Multiple Copies of Tigecycline Gene Cluster tmexC6D6-toprJ1b in Pseudomonas mendocina in a Swine Farm
Abstract
1. Introduction
2. Results
2.1. Identification of tmexCD-toprJ-Positive Strains
2.2. Antimicrobial Susceptibility of tmexC6D6-toprJ1b-Positive P. mendocina
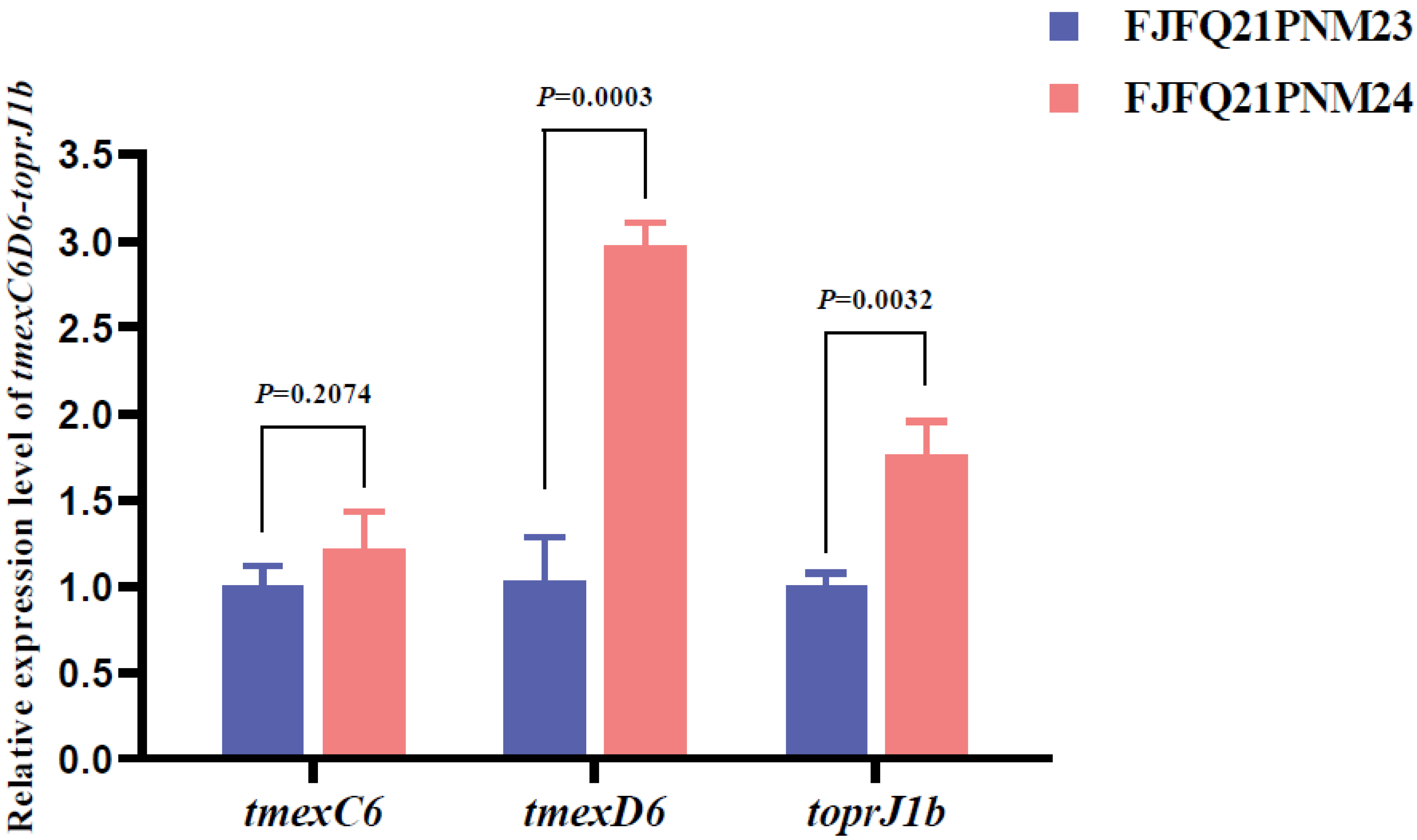
2.3. Characterization of tmexC6D6-toprJ1b-Positive P. mendocina
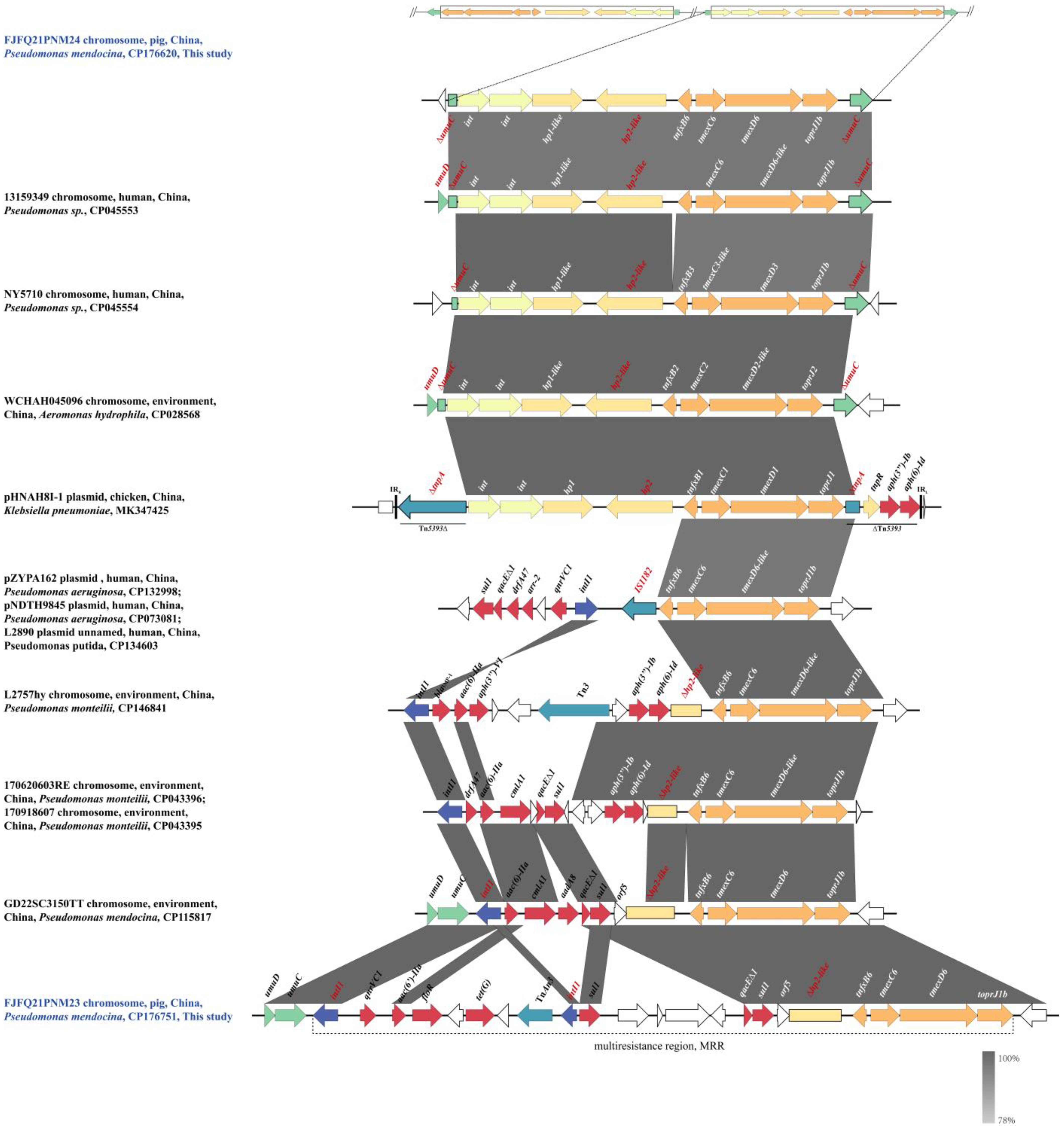
2.4. Genetic Context of tnfxB6-tmexC6D6-toprJ1b
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Antimicrobial Susceptibility Testing
4.3. Whole-Genome Sequencing (WGS) Analysis
4.4. RNA Extraction, cDNA Synthesis, and RT-qPCR
4.5. Nucleotide Sequence Accession Numbers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lv, L.; Wan, M.; Wang, C.; Gao, X.; Yang, Q.; Partridge, S.R.; Wang, Y.; Zong, Z.; Doi, Y.; Shen, J.; et al. Emergence of a Plasmid-Encoded Resistance-Nodulation-Division Efflux Pump Conferring Resistance to Multiple Drugs, Including Tigecycline, in Klebsiella pneumoniae. mBio 2020, 11, e02930-19. [Google Scholar] [CrossRef] [PubMed]
- Tasina, E.; Haidich, A.B.; Kokkali, S.; Arvanitidou, M. Efficacy and safety of tigecycline for the treatment of infectious diseases: A meta-analysis. Lancet Infect. Dis. 2011, 11, 834–844. [Google Scholar] [CrossRef]
- He, T.; Wang, R.; Liu, D.; Walsh, T.R.; Zhang, R.; Lv, Y.; Ke, Y.; Ji, Q.; Wei, R.; Liu, Z.; et al. Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat. Microbiol. 2019, 4, 1450–1456. [Google Scholar] [CrossRef]
- Wang, C.; Yang, J.; Xu, Z.; Lv, L.; Chen, S.; Hong, M.; Liu, J.H. Promoter regulatory mode evolution enhances the high multidrug resistance of tmexCD1-toprJ1. mBio 2024, 15, e0021824. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Gao, X.; Liang, X.H.; Lv, L.C.; Lu, L.T.; Yue, C.; Cui, X.X.; Yang, K.E.; Lu, D.; Liu, J.H.; et al. Pseudomonas Acts as a Reservoir of Novel Tigecycline Resistance Efflux Pump tmexC6D6-toprJ1b and tmexCD-toprJ Variants. Microbiol. Spectr. 2023, 11, e0076723. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, N.; Cai, C.; Zeng, Y.; Lu, J.; Liu, C.; Wang, H.; Zhang, Y.; Huang, L.; Zhai, W.; et al. Aeromonas spp. from hospital sewage act as a reservoir of genes resistant to last-line antibiotics. Drug Resist. Updat. 2023, 67, 100925. [Google Scholar] [CrossRef]
- Wang, C.; Gao, X.; Zhang, X.; Yue, C.; Lv, L.; Lu, L.; Liu, J.H. Emergence of two novel tmexCD-toprJ subtypes mediating tigecycline resistance in the megaplasmids from Pseudomonas putida. Microbiol. Res. 2025, 292, 128051. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhuang, Y.; Wu, C.; Jia, H.; He, F.; Ruan, Z. Global emergence of Gram-negative bacteria carrying the mobilised RND-type efflux pump gene cluster tmexCD-toprJ variants. Lancet Microbe 2024, 5, e105. [Google Scholar] [CrossRef]
- Sun, S.; Gao, H.; Liu, Y.; Jin, L.; Wang, R.; Wang, X.; Wang, Q.; Yin, Y.; Zhang, Y.; Wang, H. Co-existence of a novel plasmid-mediated efflux pump with colistin resistance gene mcr in one plasmid confers transferable multidrug resistance in Klebsiella pneumoniae. Emerg. Microbes Infect. 2020, 9, 1102–1113. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, N.; Cai, C.; Zhang, R.; Chen, S. Hospital wastewater as a reservoir for the tigecycline resistance gene cluster tmexCD-toprJ. Lancet Microbe 2023, 4, e134. [Google Scholar] [CrossRef]
- Wu, X.; Chen, G.; Wang, P.; Yang, L.; Wu, Y.; Wu, G.; Li, H.; Shao, B. Co-existence of a novel RND efflux pump tmexC6D6.2-toprJ1b and bla(OXA-4) in the extensively drug-resistant Pseudomonas aeruginosa ST233 clone. Int. J. Food Microbiol. 2025, 428, 110984. [Google Scholar] [CrossRef]
- Peng, K.; Wang, Q.; Yin, Y.; Li, Y.; Liu, Y.; Wang, M.; Qin, S.; Wang, Z.; Li, R. Plasmids Shape the Current Prevalence of tmexCD1-toprJ1 among Klebsiella pneumoniae in Food Production Chains. mSystems 2021, 6, e0070221. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Gao, X.; Lv, L.C.; Cai, Z.P.; Yang, J.; Liu, J.H. Novel tigecycline resistance gene cluster tnfxB3-tmexCD3-toprJ1b in Proteus spp. and Pseudomonas aeruginosa, co-existing with tet(X6) on an SXT/R391 integrative and conjugative element. J. Antimicrob. Chemother. 2021, 76, 3159–3167. [Google Scholar] [CrossRef]
- Wang, C.Z.; Gao, X.; Yang, Q.W.; Lv, L.C.; Wan, M.; Yang, J.; Cai, Z.P.; Liu, J.H. A Novel Transferable Resistance-Nodulation-Division Pump Gene Cluster, tmexCD2-toprJ2, Confers Tigecycline Resistance in Raoultella ornithinolytica. Antimicrob. Agents Chemother. 2021, 65, e02229-20. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, Y.; Lu, M.J.; Wang, Z.Y.; Jiao, X. Emergence of a novel tigecycline resistance gene cluster tmexC3-tmexD5-toprJ2b in Oceanimonas sp. from chicken, China. J. Antimicrob. Chemother. 2023, 78, 1311–1313. [Google Scholar] [CrossRef]
- Gao, X.; Wang, C.; Lv, L.; He, X.; Cai, Z.; He, W.; Li, T.; Liu, J.H. Emergence of a Novel Plasmid-Mediated Tigecycline Resistance Gene Cluster, tmexCD4-toprJ4, in Klebsiella quasipneumoniae and Enterobacter roggenkampii. Microbiol. Spectr. 2022, 10, e0109422. [Google Scholar] [CrossRef] [PubMed]
- Krishnasamy, V.; Otte, J.; Silbergeld, E. Antimicrobial use in Chinese swine and broiler poultry production. Antimicrob. Resist. Infect. Control 2015, 4, 17. [Google Scholar] [CrossRef]
- Mulchandani, R.; Wang, Y.; Gilbert, M.; Van Boeckel, T.P. Global trends in antimicrobial use in food-producing animals: 2020 to 2030. PLoS Glob Public Health 2023, 3, e0001305. [Google Scholar] [CrossRef]
- Peng, K.; Li, Y.; Wang, Q.; Yang, P.; Wang, Z.; Li, R. Integrative conjugative elements mediate the high prevalence of tmexCD3-toprJ1b in Proteus spp. of animal source. mSystems 2023, 8, e0042923. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Meng, N.; Wang, Z.; Li, G.; Jiao, X.; Wang, J. Distribution and Spread of the Mobilized RND Efflux Pump Gene Cluster tmexCD-toprJ in Klebsiella pneumoniae from Different Sources. Microbiol. Spectr. 2023, 11, e0536422. [Google Scholar] [CrossRef]
- Wang, Q.; Peng, K.; Liu, Y.; Xiao, X.; Wang, Z.; Li, R. Characterization of TMexCD3-TOprJ3, an RND-Type Efflux System Conferring Resistance to Tigecycline in Proteus mirabilis, and Its Associated Integrative Conjugative Element. Antimicrob. Agents Chemother. 2021, 65, e0271220. [Google Scholar] [CrossRef]
- Dong, N.; Zeng, Y.; Wang, Y.; Liu, C.; Lu, J.; Cai, C.; Liu, X.; Chen, Y.; Wu, Y.; Fang, Y.; et al. Distribution and spread of the mobilised RND efflux pump gene cluster tmexCD-toprJ in clinical Gram-negative bacteria: A molecular epidemiological study. Lancet Microbe 2022, 3, e846–e856. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, A.; Dao, T.D.; Takemura, T.; Hasebe, F.; Trang, L.T.; Thanh, N.H.; Tran, H.H.; Shibayama, K.; Kasuga, I.; Suzuki, M. A Transferable IncC-IncX3 Hybrid Plasmid Cocarrying bla(NDM-4), tet(X), and tmexCD3-toprJ3 Confers Resistance to Carbapenem and Tigecycline. mSphere 2021, 6, e0059221. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Peng, K.; Xiao, X.; Liu, Y.; Peng, D.; Wang, Z. Emergence of a multidrug resistance efflux pump with carbapenem resistance gene blaVIM-2 in a Pseudomonas putida megaplasmid of migratory bird origin. J. Antimicrob. Chemother. 2021, 76, 1455–1458. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Gao, X.; Lv, L.; Cai, Z.; Liu, J.H. IS26 Mediates the Acquisition of Tigecycline Resistance Gene Cluster tmexCD1-toprJ1 by IncHI1B-FIB Plasmids in Klebsiella pneumoniae and Klebsiella quasipneumoniae from Food Market Sewage. Antimicrob. Agents Chemother. 2021, 65, e02178-20. [Google Scholar] [CrossRef]
- Wang, C.Z.; Gao, X.; Tu, J.Y.; Lv, L.C.; Pu, W.X.; He, X.T.; Jiao, Y.X.; Deng, Y.T.; Liu, J.H. Multiple Copies of Mobile Tigecycline Resistance Efflux Pump Gene Cluster tmexC2D2.2-toprJ2 Identified in Chromosome of Aeromonas spp. Microbiol. Spectr. 2022, 10, e0346822. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, P.; Wang, H.; Zhang, J.; Zhang, Y.; Wu, Y.; Zhou, H.; Li, Y.; Shen, Z.; Chen, G.; et al. Emergence and genomic epidemiology of tigecycline resistant bacteria of fly origin across urban and rural China. Environ. Int. 2024, 193, 109099. [Google Scholar] [CrossRef]
- Zhu, J.; Lv, J.; Zhu, Z.; Wang, T.; Xie, X.; Zhang, H.; Chen, L.; Du, H. Identification of TMexCD-TOprJ-producing carbapenem-resistant Gram-negative bacteria from hospital sewage. Drug Resist. Updat. 2023, 70, 100989. [Google Scholar] [CrossRef]
- Peng, K.; Liu, Y.X.; Sun, X.; Wang, Q.; Song, L.; Wang, Z.; Li, R. Large-scale bacterial genomic and metagenomic analysis reveals Pseudomonas aeruginosa as potential ancestral source of tigecycline resistance gene cluster tmexCD-toprJ. Microbiol. Res. 2024, 285, 127747. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing—31th Edition: M100; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2021. [Google Scholar]
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 9.0; European Committee on Antimicrobial Susceptibility Testing: Växjö, Sweden, 2019. [Google Scholar]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [PubMed]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.J.; Petty, N.K.; Beatson, S.A. Easyfig: A genome comparison visualizer. Bioinformatics 2011, 27, 1009–1010. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, R.; Xia, Q.; Yu, J.; Yi, L.X.; Huang, Y.; Deng, M.; He, W.Y.; Bai, Y.; Lv, L.; et al. The evolution of infectious transmission promotes the persistence of mcr-1 plasmids. mBio 2023, 14, e0044223. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
| Strain | Species | Source | Plasmid or Chromosome | Size (bp) | Resistance Genes |
|---|---|---|---|---|---|
| FJFQ21PNM23 | Pseudomonas mendocina | Nasal swabs of swine | Chromosome | 4,779,589 | aac(6′)-IIa, qnrVC1, floR, sul1, tet(G), tmexC6D6-toprJ1b |
| Plasmid pFJFQ21PNM23 a | 142,064 | dfrA1 | |||
| FJFQ21PNM24 | Pseudomonas mendocina | Nasal swabs of swine | Chromosome | 4,785,273 | aph(6)-Id, dfrA1, aph(3″)-Ib, ant(2″)-Ia, qnrVC1, floR, sul1, sul2, tet(G), tmexC6D6-toprJ1b |
| MIC of Drug a | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | TIG | MIN | DOX | TET | CQM | CTX | CAZ | FFC | CHL | GEN | STR | CIP | SXT |
| FJFQ21PNM23 | 1 | 1 | 16 | 128 | 4 | 2 | 1 | >256 | >256 | 1 | 4 | 8 | >16/304 |
| FJFQ21PNM24 | 2 | 4 | 16 | 256 | 4 | 2 | 4 | >256 | >256 | 16 | >256 | 2 | >16/304 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, R.; Che, Y.; Wang, L.; Chen, Q.; He, B.; Qiu, J.; Wu, X.; Chen, R.; Liu, Y.; Zhou, L. Multiple Copies of Tigecycline Gene Cluster tmexC6D6-toprJ1b in Pseudomonas mendocina in a Swine Farm. Antibiotics 2025, 14, 500. https://doi.org/10.3390/antibiotics14050500
Wu R, Che Y, Wang L, Chen Q, He B, Qiu J, Wu X, Chen R, Liu Y, Zhou L. Multiple Copies of Tigecycline Gene Cluster tmexC6D6-toprJ1b in Pseudomonas mendocina in a Swine Farm. Antibiotics. 2025; 14(5):500. https://doi.org/10.3390/antibiotics14050500
Chicago/Turabian StyleWu, Renjie, Yongliang Che, Longbai Wang, Qiuyong Chen, Bing He, Jingli Qiu, Xuemin Wu, Rujing Chen, Yutao Liu, and Lunjiang Zhou. 2025. "Multiple Copies of Tigecycline Gene Cluster tmexC6D6-toprJ1b in Pseudomonas mendocina in a Swine Farm" Antibiotics 14, no. 5: 500. https://doi.org/10.3390/antibiotics14050500
APA StyleWu, R., Che, Y., Wang, L., Chen, Q., He, B., Qiu, J., Wu, X., Chen, R., Liu, Y., & Zhou, L. (2025). Multiple Copies of Tigecycline Gene Cluster tmexC6D6-toprJ1b in Pseudomonas mendocina in a Swine Farm. Antibiotics, 14(5), 500. https://doi.org/10.3390/antibiotics14050500






