Do Critically Ill Patients Undergoing Continuous Renal Replacement Therapy Require Ceftaroline Dosage Adjustments? Ceftaroline PopPK Model and Dosage Simulations with the Probability of Target Attainment Analysis Based on Retrospective Data †
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
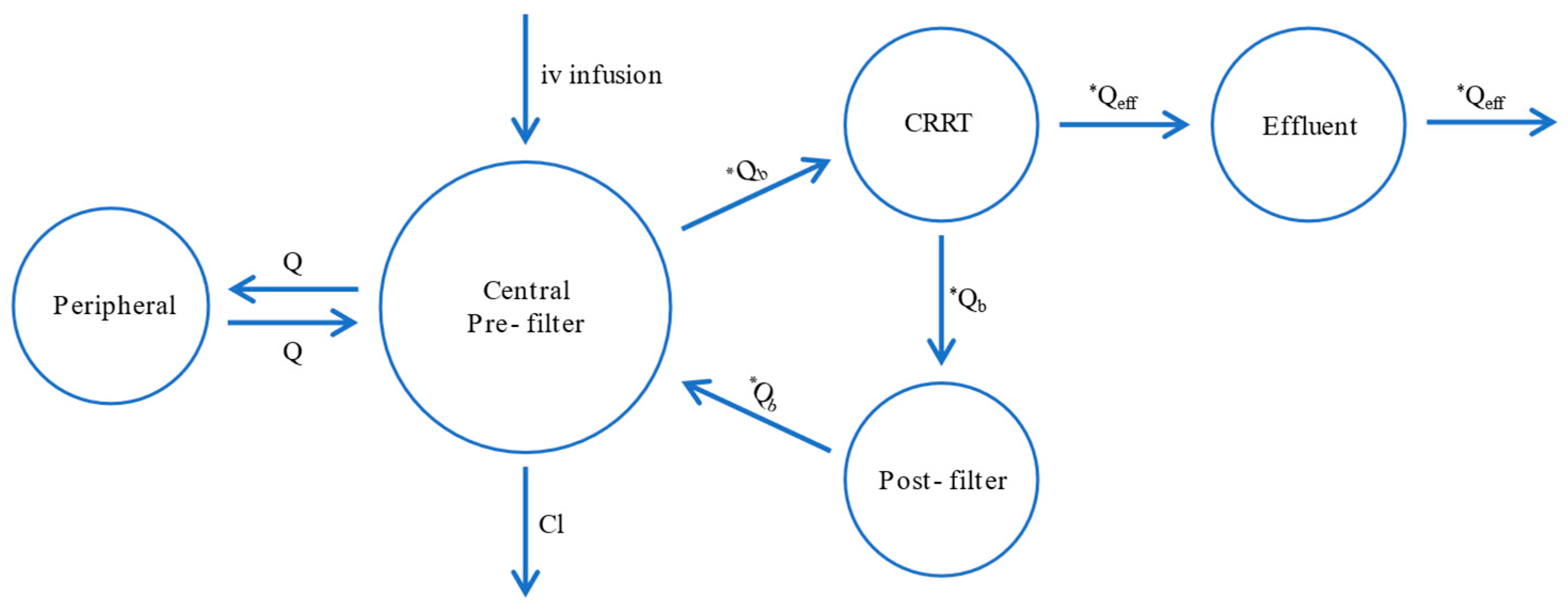
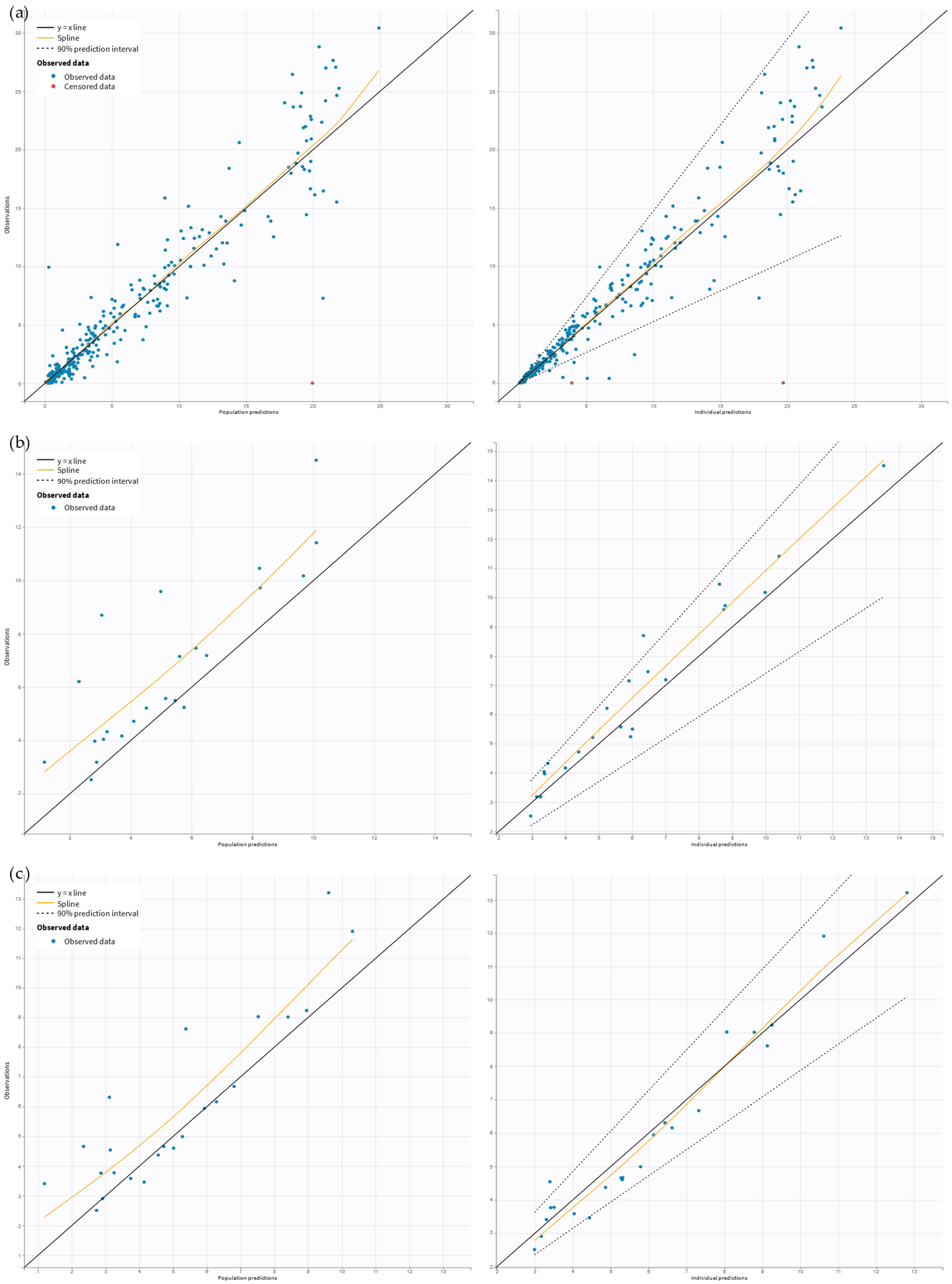
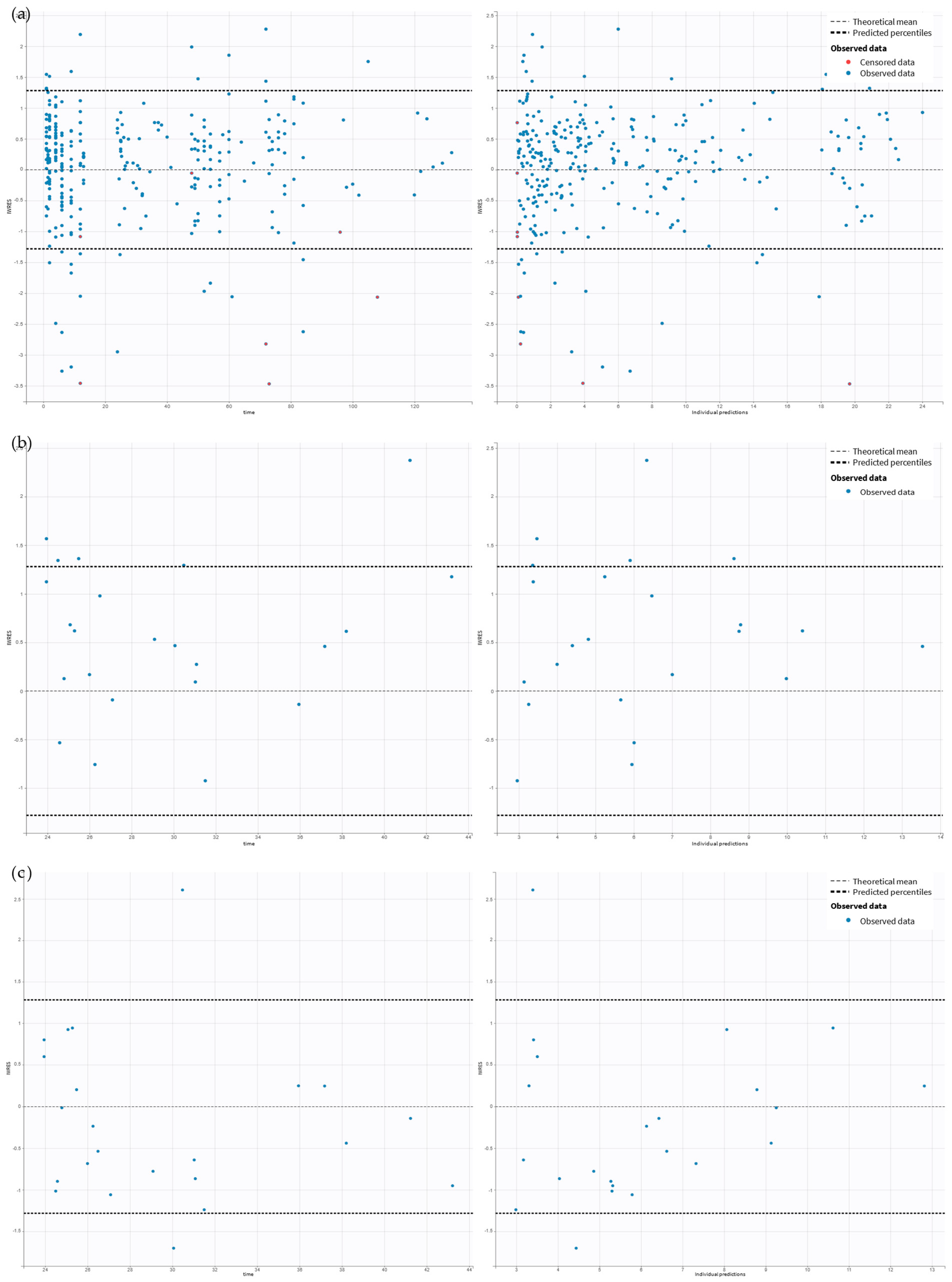
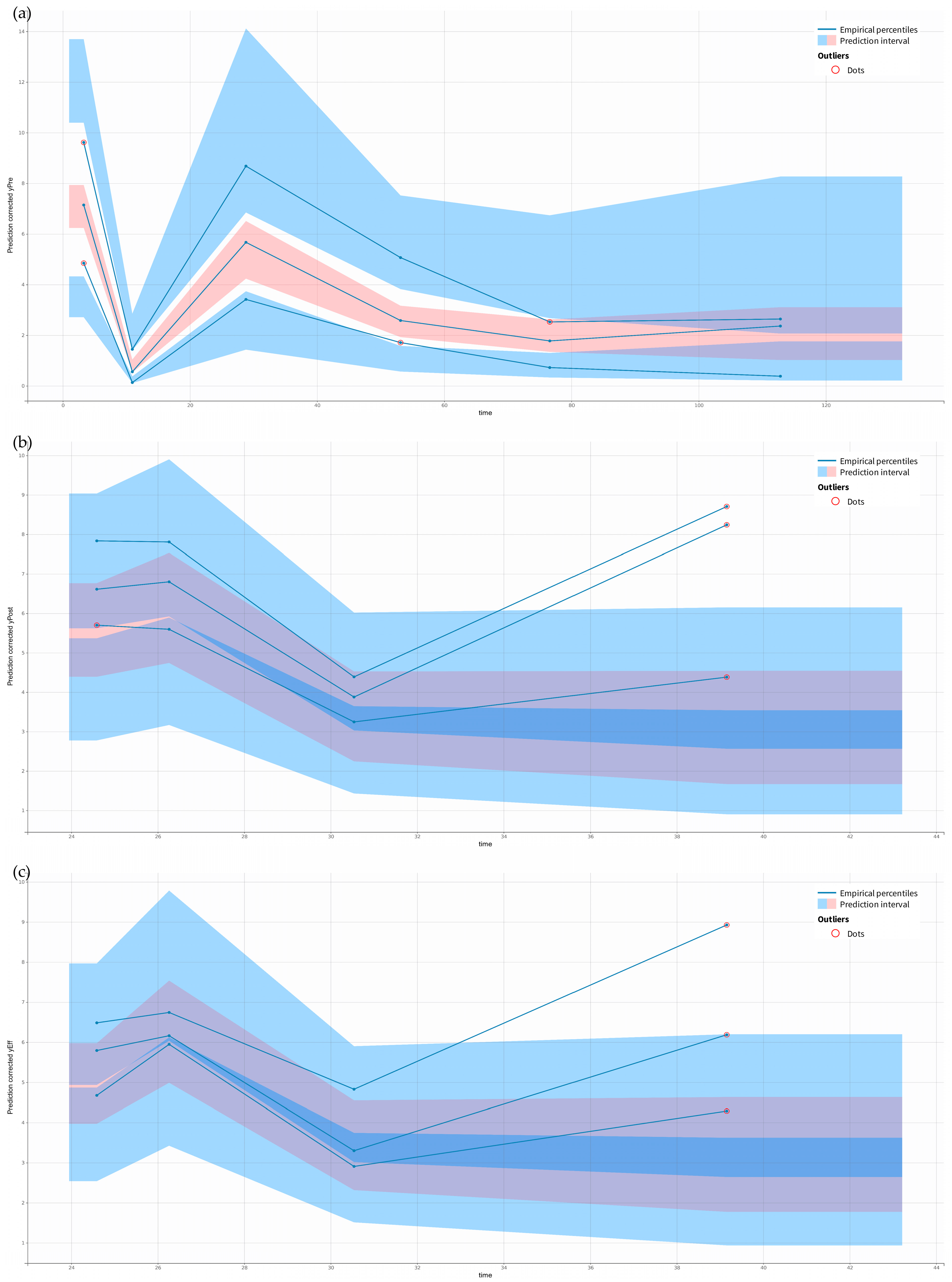
2.2. Pharmacokinetic Model Building
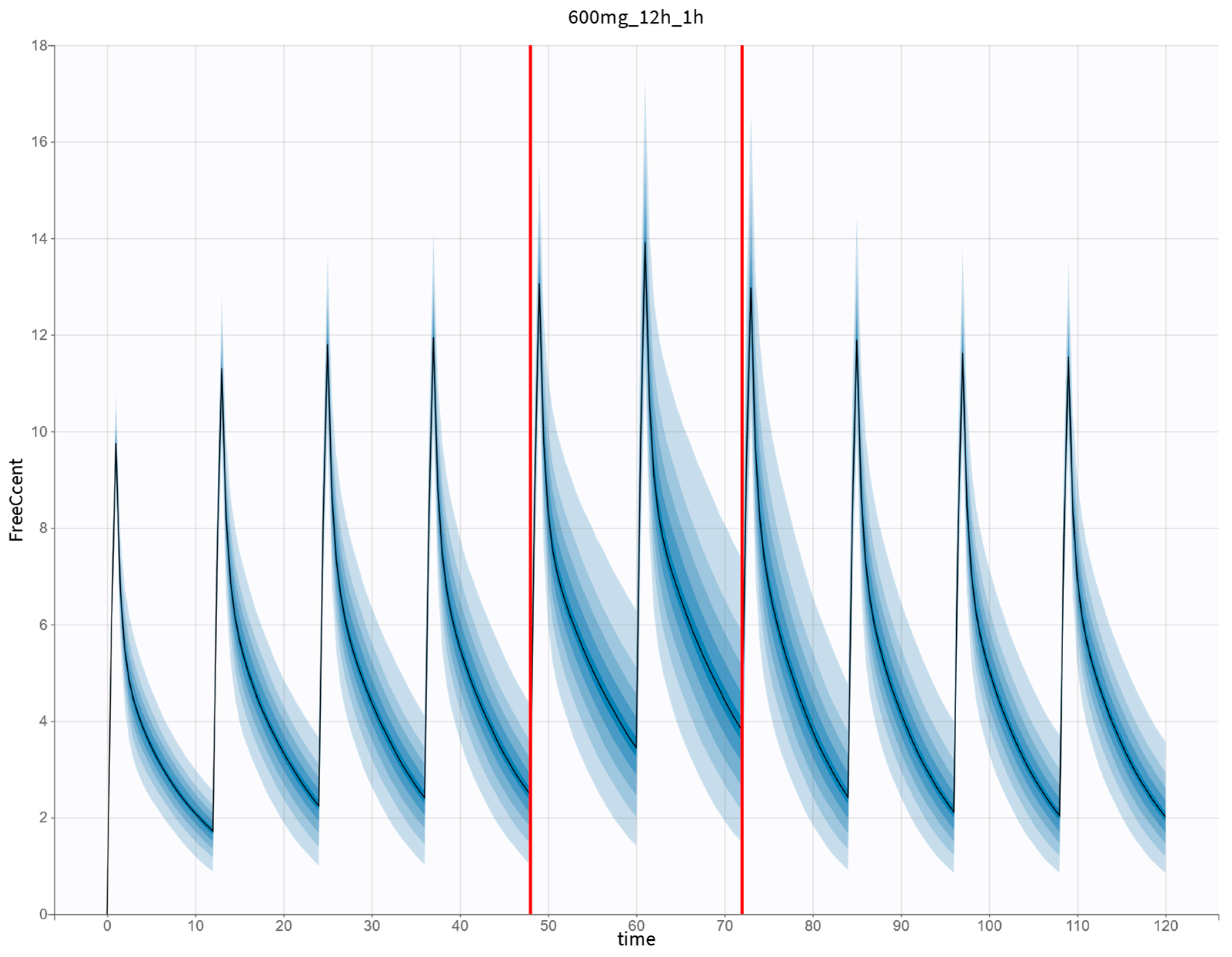
2.3. Monte Carlo Simulations
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Pharmacokinetic Analysis
4.3. Simulations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKI | Acute kidney injury |
| ARC | Augmented renal clearance |
| BICc | corrected Bayesian Information Criteria |
| CRRT | Continuous renal replacement therapy |
| Cl | Climination clearance |
| ClCr | Creatinine clearance |
| COSSAC | COnditional Sampling use for the Stepwise Approach |
| CVVHD | Continuous venovenous hemodialysis |
| CVVHDF | Continuous venovenous hemodiafiltration |
| EUCAST | The European Committee on Antimicrobial Susceptibility Testing |
| GOF | Goodness of fit |
| ICU | Intensive care unit |
| IIV | Inter-individual variability |
| IOV | Inter-occasion variability |
| MIC | Minimum inhibitory concentration |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| OFV | Objective function value |
| pcVPC | Prediction-corrected visual predictive check |
| PK | Pharmacokinetic |
| PK/PD | pharmacokinetic/pharmacodynamic |
| PopPK | Population pharmacokinetic |
| PTA | Probability of target attainment |
| SAEM | Stochastic approximation expectation-maximization |
| SmPC | Summary of product characteristics |
| Q | Inter-compartmental clearance |
| Qb | Blood flow rate |
| Qeff | Effluent flow rate |
| VCentral | Volume of the central compartment |
| VPeripheral | Volume of the peripheral compartment |
References
- Abate, G.; Wang, G.; Frisby, J. Ceftaroline: Systematic Review of Clinical Uses and Emerging Drug Resistance. Ann. Pharmacother. 2022, 56, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Steed, M.E.; Rybak, M.J. Ceftaroline: A New Cephalosporin with Activity against Resistant Gram-Positive Pathogens. Pharmacotherapy 2010, 30, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Fresán, D.; Luque, S.; Benítez-Cano, A.; Sorlí, L.; Montero, M.M.; De-Antonio, M.; Vega, V.; Roberts, J.A.; Horcajada, J.P.; Grau, S. Real-World Experience of Therapeutic Drug Monitoring and PK/PD Achievement of Ceftaroline Administered by Different Infusion Regimens in Patients with Confirmed Infections Caused by Gram-Positive Bacteria. J. Antimicrob. Chemother. 2023, 78, 2810–2815. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, S.; Williford, S.; Guo, D.; Shu, Y.; Medlin, C.; Li, M.; Yeung, S.Y.A.; Ali, F.; Jean, W.; Gopalakrishnan, M.; et al. Optimizing Ceftaroline Dosing in Critically Ill Patients Undergoing Continuous Renal Replacement Therapy. Pharmacotherapy 2021, 41, 205–211. [Google Scholar] [CrossRef]
- Zinforo | European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/zinforo (accessed on 3 January 2025).
- Gatti, M.; Pea, F. Antimicrobial Dose Reduction in Continuous Renal Replacement Therapy: Myth or Real Need? A Practical Approach for Guiding Dose Optimization of Novel Antibiotics. Clin. Pharmacokinet. 2021, 60, 1271. [Google Scholar] [CrossRef]
- Heffernan, A.J.; Mohd Sazlly Lim, S.; Lipman, J.; Roberts, J.A. A Personalised Approach to Antibiotic Pharmacokinetics and Pharmacodynamics in Critically Ill Patients. Anaesth. Crit. Care Pain. Med. 2021, 40, 100970. [Google Scholar] [CrossRef]
- Udy, A.A.; Roberts, J.A.; Lipman, J. Clinical Implications of Antibiotic Pharmacokinetic Principles in the Critically Ill. Intensive Care Med. 2013, 39, 2070–2082. [Google Scholar] [CrossRef]
- Udy, A.A.; Roberts, J.A.; Boots, R.J.; Paterson, D.L.; Lipman, J. Augmented Renal Clearance: Implications for Antibacterial Dosing in the Critically Ill. Clin. Pharmacokinet. 2010, 49, 1–16. [Google Scholar] [CrossRef]
- Udy, A.A.; Varghese, J.M.; Altukroni, M.; Briscoe, S.; McWhinney, B.C.; Ungerer, J.P.; Lipman, J.; Roberts, J.A. Subtherapeutic Initial β-Lactam Concentrations in Select Critically Ill Patients: Association between Augmented Renal Clearance and Low Trough Drug Concentrations. Chest 2012, 142, 30–39. [Google Scholar] [CrossRef]
- He, S.; Cheng, Z.; Xie, F. Population Pharmacokinetics and Dosing Optimization of Gentamicin in Critically Ill Patients Undergoing Continuous Renal Replacement Therapy. Drug Des. Dev. Ther. 2022, 16, 13–22. [Google Scholar] [CrossRef]
- EUCAST: The 2024 Breakpoint and QC Tables. Available online: https://www.eucast.org/eucast_news/news_singleview?tx_ttnews%5Btt_news%5D=570&cHash=7919d8287344766439860a24304ffaa7 (accessed on 3 January 2025).
- Stanford Antimicrobial Guidebook. Available online: https://med.stanford.edu/bugsanddrugs/guidebook.html (accessed on 3 January 2025).
- Monolix 2024R1, Lixoft SAS, a Simulations Plus Company. Available online: https://lixoft.com/products/monolix/ (accessed on 6 December 2024).
- Al-Shaer, M.H.; Maguigan, K.; Ashton, J.; Venugopalan, V.; Droege, M.E.; Philpott, C.D.; Droege, C.A.; Healy, D.P.; Mueller, E.W.; Peloquin, C.A. Applying Cefepime Population Pharmacokinetics to Critically Ill Patients Receiving Continuous Renal Replacement Therapy. Antimicrob. Agents Chemother. 2022, 66, e01611-21. [Google Scholar] [CrossRef]
- Al-Shaer, M.H.; Philpott, C.D.; Droege, C.A.; Courter, J.D.; Healy, D.P.; Droege, M.E.; Ernst, N.E.; Mueller, E.W.; Peloquin, C.A. Cefepime Population Pharmacokinetics and Target Attainment in Critically Ill Patients on Continuous Renal Replacement Therapy. Antimicrob. Agents Chemother. 2021, 65, e00144-21. [Google Scholar] [CrossRef] [PubMed]
- Chauzy, A.; Nadji, A.; Combes, J.-C.; Defrance, N.; Bouhemad, B.; Couet, W.; Chavanet, P. Cerebrospinal Fluid Pharmacokinetics of Ceftaroline in Neurosurgical Patients with an External Ventricular Drain. J. Antimicrob. Chemother. 2019, 74, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Chauzy, A.; Gregoire, N.; Ferrandière, M.; Lasocki, S.; Ashenoune, K.; Seguin, P.; Boisson, M.; Couet, W.; Marchand, S.; Mimoz, O.; et al. Population Pharmacokinetic/Pharmacodynamic Study Suggests Continuous Infusion of Ceftaroline Daily Dose in Ventilated Critical Care Patients with Early-Onset Pneumonia and Augmented Renal Clearance. J. Antimicrob. Chemother. 2022, 77, 3173–3179. [Google Scholar] [CrossRef]
- Rohatgi, A. WebPlotDigitizer User Manual, Version 4.6. 2022. Available online: https://automeris.io/WebPlotDigitizer (accessed on 2 November 2024).
- Burda, B.U.; O’Connor, E.A.; Webber, E.M.; Redmond, N.; Perdue, L.A. Estimating Data from Figures with a Web-based Program: Considerations for a Systematic Review. Res. Synth. Methods 2017, 8, 258–262. [Google Scholar] [CrossRef]
- Drevon, D.; Fursa, S.R.; Malcolm, A.L. Intercoder Reliability and Validity of WebPlotDigitizer in Extracting Graphed Data. Behav. Modif. 2017, 41, 323–339. [Google Scholar] [CrossRef]
- Alarcia-Lacalle, A.; Barrasa, H.; Maynar, J.; Canut-Blasco, A.; Gómez-González, C.; Solinís, M.Á.; Isla, A.; Rodríguez-Gascón, A. Quantification of Ceftaroline in Human Plasma Using High-Performance Liquid Chromatography with Ultraviolet Detection: Application to Pharmacokinetic Studies. Pharmaceutics 2021, 13, 959. [Google Scholar] [CrossRef]
- Adamiszak, A.; Bienert, B. Do critically ill patients undergoing continuous renal replacement therapy require ceftaroline dosage adjustments? Ceftaroline PopPK model and dosage simulations with probability of target attainment analysis based on retrospective data. In Proceedings of the 32nd PAGE Meeting, Rome, Italy, 26–28 June 2024. [Google Scholar]
- Ayral, G.; Si Abdallah, J.F.; Magnard, C.; Chauvin, J. A Novel Method Based on Unbiased Correlations Tests for Covariate Selection in Nonlinear Mixed Effects Models: The COSSAC Approach. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 318–329. [Google Scholar] [CrossRef]
- Statistical Tests for Model Building on Monolix. Available online: https://monolix.lixoft.com/tasks/tests/ (accessed on 12 March 2025).
- Bonate, P.L. A Brief Introduction to Monte Carlo Simulation. Clin. Pharmacokinet. 2001, 40, 15–22. [Google Scholar] [CrossRef]
- Roberts, J.A.; Kirkpatrick, C.M.J.; Lipman, J. Monte Carlo Simulations: Maximizing Antibiotic Pharmacokinetic Data to Optimize Clinical Practice for Critically Ill Patients. J. Antimicrob. Chemother. 2011, 66, 227–231. [Google Scholar] [CrossRef]
- Simulx 2024R1, Lixoft SAS, a Simulations Plus Company. Available online: https://www.simulations-plus.com/software/monolix/simulx/ (accessed on 6 December 2024).
- Lewis, S.J.; Mueller, B.A. Antibiotic Dosing Recommendations in Critically Ill Patients Receiving New Innovative Kidney Replacement Therapy. BMC Nephrol. 2024, 25, 73. [Google Scholar] [CrossRef]
- Fratoni, A.J.; Nicolau, D.P.; Kuti, J.L. A Guide to Therapeutic Drug Monitoring of β-Lactam Antibiotics. Pharmacotherapy 2021, 41, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Hartman, S.J.F.; Brüggemann, R.J.; Orriëns, L.; Dia, N.; Schreuder, M.F.; de Wildt, S.N. Pharmacokinetics and Target Attainment of Antibiotics in Critically Ill Children: A Systematic Review of Current Literature. Clin. Pharmacokinet. 2020, 59, 173. [Google Scholar] [CrossRef]
| Characteristics | Median [Range] or Number (%) |
|---|---|
| Gender | |
| Female | 12 (36%) |
| Male | 21 (64%) |
| Age (years) | 51 [21–77] |
| Weight (kg) | 75.5 [49–111.8] |
| ClCr (mL/min/1.73 m2) | 159 [42–309] |
| CRRT | |
| Yes | 4 (12%) |
| No | 29 (88%) |
| CRRT modality | |
| CVVHD | 2 (50%) |
| CVVHDF | 2 (50%) |
| Qb (mL/min) | 250 [250–300] |
| Qeff (mL/h) | 3200 [2700–3660] |
| Parameters | Mean Estimate (RSE%) | |
|---|---|---|
| Base Model | Final Model | |
| Cl (L/h) | 10.27 (7.0) | 11.23 (5.7) |
| ClCr on Cl | – | 0.68 (16.1) |
| IOV on Cl (%CV) | 48.12 (10.6) | 34.96 (12.3) |
| VCentral (L) | 21.26 (13.2) | 20.04 (4.1) |
| CVVHD on VCentral | – | 0.47 (18.2) |
| CVVHDF on VCentral | – | −0.42 (21.3) |
| IIV on VCentral (%CV) | 24.44 (44.3) | 3.70 (57.8) |
| Q (L/h) | 8.51 (25.9) | 8.80 (20.7) |
| CRRT on Q | – | 0.99 (26.6) |
| IIV on Q (%CV) | 50.69 (38.3) | 16.40 (58.5) |
| VPeripheral (L) | 16.72 (11.2) | 16.85 (10.5) |
| CRRT on VPeripheral | – | 0.99 (30.5) |
| IIV on VPeripheral (%CV) | 38.92 (29.0) | 38.60 (20.8) |
| VCRRT (L) | 2.18 (14.9) | 2.69 (42.3) |
| VEffluent (L) | 0.44 (17.4) | 0.60 (6.1) |
| VPost-filter (L) | 1.29 (4.23) | 1.15 (3.5) |
| bPre | 0.29 (5.0) | 0.29 (5.0) |
| bPost | 0.18 (20.6) | 0.16 (21.0) |
| bEff | 0.17 (17.3) | 0.13 (16.9) |
| Streptococcus pneumoniae (MIC = 0.25 mg/L) | Methicillin-Resistant Staphylococcus aureus (MIC = 1 mg/L) | |||
|---|---|---|---|---|
| 100%ƒT>MIC | 100%ƒT>4xMIC | 100%ƒT>MIC | 100%ƒT>4xMIC | |
| With CRRT | ||||
| CVVHD | ||||
| Qeff = 2.5 L/h | 200 mg q12 h Tinf 1 h | 200 mg q8 h Tinf 2 h | 200 mg q8 h Tinf 2 h | 800 mg q8 h Tinf 2 h |
| Qeff = 3.0 L/h | 200 mg q12 h Tinf 1 h | 300 mg q8 h Tinf 2 h | 300 mg q8 h Tinf 2 h | – |
| Qeff = 3.5 L/h | 200 mg q12 h Tinf 1 h | 300 mg q8 h Tinf 2 h | 300 mg q8 h Tinf 2 h | – |
| Qeff = 4.0 L/h | 200 mg q12 h Tinf 1 h | 300 mg q8 h Tinf 2 h | 300 mg q8 h Tinf 2 h | – |
| CVVHDF | ||||
| Qeff = 2.5 L/h | 200 mg q12 h Tinf 1 h | 300 mg q8 h Tinf 2 h | 300 mg q8 h Tinf 2 h | – |
| Qeff = 3.0 L/h | 200 mg q12 h Tinf 1 h | 300 mg q8 h Tinf 2 h | 300 mg q8 h Tinf 2 h | – |
| Qeff = 3.5 L/h | 200 mg q12 h Tinf 1 h | 300 mg q8 h Tinf 2 h | 300 mg q8 h Tinf 2 h | – |
| Qeff = 4.0 L/h | 300 mg q12 h Tinf 1 h | 400 mg q8 h Tinf 2 h | 400 mg q8 h Tinf 2 h | – |
| Without CRRT | ||||
| ClCr | ||||
| 80 mL/min/1.73 m2 | 300 mg q12 h Tinf 1 h | 400 mg q8 h Tinf 2 h | 400 mg q8 h Tinf 2 h | – |
| 130 mL/min/1.73 m2 | 200 mg q8 h Tinf 2 h | 800 mg q8 h Tinf 2 h | 800 mg q8 h Tinf 2 h | – |
| 210 mL/min/1.73 m2 | 600 mg q8 h Tinf 2 h | – | – | – |
| 300 mL/min/1.73 m2 | 800 mg q8 h Tinf 2 h | – | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamiszak, A.; Pietrzkiewicz, K.; Bartkowska-Śniatkowska, A.; Smuszkiewicz, P.; Kusza, K.; Grześkowiak, E.; Bienert, A. Do Critically Ill Patients Undergoing Continuous Renal Replacement Therapy Require Ceftaroline Dosage Adjustments? Ceftaroline PopPK Model and Dosage Simulations with the Probability of Target Attainment Analysis Based on Retrospective Data. Antibiotics 2025, 14, 347. https://doi.org/10.3390/antibiotics14040347
Adamiszak A, Pietrzkiewicz K, Bartkowska-Śniatkowska A, Smuszkiewicz P, Kusza K, Grześkowiak E, Bienert A. Do Critically Ill Patients Undergoing Continuous Renal Replacement Therapy Require Ceftaroline Dosage Adjustments? Ceftaroline PopPK Model and Dosage Simulations with the Probability of Target Attainment Analysis Based on Retrospective Data. Antibiotics. 2025; 14(4):347. https://doi.org/10.3390/antibiotics14040347
Chicago/Turabian StyleAdamiszak, Arkadiusz, Krzysztof Pietrzkiewicz, Alicja Bartkowska-Śniatkowska, Piotr Smuszkiewicz, Krzysztof Kusza, Edmund Grześkowiak, and Agnieszka Bienert. 2025. "Do Critically Ill Patients Undergoing Continuous Renal Replacement Therapy Require Ceftaroline Dosage Adjustments? Ceftaroline PopPK Model and Dosage Simulations with the Probability of Target Attainment Analysis Based on Retrospective Data" Antibiotics 14, no. 4: 347. https://doi.org/10.3390/antibiotics14040347
APA StyleAdamiszak, A., Pietrzkiewicz, K., Bartkowska-Śniatkowska, A., Smuszkiewicz, P., Kusza, K., Grześkowiak, E., & Bienert, A. (2025). Do Critically Ill Patients Undergoing Continuous Renal Replacement Therapy Require Ceftaroline Dosage Adjustments? Ceftaroline PopPK Model and Dosage Simulations with the Probability of Target Attainment Analysis Based on Retrospective Data. Antibiotics, 14(4), 347. https://doi.org/10.3390/antibiotics14040347







