Cefiderocol Antimicrobial Susceptibility Testing by Disk Diffusion: Influence of Agar Media and Inhibition Zone Morphology in K. pneumoniae Metallo-β-lactamase
Abstract
1. Introduction
- Class A carbapenemases (e.g., KPC)–these enzymes hydrolyze carbapenems but can be inhibited by certain β-lactamase inhibitors;
- Class B metallo-β-lactamases (MBLs) (e.g., NDM, VIM, IMP)–these enzymes require zinc ions for activity and are resistant to available for treatment β-lactamase inhibitors;
- Class C carbapenemases (e.g., OXA-48-like)–these are particularly problematic due to their ability to hydrolyze a broad range of β-lactams [4].
1.1. Challenges in Cefiderocol Susceptibility Testing
1.1.1. Biological Factors Affecting Susceptibility Testing
1.1.2. Technical Challenges in Standardizing Cefiderocol AST
- Broth microdilution—the reference method recommended by EUCAST and CLSI, but labor-intensive and costly.
- Gradient strip diffusion—provides an MIC value but may be affected by iron availability.
1.1.3. Economic Barriers to Cefiderocol AST
1.1.4. Organizational Factors Affecting AST in Clinical Laboratory
1.2. Study Objective
2. Results
2.1. Differences Between Agar Media
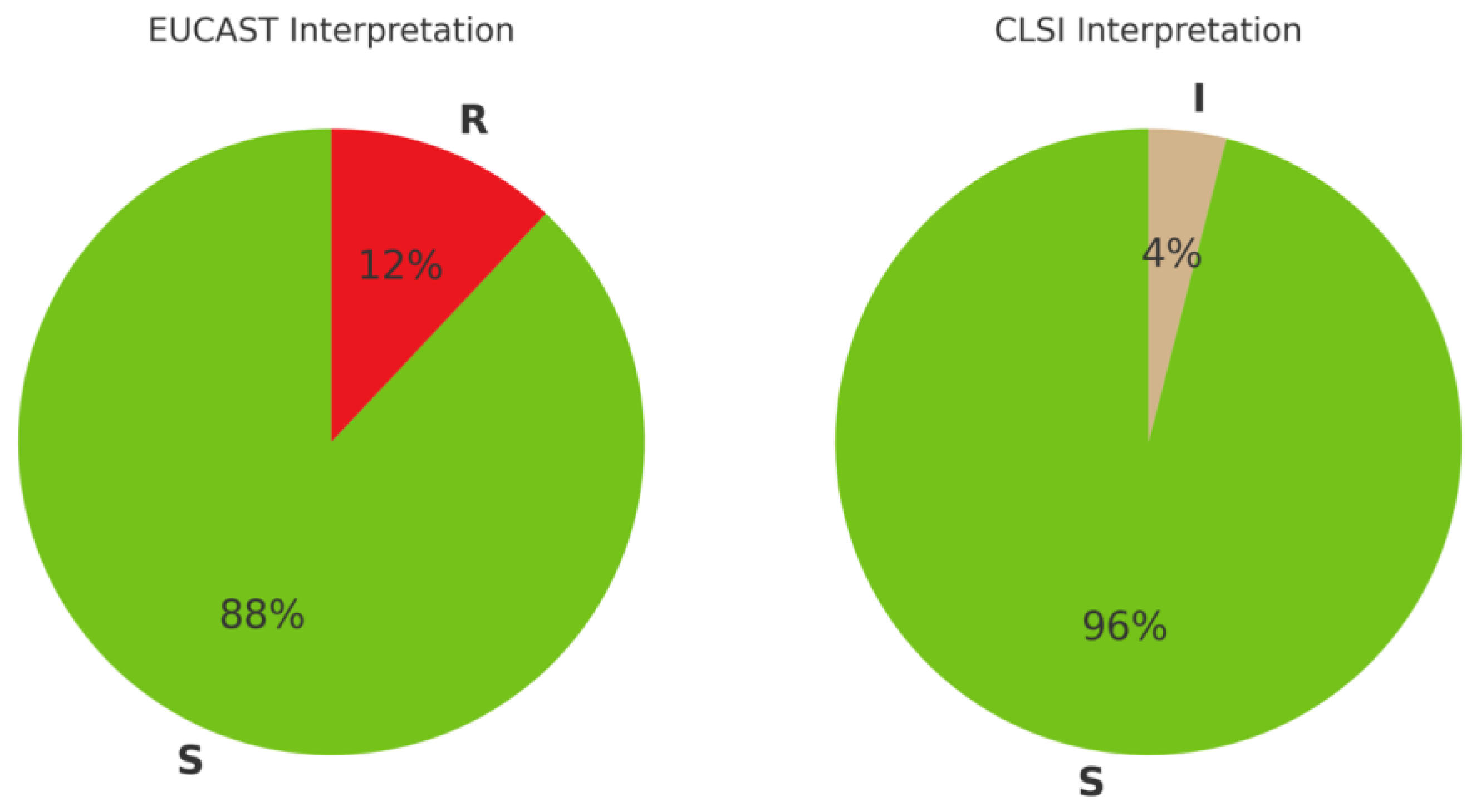
2.2. Antimicrobial Susceptibility Interpretation
3. Discussion
Strengths and Limitations
4. Materials and Methods
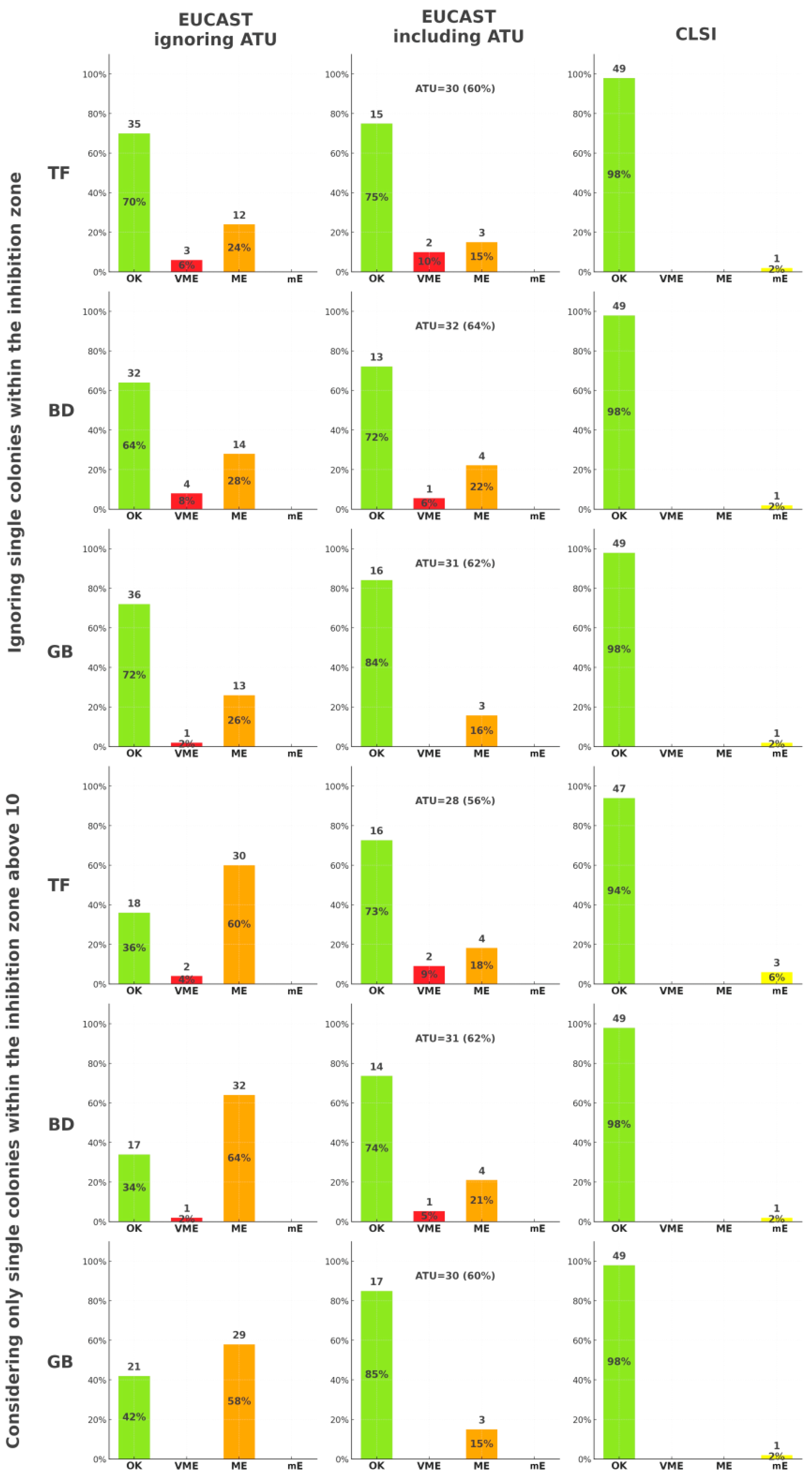
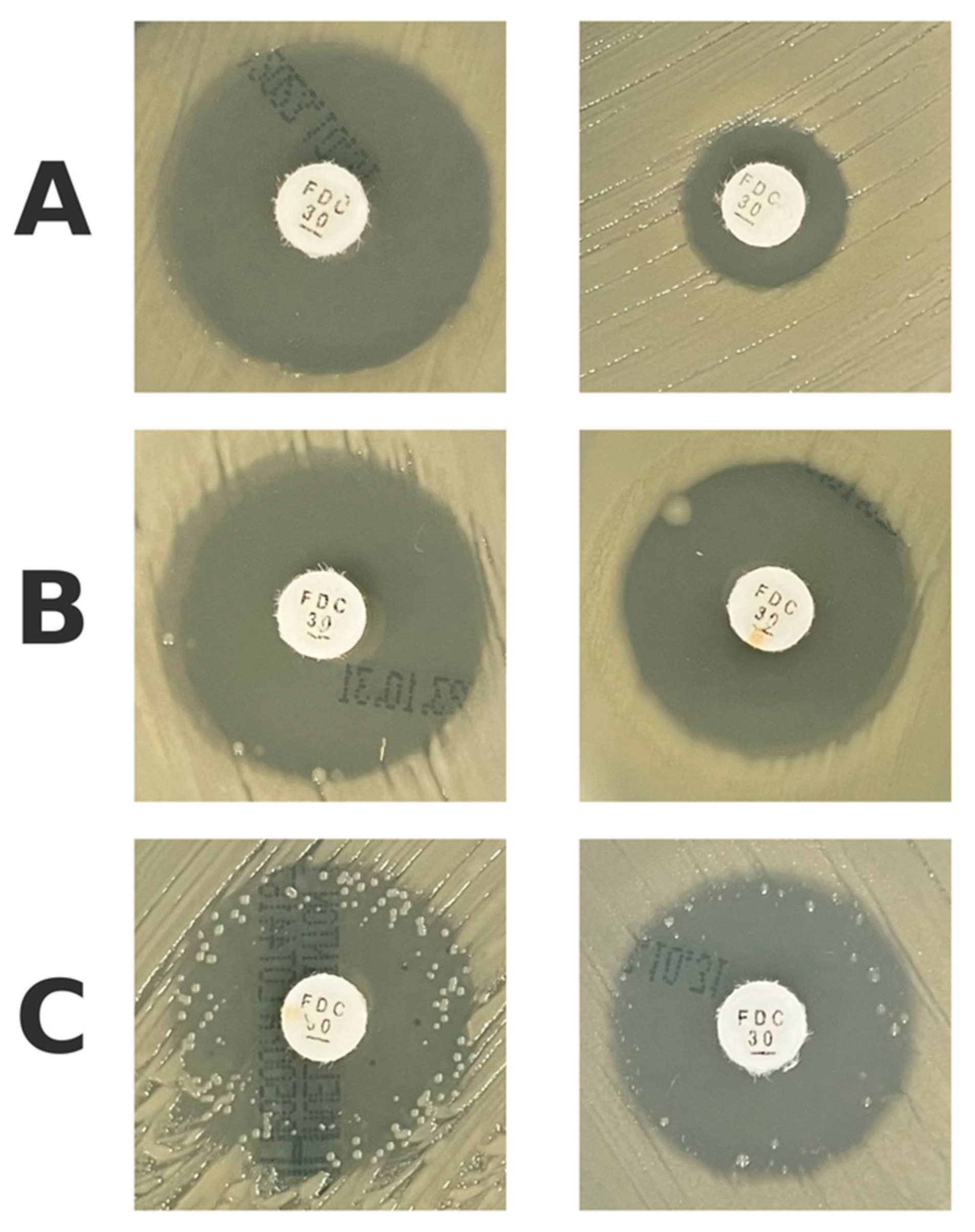
4.1. Assessing the Isolated Colonies Within the Inhibition Zone
4.2. Antimicrobial Susceptibility Testing Errors
- Very Major Error (VME)—a resistant isolate falsely categorized as susceptible by disk diffusion;
- Major Error (ME)—a susceptible isolate falsely categorized as resistant by disk diffusion;
- Minor Error (mE)—an intermediate isolate falsely categorized as susceptible or resistant, or vice versa (applicable only to CLSI).
4.3. Data Analysis
4.4. Ethical Issues
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AST | Antimicrobial Susceptibility Testing |
| ATU | Area of Technical Uncertainty |
| CAB | Columbia Agar with 5% Blood |
| CHA | Chromogenic Agar |
| CLSI | Clinical and Laboratory Standards Institute |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| IMP | Imipenemase Metallo-β-lactamase |
| KPC | Klebsiella pneumoniae carbapenemase |
| MBL | Metallo-β-lactamase |
| MCA | MacConkey Agar |
| MDR | Multidrug Resistance |
| ME | Major Error |
| mE | Minor Error |
| MHA | Mueller-Hinton Agar |
| MIC | Minimal Inhibitory Concentration |
| NDM | New Delhi metallo-β-lactamase |
| OXA | Oxacillinase |
| VIM | Verona Integron-encoded Metallo-β-lactamase |
| VME | Very Major Error |
| WHO | World Health Organization |
References
- World Health Organization. WHO Bacterial Priority List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Gajic, I.; Tomic, N.; Lukovic, B.; Jovicevic, M.; Kekic, D.; Petrovic, M.; Jankovic, M.; Trudic, A.; Mitic Culafic, D.; Milenkovic, M.; et al. A Comprehensive Overview of Antibacterial Agents for Combating Multidrug-Resistant Bacteria: The Current Landscape, Development, Future Opportunities, and Challenges. Antibiotics 2025, 14, 221. [Google Scholar] [CrossRef]
- Ambler, R.P.; Coulson, A.F.; Frère, J.M.; Ghuysen, J.M.; Joris, B.; Forsman, M.; Levesque, R.C.; Tiraby, G.; Waley, S.G. A standard numbering scheme for the class A beta-lactamases. Biochem. J. 1991, 276, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Brauncajs, M.; Bielec, F.; Macieja, A.; Pastuszak-Lewandoska, D. Carbapenem-Resistant Gram-Negative Fermenting and Non-Fermenting Rods Isolated from Hospital Patients in Poland—What Are They Susceptible to? Biomedicines 2022, 10, 3049. [Google Scholar] [CrossRef]
- Domingues, S.; Lima, T.; Saavedra, M.J.; Da Silva, G.J. An Overview of Cefiderocol’s Therapeutic Potential and Underlying Resistance Mechanisms. Life 2023, 13, 1427. [Google Scholar] [CrossRef]
- Gijón Cordero, D.; Castillo-Polo, J.A.; Ruiz-Garbajosa, P.; Cantón, R. Antibacterial spectrum of cefiderocol. Rev. Esp. Quimioter. 2022, 35, 20–27. [Google Scholar] [CrossRef]
- Kayama, S.; Kawakami, S.; Kondo, K.; Kitamura, N.; Yu, L.; Hayashi, W.; Yahara, K.; Sugawara, Y.; Sugai, M. In vitro activity of cefiderocol against carbapenemase-producing and meropenem-non-susceptible Gram-negative bacteria collected in the Japan Antimicrobial Resistant Bacterial Surveillance. J. Glob. Antimicrob. Resist. 2024, 38, 12–20. [Google Scholar] [CrossRef]
- Brauncajs, M.; Bielec, F.; Macieja, A.; Pastuszak-Lewandoska, D. Cefiderocol—An effective antimicrobial for MDR infections but a challenge for routine antimicrobial susceptibility testing. Adv. Med. Sci. 2024, 69, 256–263. [Google Scholar] [CrossRef]
- Kimbrough, J.H.; Maher, J.M.; Sader, H.S.; Castanheira, M.; Mendes, R.E. In vitro activity assessment of cefiderocol against Enterobacterales, Pseudomonas aeruginosa, and Acinetobacter spp., including β-lactam nonsusceptible molecularly characterized isolates, collected from 2020 to 2021 in the United States and European hospitals. Microbiol. Spectr. 2024, 12, e0147424. [Google Scholar]
- Stracquadanio, S.; Nicolosi, A.; Marino, A.; Calvo, M.; Stefani, S. Issues with Cefiderocol Testing: Comparing Commercial Methods to Broth Microdilution in Iron-Depleted Medium—Analyses of the Performances, ATU, and Trailing Effect According to EUCAST Initial and Revised Interpretation Criteria. Diagnostics 2024, 14, 2318. [Google Scholar] [CrossRef]
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 15.0. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_15.0_Breakpoint_Tables.pdf (accessed on 1 April 2025).
- CLSI. M39: Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data, 5th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2025. [Google Scholar]
- Hackel, M.A.; Tsuji, M.; Yamano, Y.; Echols, R.; Karlowsky, J.A.; Sahm, D.F. Reproducibility of broth microdilution MICs for the novel siderophore cephalosporin, cefiderocol, determined using iron-depleted cation-adjusted Mueller-Hinton broth. Diagn. Microbiol. Infect. Dis. 2019, 94, 321–325. [Google Scholar] [CrossRef]
- García-Rivera, C.; Sánchez-Bautista, A.; Parra-Grande, M.; Ricart-Silvestre, A.; Ventero, M.P.; Tyshkovska, I.; Merino, E.; Rodríguez Díaz, J.C. Comparison of Different Methods for Assaying the In Vitro Activity of Cefiderocol against Carbapenem-Resistant Pseudomonas aeruginosa Strains: Influence of Bacterial Inoculum. Antibiotics 2024, 13, 663. [Google Scholar] [CrossRef] [PubMed]
- Jeannot, K.; Gaillot, S.; Triponney, P.; Portets, S.; Pourchet, V.; Fournier, D.; Potron, A. Performance of the Disc Diffusion Method, MTS Gradient Tests and Two Commercially Available Microdilution Tests for the Determination of Cefiderocol Susceptibility in Acinetobacter spp. Microorganisms 2023, 11, 1971. [Google Scholar] [CrossRef] [PubMed]
- Jeannot, K.; Dortet, L.; CA-SFM group. Cefiderocol susceptibility testing: The challenge of disc diffusion. Clin. Microbiol. Infect. 2025, S1198-743X(24)00644-X. [Google Scholar] [CrossRef]
- Jain, C.; Nikita, N. Evaluation of Chromogenic Agar Media for Isolation, Identification and Direct Antibiotic Susceptibility Testing of Uropathogens. Int. J. Pharm. Res. Allied. Sci. 2023, 12, 7–12. [Google Scholar] [CrossRef]
- Willey, J.M.; Sandman, K.; Wood, D. Prescott’s Microbiology, 11th ed.; McGraw Hill: New York, NY, USA, 2020. [Google Scholar]
- Bielec, F.; Brauncajs, M.; Pastuszak-Lewandoska, D. Comparison of Substance Sources in Experimental Antimicrobial Susceptibility Testing. Sci. Pharm. 2023, 91, 10. [Google Scholar] [CrossRef]
- Isler, B.; Vatansever, C.; Özer, B.; Çınar, G.; Aslan, A.T.; Falconer, C.; Bauer, M.J.; Forde, B.; Şimşek, F.; Tülek, N.; et al. Higher rates of cefiderocol resistance among NDM producing Klebsiella bloodstream isolates applying EUCAST over CLSI breakpoints. Infect. Dis. 2023, 55, 607–613. [Google Scholar] [CrossRef]
- Åhman, J.; Matuschek, E.; Kahlmeter, G. Evaluation of ten brands of pre-poured Mueller-Hinton agar plates for EUCAST disc diffusion testing. Clin. Microbiol. Infect. 2022, 28, 1499.e1–1499.e5. [Google Scholar] [CrossRef]
- Potter, R.F.; Wallace, M.A.; Muenks, C.E.; Alvarado, K.; Yarbrough, M.L.; Burnham, C.D. Evaluation of Variability in Interpretation of Disk Diffusion Testing for Cefiderocol Using Different Brands of Mueller-Hinton Agar. J. Appl. Lab. Med. 2023, 8, 523–534. [Google Scholar] [CrossRef]
- EUCAST. Antimicrobial Susceptibility Testing EUCAST Disk Diffusion Method, Version 13.0. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/2025_manuals/Manual_v_13.0_EUCAST_Disk_Test_2025.pdf (accessed on 1 April 2025).
- Morris, C.P.; Bergman, Y.; Tekle, T.; Fissel, J.A.; Tamma, P.D.; Simner, P.J. Cefiderocol Antimicrobial Susceptibility Testing against Multidrug-Resistant Gram-Negative Bacilli: A Comparison of Disk Diffusion to Broth Microdilution. J. Clin. Microbiol. 2020, 59, e01649-20. [Google Scholar] [CrossRef]
- Bovo, F.; Lazzarotto, T.; Ambretti, S.; Gaibani, P. Comparison of Broth Microdilution, Disk Diffusion and Strip Test Methods for Cefiderocol Antimicrobial Susceptibility Testing on KPC-Producing Klebsiella pneumoniae. Antibiotics 2023, 12, 614. [Google Scholar] [CrossRef]
- Bhalodi, A.A.; Oppermann, N.; Campeau, S.A.; Humphries, R.M. Variability of Beta-Lactam Broth Microdilution for Pseudomonas aeruginosa. Antimicrob. Agents. Chemother. 2021, 65, e0064021. [Google Scholar] [CrossRef] [PubMed]
- Bianco, G.; Boattini, M.; Comini, S.; Banche, G.; Cavallo, R.; Costa, C. Disc Diffusion and ComASP® Cefiderocol Microdilution Panel to Overcome the Challenge of Cefiderocol Susceptibility Testing in Clinical Laboratory Routine. Antibiotics 2023, 12, 604. [Google Scholar] [CrossRef]
- Castillo-Polo, J.A.; Hernández-García, M.; Maruri-Aransolo, A.; Morosini, M.I.; Ruiz-Garbajosa, P.; Cantón, R. Cefiderocol AST in a real-life Klebsiella pneumoniae collection: Challenges in the ATU range. J. Antimicrob. Chemother. 2025, 80, 797–801. [Google Scholar] [CrossRef]
- Baltas, I.; Patel, T.; Lima Soares, A. Resistance profiles of carbapenemase-producing Enterobacterales in a large centre in England: Are we already losing cefiderocol?-right to reply-authors’ response. J Antimicrob. Chemother. 2025, 80, 1464–1465. [Google Scholar] [CrossRef]
- Ersoy, S.C.; Heithoff, D.M.; Barnes, L., 5th; Tripp, G.K.; House, J.K.; Marth, J.D.; Smith, J.W.; Mahan, M.J. Correcting a Fundamental Flaw in the Paradigm for Antimicrobial Susceptibility Testing. EBioMedicine 2017, 20, 173–181. [Google Scholar] [CrossRef]
- Heithoff, D.M.; Barnes, L.; Mahan, S.P.; Fried, J.C.; Fitzgibbons, L.N.; House, J.K.; Mahan, M.J. Re-evaluation of FDA-approved antibiotics with increased diagnostic accuracy for assessment of antimicrobial resistance. Cell Rep. Med. 2023, 4, 101023. [Google Scholar] [CrossRef]
- Nassar, M.S.M.; Hazzah, W.A.; Bakr, W.M.K. Evaluation of antibiotic susceptibility test results: How guilty a laboratory could be? J. Egypt. Public Health Assoc. 2019, 94, 4. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Pawar, J.S.; Akhter, N.; Lucy, I.B. Conventional methods and future trends in antimicrobial susceptibility testing. Saudi J. Biol. Sci. 2023, 30, 103582. [Google Scholar] [CrossRef]
- Bedenić, B.; Luxner, J.; Zarfel, G.; Benčić, A.; Sardelić, S.; Anušić, M.; Vraneš, J.; Dobretzberger, V.; Barišić, I.; Grisold, A. Characterization of Klebsiella pneumoniae Isolates Resistant to Cefiderocol from Hospitals and Outpatient Settings in Croatia. Antibiotics 2025, 14, 154. [Google Scholar] [CrossRef]
- Kammineni, C.; Vamsi, S.; Basireddy, S.R. Surveillance of In Vitro Activity of Cefiderocol Against Carbapenem-Resistant Gram-Negative Clinical Isolates in a Tertiary Care Hospital. Cureus 2024, 16, e67164. [Google Scholar] [CrossRef]
| Organization | MIC Breakpoints [mg/L] | Zone Diameter Breakpoints [mm] (Disk Content 30 µg) | |||||
|---|---|---|---|---|---|---|---|
| S | I | R | S | I | R | ATU | |
| EUCAST | ≤2 | - | >2 | ≥23 | - | <23 | 21–23 |
| CLSI | ≤4 | 8 | ≥16 | ≥16 | 9–15 | ≤8 | - |
| Agar Medium 1 | Agar Medium 2 | Ignoring Single Colonies Within the Inhibition Zone | Considering All Single Colonies Within the Inhibition Zone | Considering Only Single Colonies Within the Inhibition Zone Up to 10 | Considering Only Single Colonies Within the Inhibition Zone Above 10 | ||||
|---|---|---|---|---|---|---|---|---|---|
| t | p | t | p | t | p | t | p | ||
| CAB, TF | CAB, BD | 0.286 | 0.775 | −0.007 | 0.995 | 0.325 | 0.746 | −0.129 | 0.897 |
| CAB, TF | CAB, GB | 0.332 | 0.741 | −0.390 | 0.697 | −0.080 | 0.937 | −0.064 | 0.949 |
| CAB, TF | MCA, TF | 0.153 | 0.879 | −0.797 | 0.428 | −0.619 | 0.537 | −0.105 | 0.916 |
| CAB, TF | MCA, BD | 0.139 | 0.889 | −0.530 | 0.597 | −0.273 | 0.786 | −0.202 | 0.840 |
| CAB, TF | MCA, GB | 0.061 | 0.951 | −2.185 | 0.031 * | −1.734 | 0.086 | −0.539 | 0.591 |
| CAB, TF | CHA, TF | −0.291 | 0.771 | 0.462 | 0.645 | 0.418 | 0.677 | −0.138 | 0.890 |
| CAB, TF | CHA, BD | −0.954 | 0.342 | −0.865 | 0.389 | −0.818 | 0.415 | −0.909 | 0.366 |
| CAB, TF | CHA, GB | −1.089 | 0.279 | −1.432 | 0.155 | −0.971 | 0.334 | −1.553 | 0.124 |
| CAB, BD | CAB, GB | 0.052 | 0.959 | −0.369 | 0.713 | −0.394 | 0.695 | 0.070 | 0.944 |
| CAB, BD | MCA, TF | −0.141 | 0.888 | −0.760 | 0.449 | −0.900 | 0.370 | 0.027 | 0.979 |
| CAB, BD | MCA, BD | −0.147 | 0.883 | −0.507 | 0.613 | −0.557 | 0.579 | −0.083 | 0.934 |
| CAB, BD | MCA, GB | −0.232 | 0.817 | −2.093 | 0.039 * | −1.931 | 0.056 | −0.452 | 0.652 |
| CAB, BD | CHA, TF | −0.586 | 0.560 | 0.454 | 0.651 | 0.081 | 0.935 | −0.020 | 0.984 |
| CAB, BD | CHA, BD | −1.252 | 0.214 | −0.828 | 0.410 | −1.076 | 0.284 | −0.859 | 0.392 |
| CAB, BD | CHA, GB | −1.402 | 0.164 | −1.376 | 0.172 | −1.217 | 0.226 | −1.578 | 0.118 |
| CAB, GB | MCA, TF | −0.191 | 0.849 | −0.402 | 0.689 | −0.526 | 0.600 | −0.044 | 0.965 |
| CAB, GB | MCA, BD | −0.196 | 0.845 | −0.166 | 0.868 | −0.195 | 0.846 | −0.150 | 0.881 |
| CAB, GB | MCA, GB | −0.280 | 0.780 | −1.801 | 0.075 | −1.617 | 0.109 | −0.514 | 0.608 |
| CAB, GB | CHA, TF | −0.626 | 0.533 | 0.842 | 0.402 | 0.485 | 0.629 | −0.084 | 0.933 |
| CAB, GB | CHA, BD | −1.280 | 0.204 | −0.486 | 0.628 | −0.726 | 0.469 | −0.908 | 0.366 |
| CAB, GB | CHA, GB | −1.428 | 0.157 | −1.061 | 0.291 | −0.878 | 0.382 | −1.614 | 0.110 |
| MCA, TF | MCA, BD | −0.010 | 0.992 | 0.205 | 0.838 | 0.292 | 0.771 | −0.109 | 0.913 |
| MCA, TF | MCA, GB | −0.093 | 0.926 | −1.431 | 0.155 | −1.119 | 0.266 | −0.481 | 0.632 |
| MCA, TF | CHA, TF | −0.456 | 0.649 | 1.242 | 0.217 | 1.002 | 0.319 | −0.045 | 0.964 |
| MCA, TF | CHA, BD | −1.138 | 0.258 | −0.103 | 0.919 | −0.228 | 0.820 | −0.886 | 0.378 |
| MCA, TF | CHA, GB | −1.287 | 0.201 | −0.692 | 0.491 | −0.389 | 0.698 | −1.609 | 0.111 |
| MCA, BD | MCA, GB | −0.081 | 0.935 | −1.503 | 0.136 | −1.308 | 0.194 | −0.354 | 0.724 |
| MCA, BD | CHA, TF | −0.435 | 0.664 | 0.952 | 0.344 | 0.645 | 0.521 | 0.056 | 0.956 |
| MCA, BD | CHA, BD | −1.101 | 0.274 | −0.291 | 0.771 | −0.490 | 0.625 | −0.764 | 0.447 |
| MCA, BD | CHA, GB | −1.243 | 0.217 | −0.830 | 0.409 | −0.635 | 0.527 | −1.454 | 0.149 |
| MCA, GB | CHA, TF | −0.362 | 0.718 | 2.577 | 0.011 * | 2.050 | 0.043 * | 0.388 | 0.699 |
| MCA, GB | CHA, BD | −1.040 | 0.301 | 1.265 | 0.209 | 0.837 | 0.404 | −0.471 | 0.639 |
| MCA, GB | CHA, GB | −1.183 | 0.240 | 0.664 | 0.508 | 0.664 | 0.508 | −1.183 | 0.240 |
| CHA, TF | CHA, BD | −0.676 | 0.501 | −1.293 | 0.199 | −1.176 | 0.242 | −0.772 | 0.442 |
| CHA, TF | CHA, GB | −0.801 | 0.425 | −1.838 | 0.069 | −1.318 | 0.191 | −1.410 | 0.162 |
| CHA, BD | CHA, GB | −0.095 | 0.924 | −0.567 | 0.572 | −0.156 | 0.876 | −0.589 | 0.558 |
| Agar Medium | Ignoring Single Colonies Within the Inhibition Zone | Considering All Single Colonies Within the Inhibition Zone | Considering Only Single Colonies Within the Inhibition Zone Up to 10 | Considering Only Single Colonies Within the Inhibition Zone Above 10 | ||||
|---|---|---|---|---|---|---|---|---|
| ρ | p | ρ | p | ρ | p | ρ | p | |
| CBA, TF | −0.504 | 0.000190 * | −0.153 | 0.289484 | −0.106 | 0.462408 | −0.493 | 0.000272 * |
| CBA, BD | −0.493 | 0.000272 * | −0.053 | 0.715205 | −0.004 | 0.977778 | −0.500 | 0.000222 * |
| CBA, GB | −0.612 | 0.000002 * | −0.157 | 0.277542 | −0.097 | 0.503243 | −0.623 | 0.000001 * |
| MCA, TF | −0.464 | 0.000683 | −0.199 | 0.165289 | −0.118 | 0.413014 | −0.487 | 0.000339 |
| MCA, BD | −0.398 | 0.004205 | −0.261 | 0.066671 | −0.225 | 0.115777 | −0.404 | 0.003611 |
| MCA, GB | −0.476 | 0.000482 | −0.236 | 0.098908 | −0.236 | 0.098908 | −0.476 | 0.000482 |
| CHA, TF | −0.464 | 0.000691 | −0.084 | 0.562990 | 0.037 | 0.799958 | −0.471 | 0.000550 |
| CHA, BD | −0.308 | 0.029583 | −0.074 | 0.607392 | −0.027 | 0.854732 | −0.305 | 0.031158 |
| CHA, GB | −0.482 | 0.000396 | −0.150 | 0.300036 | −0.150 | 0.300036 | −0.482 | 0.000396 |
| Manufacturer | Becton Dickinson | Graso Biotech | Thermo Fisher |
|---|---|---|---|
| Media type | Columbia Agar with 5% Sheep Blood | ||
| Product name | Columbia Agar with 5% Sheep Blood | Columbia Agar with 5% Sheep Blood | Columbia Agar with Sheep Blood PLUS |
| Catalog number | 254,005 | 1190PD90 | PB5039A |
| Formulation | Pancreatic digest of casein 12.0 g/L, Peptic digest of animal tissue 5.0 g/L, Yeast extract 3.0 g/L, Beef extract 3.0 g/L, Corn starch 1.0 g/L, Sodium chloride 5.0 g/L, Agar 13.5 g/L, Defibrinated sheep blood 5% | Enzymatic casein hydrolysate 5.0 g/L Enzymatic hydrolysate of animal tissues 8.0 g/L Yeast extract 10.0 g/L Agar 14.0 g/L Sodium chloride 5.0 g/L Corn starch 1.0 g/L Defibrinated sheep blood 50.0 mL/L | Special peptone 23.0 g/L Starch 1.0 g/L Sodium chloride 5.0 g/L Agar 10.0 g/L Defibrinated sheep blood 50.0 mL/L |
| Media type | MacConkey Agar | ||
| Product name | MacConkey II Agar | MacConkey Agar + Crystal Violet | MacConkey Agar No. 3 |
| Catalog number | 254,025 | 1020PD90 | PO5002A |
| Formulation | Pancreatic digest of gelatin 17.0 g/L Pancreatic digest of casein 1.5 g/L Peptic digest of animal tissue 1.5 g/L Lactose 10.0 g/L Bile salts 1.5 g/L Sodium chloride 5.0 g/L Neutral red 0.03 g/L Crystal violet 0.001 g/L Agar 13.5 g/L | Enzymatic gelatin hydrolysate 17.0 g/L Enzymatic casein hydrolysate 1.5 g/L Enzymatic hydrolysate of animal tissues 1.5 g/L Lactose 10.0 g/L Bile salts 1.5 g/L Sodium chloride 5.0 g/L Neutral red 0.03 g/L Crystal violet 0.001 g/L Agar 13.5 g/L | Peptone 20.0 g/L Lactose 10.0 g/L Bile salts No. 3 1.5 g/L Sodium chloride 5.0 g/L Neutral red 0.03 g/L Crystal violet 0.001 g/L Agar 15.0 g/L |
| Media type | Chromogenic Agar | ||
| Product name | CHROMagar Orientation | CHROMagar Orientation | Brilliance UTI Clarity |
| Catalog number | 254,489 | 1410PD90 | PO5159A |
| Formulation | Chromopeptone 16.1 g/L Chromogen mix 1.3 g/L Agar 15 g/L | Chromogenic mixture 1.0 g/L Peptone and yeast extract 17.0 g/L Agar 15.0 g/L | Peptone 9.0 g/L Chromogenic mix 17.0 g/L Tryptophan 1.0 g/L Agar 10.0 g/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saar, M.; Wawrzyk, A.; Pastuszak-Lewandoska, D.; Bielec, F. Cefiderocol Antimicrobial Susceptibility Testing by Disk Diffusion: Influence of Agar Media and Inhibition Zone Morphology in K. pneumoniae Metallo-β-lactamase. Antibiotics 2025, 14, 527. https://doi.org/10.3390/antibiotics14050527
Saar M, Wawrzyk A, Pastuszak-Lewandoska D, Bielec F. Cefiderocol Antimicrobial Susceptibility Testing by Disk Diffusion: Influence of Agar Media and Inhibition Zone Morphology in K. pneumoniae Metallo-β-lactamase. Antibiotics. 2025; 14(5):527. https://doi.org/10.3390/antibiotics14050527
Chicago/Turabian StyleSaar, Maciej, Anna Wawrzyk, Dorota Pastuszak-Lewandoska, and Filip Bielec. 2025. "Cefiderocol Antimicrobial Susceptibility Testing by Disk Diffusion: Influence of Agar Media and Inhibition Zone Morphology in K. pneumoniae Metallo-β-lactamase" Antibiotics 14, no. 5: 527. https://doi.org/10.3390/antibiotics14050527
APA StyleSaar, M., Wawrzyk, A., Pastuszak-Lewandoska, D., & Bielec, F. (2025). Cefiderocol Antimicrobial Susceptibility Testing by Disk Diffusion: Influence of Agar Media and Inhibition Zone Morphology in K. pneumoniae Metallo-β-lactamase. Antibiotics, 14(5), 527. https://doi.org/10.3390/antibiotics14050527








