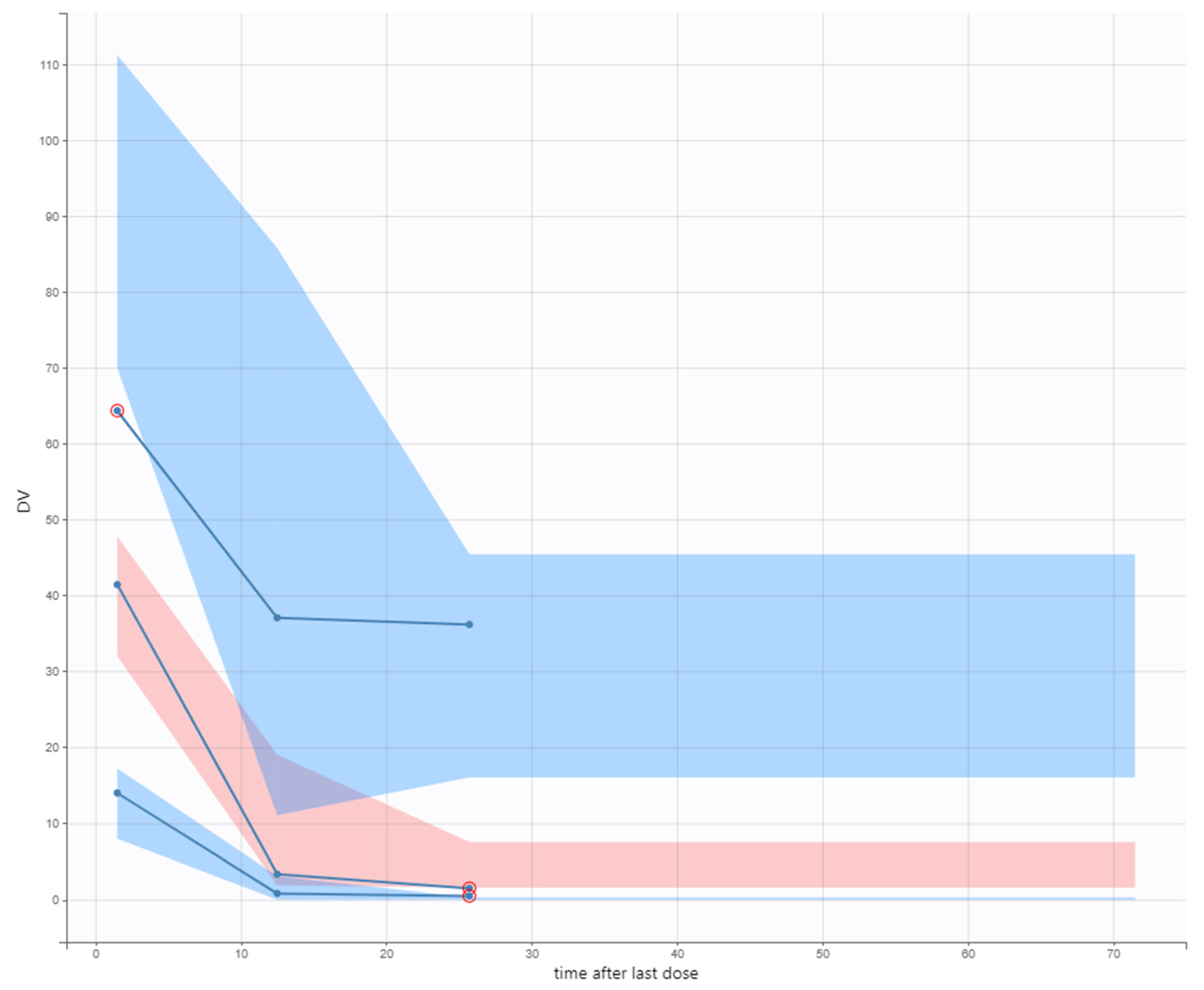
2.3. Model Evaluation
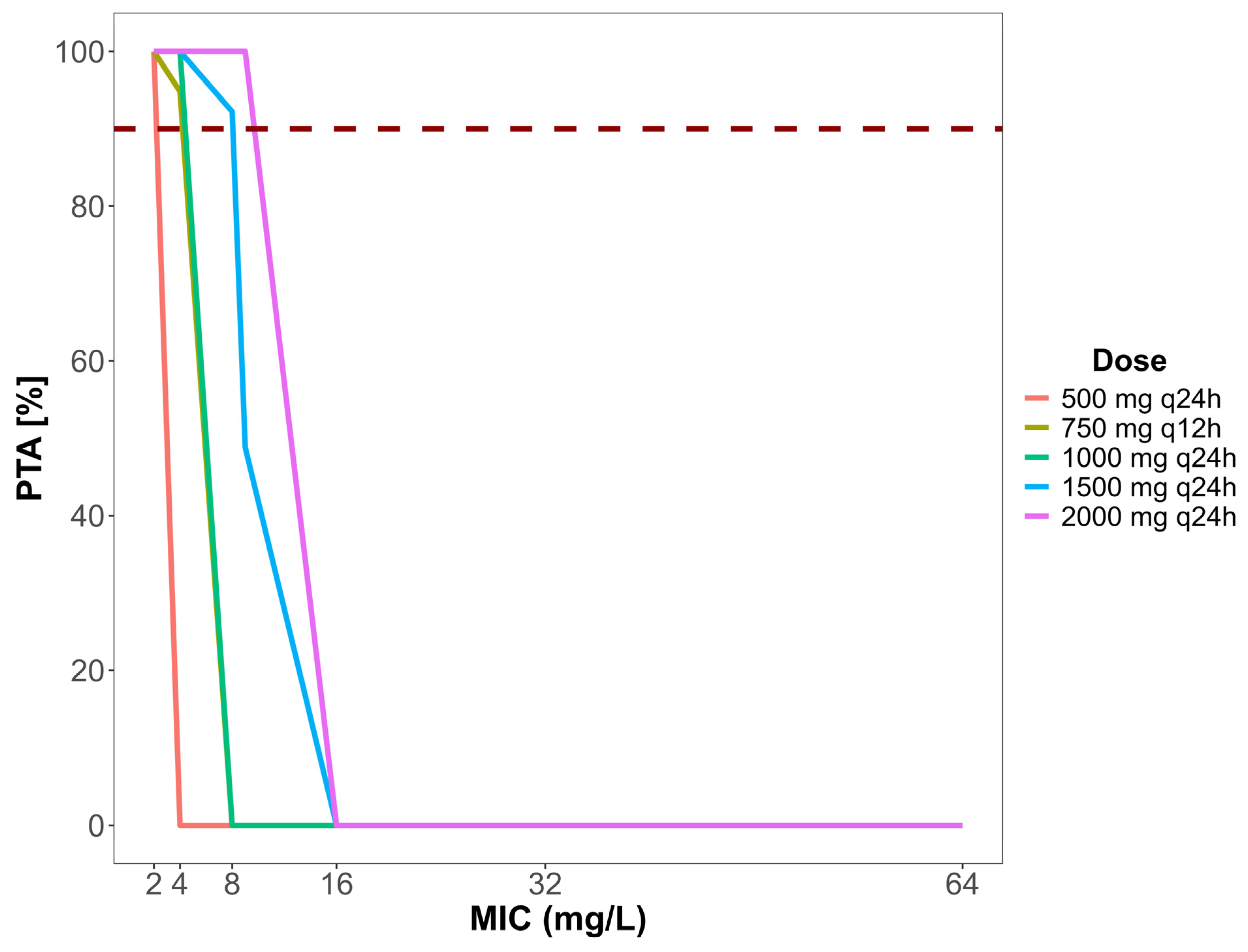
2.4. Dose Simulation and Target Attainment
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Blood Samples and Amikacin Quantification
4.3. Data Collection
4.4. Population Pharmacokinetic (popPK) Analysis
4.4.1. Model Construction and Evaluation
4.4.2. Evaluation of Amikacin Doses by Monte Carlo Simulation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harikumar, G.; Krishanan, K. The Growing Menace of Drug Resistant Pathogens and Recent Strategies to Overcome Drug Resistance: A Review. J. King Saud. Univ. Sci. 2022, 34, 101979. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Van Boeckel, T.P.; Carmeli, Y.; Cosgrove, S.; Laxminarayan, R. Global Incidence in Hospital-Associated Infections Resistant to Antibiotics: An Analysis of Point Prevalence Surveys from 99 Countries. PLoS Med. 2023, 20, e1004178. [Google Scholar] [CrossRef] [PubMed]
- ECDC European Centre for Disease Prevention and Control. Economic Evaluations of Interventions to Prevent Healthcare-Associated Infections; ECDC: Solna, Stockholm, 2017. [CrossRef]
- Kato, H.; Hagihara, M.; Hirai, J.; Sakanashi, D.; Suematsu, H.; Nishiyama, N.; Koizumi, Y.; Yamagishi, Y.; Matsuura, K.; Mikamo, H. Evaluation of Amikacin Pharmacokinetics and Pharmacodynamics for Optimal Initial Dosing Regimen. Drugs R&D 2017, 17, 177–187. [Google Scholar] [CrossRef]
- Pérez-Blanco, J.S.; Sáez Fernández, E.M.; Calvo, M.V.; Lanao, J.M.; Martín-Suárez, A. Evaluation of Current Amikacin Dosing Recommendations and Development of an Interactive Nomogram: The Role of Albumin. Pharmaceutics 2021, 13, 264. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Alffenaar, J.-W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial Therapeutic Drug Monitoring in Critically Ill Adult Patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- Abdul–Aziz, M.H.; Brady, K.; Cotta, M.O.; Roberts, J.A. Therapeutic Drug Monitoring of Antibiotics: Defining the Therapeutic Range. Ther. Drug Monit. 2022, 44, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, S.; Hadi, A.M.; Najmeddin, F.; Shahrami, B.; Rouini, M.R.; Najafi, A.; Mojtahedzadeh, M. Evaluation of Amikacin Dosing Schedule in Critically Ill Elderly Patients with Different Stages of Renal Dysfunction. Eur. J. Hosp. Pharm. 2022, 29, e67–e71. [Google Scholar] [CrossRef]
- Marsot, A.; Hraiech, S.; Cassir, N.; Daviet, F.; Parzy, G.; Blin, O.; Papazian, L.; Guilhaumou, R. Aminoglycosides in Critically Ill Patients: Which Dosing Regimens for Which Pathogens? Int. J. Antimicrob. Agents 2020, 56, 106124. [Google Scholar] [CrossRef]
- Medellín-Garibay, S.E.; Romano-Aguilar, M.; Parada, A.; Suárez, D.; Romano-Moreno, S.; Barcia, E.; Cervero, M.; García, B. Amikacin Pharmacokinetics in Elderly Patients with Severe Infections. Eur. J. Pharm. Sci. 2022, 175, 106219. [Google Scholar] [CrossRef]
- Avent, M.L.; Rogers, B.A.; Cheng, A.C.; Paterson, D.L. Current Use of Aminoglycosides: Indications, Pharmacokinetics and Monitoring for Toxicity. Intern. Med. J. 2011, 41, 441–449. [Google Scholar] [CrossRef]
- Li, S.; Zhu, S.; Xie, F. Population Pharmacokinetic/Pharmacodynamic Evaluations of Amikacin Dosing in Critically Ill Patients Undergoing Continuous Venovenous Hemodiafiltration. J. Pharm. Pharmacol. 2023, 75, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J.; Abate, B.J.; Kang, S.L.; Ruffing, M.J.; Lerner, S.A.; Drusano, G.L. Prospective Evaluation of the Effect of an Aminoglycoside Dosing Regimen on Rates of Observed Nephrotoxicity and Ototoxicity. Antimicrob. Agents Chemother. 1999, 43, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- van Altena, R.; Dijkstra, J.A.; van der Meer, M.E.; Borjas Howard, J.F.; Kosterink, J.G.W.; van Soolingen, D.; van der Werf, T.S.; Alffenaar, J.W.C. Reduced Chance of Hearing Loss Associated with Therapeutic Drug Monitoring of Aminoglycosides in the Treatment of Multidrug-Resistant Tuberculosis. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Boidin, C.; Bourguignon, L.; Cohen, S.; Roger, C.; Lefrant, J.Y.; Roberts, J.A.; Allaouchiche, B.; Lepape, A.; Friggeri, A.; Goutelle, S. Amikacin Initial Dose in Critically Ill Patients: A Nonparametric Approach to Optimize a Priori Pharmacokinetic/Pharmacodynamic Target Attainments in Individual Patients. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Gatti, M.; Rinaldi, M.; Laici, C.; Siniscalchi, A.; Viale, P.; Pea, F. Role of a Real-Time TDM-Based Expert Clinical Pharmacological Advice Program in Optimizing the Early Pharmacokinetic/Pharmacodynamic Target Attainment of Continuous Infusion Beta-Lactams Among Orthotopic Liver Transplant Recipients with Documented or Suspected Gram-Negative Infections. Antibiotics 2023, 12, 1599. [Google Scholar] [CrossRef]
- Brunet, M.; van Gelder, T.; Åsberg, A.; Haufroid, V.; Hesselink, D.A.; Langman, L.; Lemaitre, F.; Marquet, P.; Seger, C.; Shipkova, M.; et al. Therapeutic Drug Monitoring of Tacrolimus-Personalized Therapy. Ther. Drug Monit. 2019, 41, 261–307. [Google Scholar] [CrossRef]
- de Velde, F.; Mouton, J.W.; de Winter, B.C.M.; van Gelder, T.; Koch, B.C.P. Clinical Applications of Population Pharmacokinetic Models of Antibiotics: Challenges and Perspectives. Pharmacol. Res. 2018, 134, 280–288. [Google Scholar] [CrossRef]
- Dasgupta, A.; Krasowski, M. Therapeutic Drug Monitoring Data, 4th ed.; Academic Press: Cambridge, MA, USA, 2019; ISBN 9780128158500. [Google Scholar]
- Clarke, W.; Dasgupta, A. Clinical Challenges in Therapeutic Drug Monitoring; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780128020258. [Google Scholar]
- Ette, E.I.; Williams, P.J. Pharmacometrics: The Science of Quantitative Pharmacology; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 9780471677833. [Google Scholar]
- Aquino, M.; Tinoco, M.; Bicker, J.; Falcão, A.; Rocha, M.; Fortuna, A. Therapeutic Drug Monitoring of Amikacin in Neutropenic Oncology Patients. Antibiotics 2023, 12, 373. [Google Scholar] [CrossRef]
- Roger, C.; Louart, B.; Elotmani, L.; Barton, G.; Escobar, L.; Koulenti, D.; Lipman, J.; Leone, M.; Muller, L.; Boutin, C.; et al. An International Survey on Aminoglycoside Practices in Critically Ill Patients: The AMINO III Study. Ann. Intensive Care 2021, 11, 49. [Google Scholar] [CrossRef]
- Sadeghi, K.; Hamishehkar, H.; Najmeddin, F.; Ahmadi, A.; Hazrati, E.; Honarmand, H.; Mojtahedzadeh, M. High-Dose Amikacin for Achieving Serum Target Levels in Critically Ill Elderly Patients. Infect. Drug Resist. 2018, 11, 223–228. [Google Scholar] [CrossRef]
- Aréchiga-Alvarado, N.A.; Medellín-Garibay, S.E.; Milán-Segovia, R.d.C.; Ortiz-Álvarez, A.; Magaña-Aquino, M.; Romano-Moreno, S. Population Pharmacokinetics of Amikacin Administered Once Daily in Patients with Different Renal Functions. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef] [PubMed]
- Coste, A.; Deslandes, G.; Jalin, L.; Corvec, S.; Caillon, J.; Boutoille, D.; Grégoire, M.; Bretonnière, C. PK/PD Targets of Amikacin and Gentamicin in ICU Patients. Med. Mal. Infect. 2020, 50, 709–714. [Google Scholar] [CrossRef] [PubMed]
- EUCAST The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 12.0. Available online: http://www.eucast.org (accessed on 17 July 2022).
- Marsot, A.; Guilhaumou, R.; Riff, C.; Blin, O. Amikacin in Critically Ill Patients: A Review of Population Pharmacokinetic Studies. Clin. Pharmacokinet. 2017, 56, 127–138. [Google Scholar] [CrossRef]
- Coste, A.; Bellouard, R.; Deslandes, G.; Jalin, L.; Roger, C.; Ansart, S.; Dailly, E.; Bretonnière, C.; Grégoire, M. Development of a Predictive Dosing Nomogram to Achieve PK/PD Targets of Amikacin Initial Dose in Critically Ill Patients: A Non-Parametric Approach. Antibiotics 2023, 12, 123. [Google Scholar] [CrossRef]
- Mabilat, C.; Gros, M.F.; Nicolau, D.; Mouton, J.W.; Textoris, J.; Roberts, J.A.; Cotta, M.O.; van Belkum, A.; Caniaux, I. Diagnostic and Medical Needs for Therapeutic Drug Monitoring of Antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 39, 791–797. [Google Scholar] [CrossRef]
- Illamola, S.M.; Huynh, H.Q.; Liu, X.; Bhakt, Z.N.; Sherwin, C.M.; Liou, T.G.; Carveth, H.; Young, D.C. Population Pharmacokinetics of Amikacin in Adult Patients with Cystic Fibrosis. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Kato, H.; Parker, S.L.; Roberts, J.A.; Hagihara, M.; Asai, N.; Yamagishi, Y.; Paterson, D.L.; Mikamo, H. Population Pharmacokinetics Analysis of Amikacin Initial Dosing Regimen in Elderly Patients. Antibiotics 2021, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Oda, K.; Nishihara, M.; Neo, M. A Simulation Study on Model-Informed Precision Dosing of Amikacin for Achieving Target Area under the Concentration–Time Curve. Br. J. Clin. Pharmacol. 2024, 90, 1173–1182. [Google Scholar] [CrossRef]
- Fukumoto, S.; Ohbayashi, M.; Okada, A.; Kohyama, N.; Tamatsukuri, T.; Inoue, H.; Kato, A.; Kotani, T.; Sagara, H.; Dohi, K.; et al. Population Pharmacokinetic Model and Dosing Simulation of Meropenem Using Measured Creatinine Clearance for Patients with Sepsis. Ther. Drug Monit. 2023, 45, 392–399. [Google Scholar] [CrossRef]
- Cockcroft, D.W.; Gault, H. Prediction of Creatinine Clearance from Serum Creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- De Winter, S.; van Hest, R.; Dreesen, E.; Annaert, P.; Wauters, J.; Meersseman, W.; Van den Eede, N.; Desmet, S.; Verelst, S.; Vanbrabant, P.; et al. Quantification and Explanation of the Variability of First-Dose Amikacin Concentrations in Critically Ill Patients Admitted to the Emergency Department: A Population Pharmacokinetic Analysis. Eur. J. Drug Metab. Pharmacokinet. 2021, 46, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Duong, A.; Simard, C.; Wang, Y.L.; Williamson, D.; Marsot, A. Aminoglycosides in the Intensive Care Unit: What Is New in Population PK Modeling? Antibiotics 2021, 10, 507. [Google Scholar] [CrossRef] [PubMed]
- Siebinga, H.; Robb, F.; Thomson, A.H. Population Pharmacokinetic Evaluation and Optimization of Amikacin Dosage Regimens for the Management of Mycobacterial Infections. J. Antimicrob. Chemother. 2020, 75, 2933–2940. [Google Scholar] [CrossRef]
- Ahamadi, M.; Largajolli, A.; Diderichsen, P.M.; de Greef, R.; Kerbusch, T.; Witjes, H.; Chawla, A.; Davis, C.B.; Gheyas, F. Operating Characteristics of Stepwise Covariate Selection in Pharmacometric Modeling. J Pharmacokinet Pharmacodyn 2019, 46, 273–285. [Google Scholar] [CrossRef]
- Steffens, N.A.; Petreceli, R.R.; Azevedo, V.C.; França, A.S.; Hahn, R.Z.; Bondan, A.P.; Linden, R.; Charão, M.F.; Schwarzbold, A.d.V.; Brucker, N. Amikacin Therapeutic Drug Monitoring: Evaluation of Therapy Performance and Analytical Techniques in a Developing Country Setting. Clin Biochem. 2025, 136, 110874. [Google Scholar] [CrossRef] [PubMed]
- Carrié, C.; Delzor, F.; Roure, S.; Dubuisson, V.; Petit, L.; Molimard, M.; Breilh, D.; Biais, M. Population Pharmacokinetic Study of the Suitability of Standard Dosing Regimens of Amikacin in Critically Ill Patients with Open-Abdomen and Negative-Pressure Wound Therapy. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Duszynska, W.; Taccone, F.S.; Hurkacz, M.; Kowalska-Krochmal, B.; Wiela-Hojeńska, A.; Kübler, A. Therapeutic Drug Monitoring of Amikacin in Septic Patients. Crit. Care 2013, 17, R165. [Google Scholar] [CrossRef]
- Boyer, A.; Timsit, J.-F.; Klouche, K.; Canet, E.; Phan, T.; Bohé, J.; Rubin, S.; Orieux, A.; Lautrette, A.; Gruson, D.; et al. Aminoglycosides in Critically Ill Septic Patients With Acute Kidney Injury Receiving Continuous Renal Replacement Therapy: A Multicenter, Observational Study. Clin. Ther. 2021, 43, 1116–1124. [Google Scholar] [CrossRef]
- Usman, M.; Frey, O.R.; Hempel, G. Population Pharmacokinetics of Meropenem in Elderly Patients: Dosing Simulations Based on Renal Function. Eur. J. Clin. Pharmacol. 2017, 73, 333–342. [Google Scholar] [CrossRef]
- Blaser, J.; Rüttimann, S.; Bhend, H.; Lüthy, R. Increase of Amikacin Half-Life during Therapy in Patients with Renal Insufficiency. Antimicrob. Agents Chemother. 1983, 23, 888–891. [Google Scholar] [CrossRef]
- Dasgupta, A.; Krasowski, M.D. Therapeutic Drug Monitoring of Antimicrobial, Antifungal and Antiviral Agents. In Therapeutic Drug Monitoring Data; Elsevier: Amsterdam, The Netherlands, 2020; pp. 159–197. [Google Scholar]
- da Silva, A.C.C.; de Lima Feltraco Lizot, L.; Bastiani, M.F.; Antunes, M.V.; Brucker, N.; Linden, R. Ready for TDM: Simultaneous Quantification of Amikacin, Vancomycin and Creatinine in Human Plasma Employing Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry. Clin. Biochem. 2019, 70, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Nephrological Calculator from the Website of the Brazilian Society of Nephrology. Cockcroft & Gault–SBN. Available online: https://sbn.org.br/medicos/utilidades/calculadoras-nefrologicas/cockcroft-gault/ (accessed on 21 December 2024).

| Parameter | Mean ± SD | Median [Range] |
|---|---|---|
| Male/Female | 33/6 | - |
| Age (Years) | 46.33 (16.75) | 51 [4–75] |
| Pediatric | 2 | - |
| Adult | 28 | - |
| Elderly | 9 | - |
| Weight (kg) | 70.29 (25.07) | 69.40 [15.60–143.80] |
| BMI (kg/m2) | 24.57 (7.62) | 23.20 [10.60–50.70] |
| Serum creatinine (mg/dL) | 1.48 (1.55) | 0.93 [0.16–8.35] |
| CrCl (mL/min) 1 | 100.3 (102.2) | 79.01 [12.97–517.97] |
| Microorganisms isolated | n | |
| Pseudomonas aeruginosa | 7 | |
| Acinetobacter baumannii | 1 | |
| Klebsiella pneeumoniae | 20 | |
| Escherichia coli | 2 | |
| Morganella morganii | 2 | |
| Enterobacter | 1 | |
| Staphylococcus haemophilus | 1 | |
| Enterococcus faecalis | 1 | |
| Vancomycin resistant Enterococcus | 1 | |
| Methicillin resistant Staphylococcus aureus | 1 |
| Parameter | Final Estimate | RSE (%) | Bootstrap Mean | Median | Difference (%) |
|---|---|---|---|---|---|
| Cl (L/h) | 1.49 | 12.96 | 1.47 | 1.49 | −1.29 |
| Vd (L) | 23.18 | 23.73 | 24.08 | 23.86 | 3.91 |
| βCrCl on Cl | 0.004 | 57.15 | 0.005 | 0.0041 | 24.93 |
| IIV (%CV) | |||||
| ΩCl | 0.67 (74.8) | 17.01 | 0.63 | 0.62 | −6.21 |
| ΩVd | 0.47 (49.89) | 37.69 | 0.41 | 0.42 | −12.51 |
| Proportional error model | 0.38 | 14.86 | 0.39 | 0.39 | 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steffens, N.A.; Zimmermann, E.S.; Azeredo, F.J.; Linden, R.; Finatto, L.J.; Hahn, R.Z.; Schwarzbold, A.V.; Pacheco, L.S.; Brucker, N. Therapeutic Drug Monitoring-Based Population Pharmacokinetics of Amikacin in Patients at a Teaching Hospital. Antibiotics 2025, 14, 531. https://doi.org/10.3390/antibiotics14060531
Steffens NA, Zimmermann ES, Azeredo FJ, Linden R, Finatto LJ, Hahn RZ, Schwarzbold AV, Pacheco LS, Brucker N. Therapeutic Drug Monitoring-Based Population Pharmacokinetics of Amikacin in Patients at a Teaching Hospital. Antibiotics. 2025; 14(6):531. https://doi.org/10.3390/antibiotics14060531
Chicago/Turabian StyleSteffens, Nadine Arnold, Estevan Sonego Zimmermann, Francine Johansson Azeredo, Rafael Linden, Luis Junior Finatto, Roberta Zilles Hahn, Alexandre Vargas Schwarzbold, Liliane Souto Pacheco, and Natália Brucker. 2025. "Therapeutic Drug Monitoring-Based Population Pharmacokinetics of Amikacin in Patients at a Teaching Hospital" Antibiotics 14, no. 6: 531. https://doi.org/10.3390/antibiotics14060531
APA StyleSteffens, N. A., Zimmermann, E. S., Azeredo, F. J., Linden, R., Finatto, L. J., Hahn, R. Z., Schwarzbold, A. V., Pacheco, L. S., & Brucker, N. (2025). Therapeutic Drug Monitoring-Based Population Pharmacokinetics of Amikacin in Patients at a Teaching Hospital. Antibiotics, 14(6), 531. https://doi.org/10.3390/antibiotics14060531