Antifungal Effects of the Phloroglucinol Derivative DPPG Against Pathogenic Aspergillus fumigatus
Abstract
1. Introduction
2. Results
2.1. Distribution of DAPG Biosynthetic Clusters in Skin
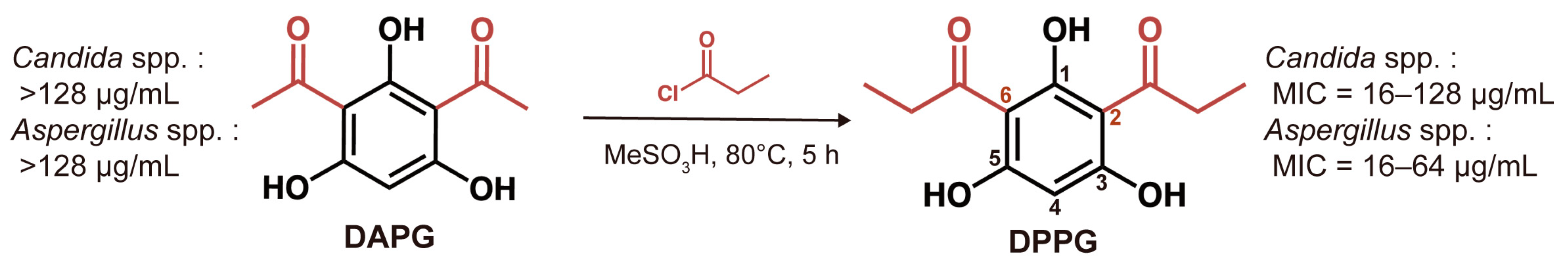
2.2. In Vitro Antifungal Activity of DAPG and DPPG
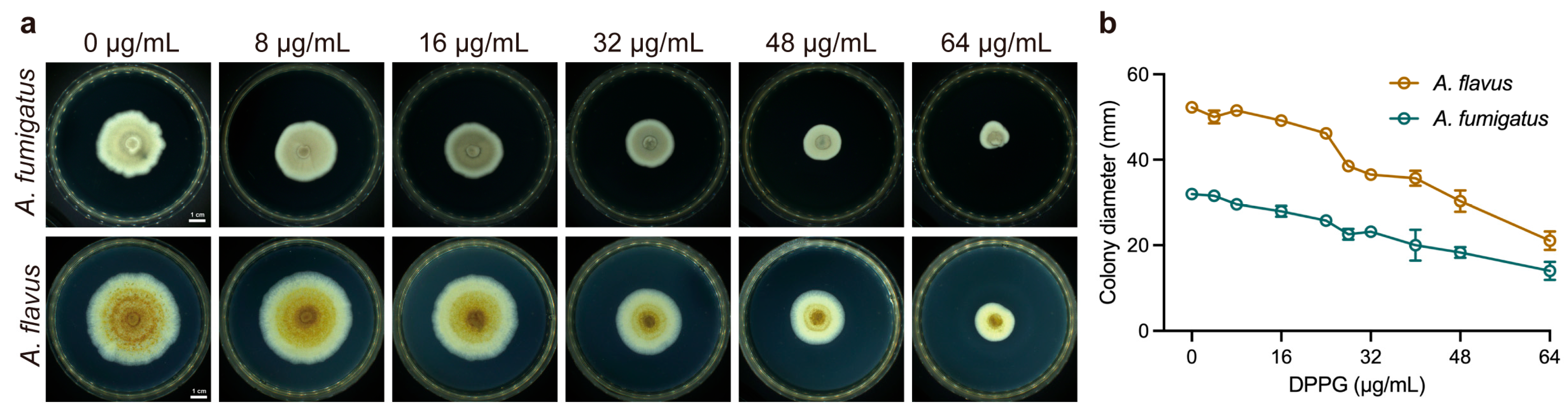
2.3. Inhibitory Effect on Hyphal Growth of DPPG
2.4. Inhibitory Effect on Spore Germination of DPPG
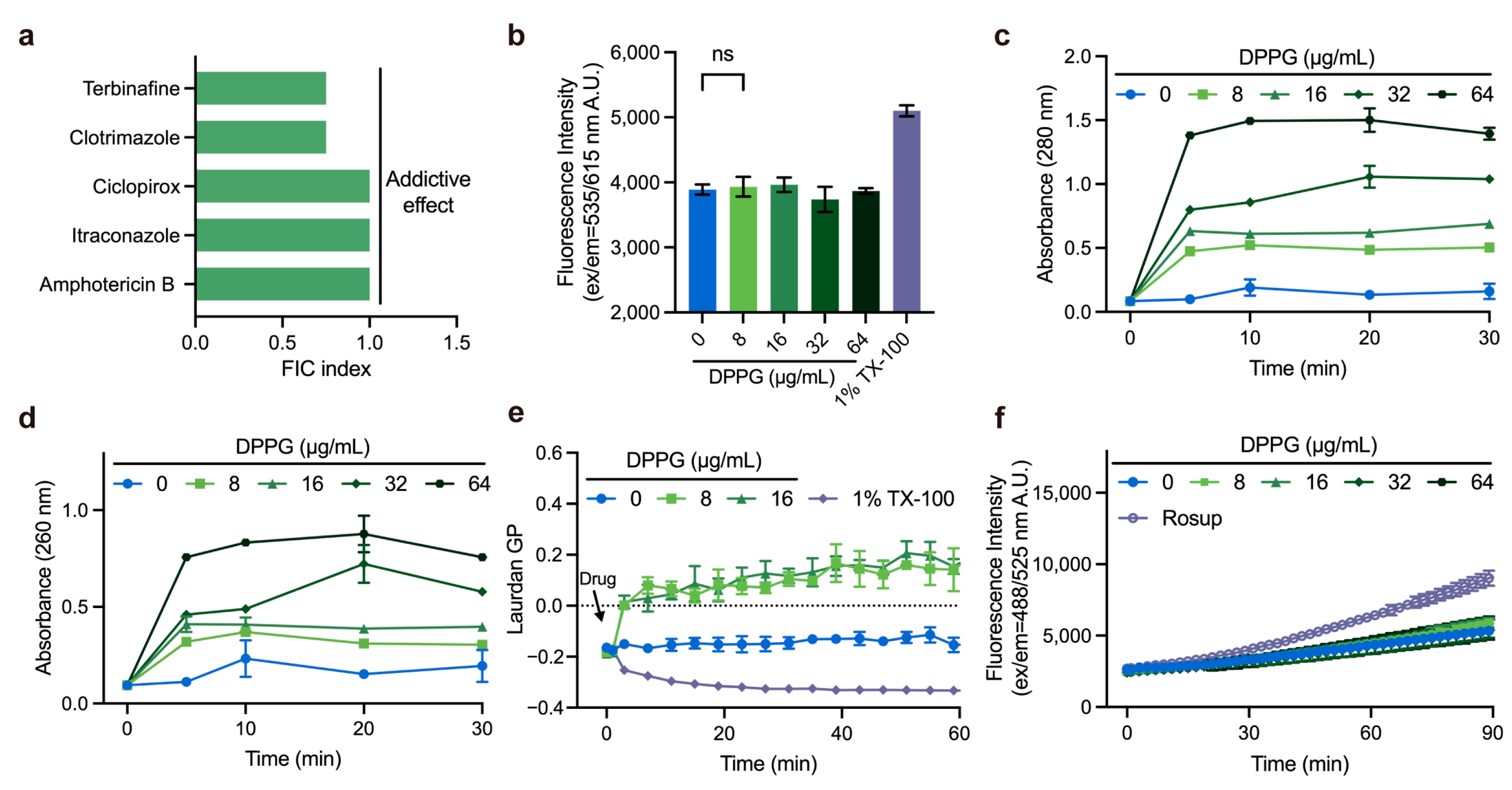
2.5. Membrane-Targeting Property of DPPG
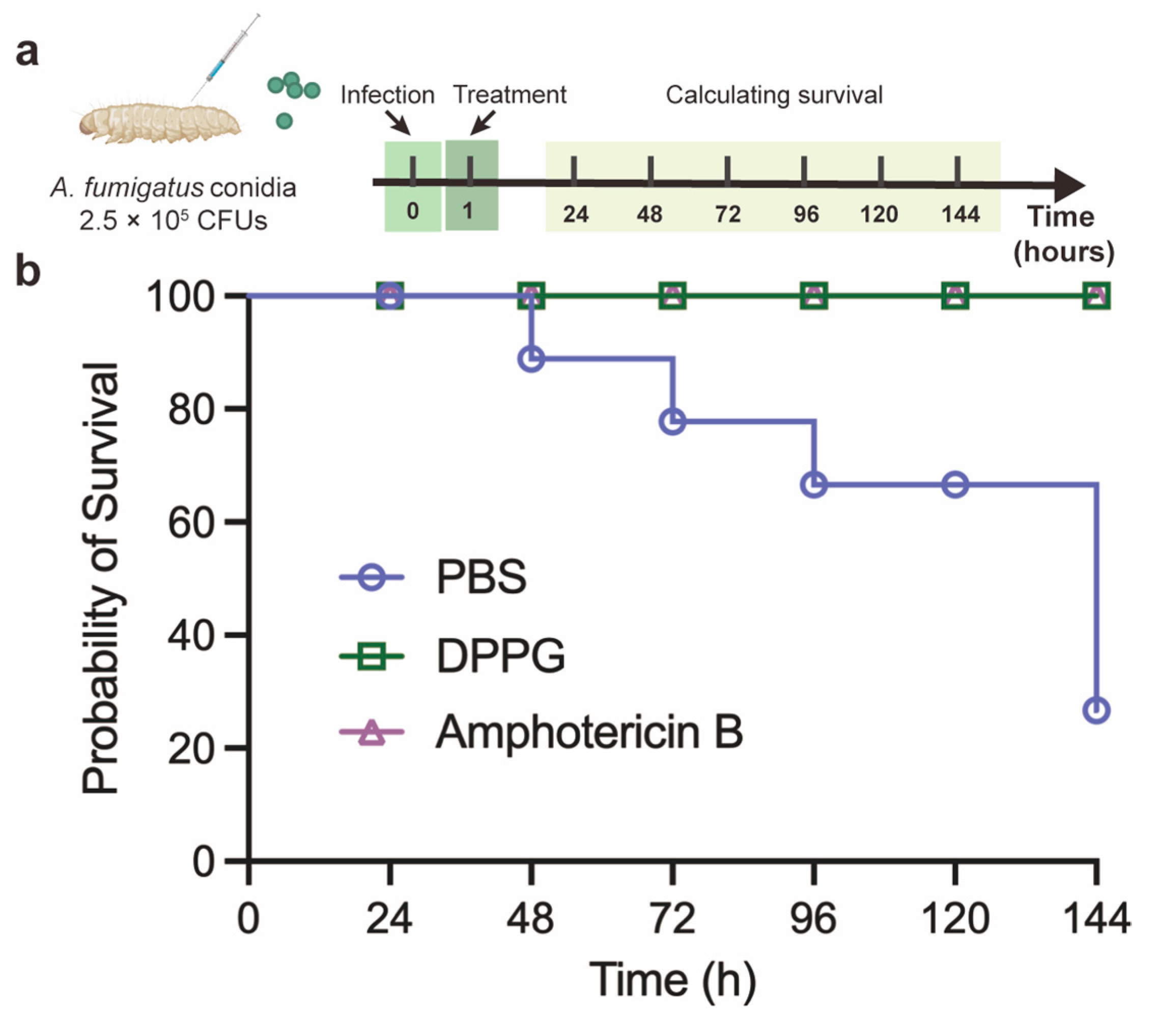
2.6. In Vivo Antifungal Activity of DPPG in G. mellonella Model
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Biosynthetic Gene Clusters Analysis
4.3. Fungal Strains and Culture Conditions
4.4. Synthesis of the Derivative DPPG
4.5. Preparation of Spore Suspension
4.6. Antimicrobial-Susceptibility Test
4.7. Checkerboard Assay
4.8. Hyphal Growth Assay
4.9. Spore Germination Assay
4.10. Membrane Integrity Assays
4.11. Cytoplasmic Content Leakage Test
4.12. ROS Measurement
4.13. G. Mellonella Infection Model
4.14. Molecular Docking
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DPPG | 2,4-diproylphloroglucinol |
| DAPG | 2,4-diacetylchloroglucoside |
| RPMI | Roswell Park Memorial Institute |
| MOPS | 4-Morpholinepropanesulfonic acid |
| PBS | phosphate buffered saline |
| PDA | potato dextrose agar |
| SDA | sabouraud agar |
| DCFH-DA | 2′,7′-dichlorodihydrofluorescein diacetate |
| PI | propidium iodide |
| ROS | reactive oxidant species |
| CLSI | Clinical and Laboratory Standards Institute |
| FIC | fractional inhibitory concentration |
| MIC | minimum inhibitory concentration |
| CFU | colony-forming unit |
| GP | generalized polarization |
| BGC | biosynthetic gene cluster |
References
- Denning, D.W. Global incidence and mortality of severe fungal disease. Lancet. Infect. Dis. 2024, 24, e428–e438. [Google Scholar] [CrossRef] [PubMed]
- Casalini, G. The WHO fungal priority pathogens list: A crucial reappraisal to review the prioritisation. Lancet Microbe 2024, 5, 717–724. [Google Scholar] [CrossRef] [PubMed]
- William, E.D. Introduction to Antifungal Drugs. Clin. Infect. Dis. 2000, 30, 653–657. [Google Scholar]
- Perfect, J. The antifungal pipeline: A reality check. Nat. Rev. Drug Discov. 2017, 16, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Al, M.O. FDA-approved antibacterials and echinocandins. Antibiotics 2025, 14, 166. [Google Scholar] [CrossRef]
- Debergh, H.; Becker, P.; Vercammen, F.; Lagrou, K.; Haesendonck, R.; Saegerman, C.; Packeu, A. Pulmonary aspergillosis in Humboldt penguins–susceptibility patterns and molecular epidemiology of clinical and environmental Aspergillus fumigatus isolates from a Belgian zoo, 2017–2022. Antibiotics 2023, 12, 584. [Google Scholar] [CrossRef]
- Arastehfar, A.; Carvalho, A.; Houbraken, J.; Lombardi, L.; Garcia-Rubio, R.; Jenks, J.; Rivero-Menendez, O.; Aljohani, R.; Jacobsen, I.; Berman, J.; et al. Aspergillus fumigatus and aspergillosis: From basics to clinics. Stud. Mycol. 2021, 100, 100115. [Google Scholar] [CrossRef]
- Hollomon, D. Does agricultural use of azole fungicides contribute to resistance in the human pathogen Aspergillus fumigatus? Pest. Manag. Sci. 2017, 73, 1987–1993. [Google Scholar] [CrossRef]
- Jenks, J.D.; Mehta, S.R.; Hoenigl, M. Broad spectrum triazoles for invasive mould infections in adults: Which drug and when? Med. Mycol. 2019, 57, S168–S178. [Google Scholar] [CrossRef]
- Snelders, E.; Camps, S.M.; Karawajczyk, A. Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus. PLoS ONE 2012, 7, e31801. [Google Scholar] [CrossRef]
- Robbins, N.; Cowen, L.E. Antifungal discovery. Curr. Opin. Microbiol. 2022, 69, 102198. [Google Scholar] [CrossRef] [PubMed]
- Calderone, R.; Sun, N.; Gay, A.F.; Groutas, W.; Weerawarna, P.; Prasad, S.; Alex, D.; Li, D. Antifungal drug discovery: The process and outcomes. Future Microbiol. 2014, 9, 791–805. [Google Scholar] [CrossRef]
- Cavassin, F.B.; Baú-Carneiro, J.L.; Vilas-Boas, R.R.; Queiroz-Telles, F. Sixty years of amphotericin B: An overview of the main antifungal agent used to treat invasive fungal infections. Infect. Dis. Ther. 2021, 10, 115–147. [Google Scholar] [CrossRef]
- Salama, E.A.; Elgammal, Y.; Utturkar, S.M.; Lanman, N.A.; Hazbun, T.R.; Seleem, M.N. Overcoming amphotericin B resistance in Candida auris using the antiemetic drug rolapitant. Antimicrob. Agents Chemother. 2024, 68, e00556-24. [Google Scholar] [CrossRef]
- Maji, A.; Soutar, C.P.; Zhang, J.; Lewandowska, A.; Uno, B.E.; Yan, S.; Shelke, Y.; Murhade, G.; Nimerovsky, E.; Borcik, C.G.; et al. Tuning sterol extraction kinetics yields a renal-sparing polyene antifungal. Nature 2023, 623, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Omelchuk, O.; Bychkova, E.; Efimova, S.; Grammatikova, N.; Zatonsky, G.; Dezhenkova, L.; Solovieva, S.; Ostroumova, O.; Tevyashova, A.; Shchekotikhin, A. Mono-N-alkylation of amphotericin B and nystatin A1 and its amides: Effect on the in vitro activity, cytotoxicity and permeabilization of model membranes. Antibiotics 2024, 13, 1177. [Google Scholar] [CrossRef]
- Haas, D.; Défago, G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 2005, 3, 307–319. [Google Scholar] [CrossRef]
- Mohammed, A.F.; Oloyede, A.R.; Odeseye, A.O. Biological control of bacterial wilt of tomato caused by Ralstonia solanacearum using Pseudomonas species isolated from the rhizosphere of tomato plants. Arch. Phytopathol. Plant Prot. 2020, 53, 1–6. [Google Scholar] [CrossRef]
- Sharifi-Tehrani, A.; Zala, M.; Natsch, A.; Moënne-Loccoz, Y.; Défago, G. Biocontrol of soil-borne fungal plant diseases by 2,4-diacetylphloroglucinol-producing fluorescent pseudomonads with different restriction profiles of amplified 16S rDNA. Eur. J. Plant Pathol. 1998, 104, 631–643. [Google Scholar] [CrossRef]
- De Souza, J.T.; Arnould, C.; Deulvot, C.; Lemanceau, P.; Gianinazzi-Pearson, V.; Raaijmakers, J.M. Effect of 2,4-diacetylphloroglucinol on Pythium: Cellular responses and variation in sensitivity among propagules and species. Phytopathology 2003, 93, 966–975. [Google Scholar] [CrossRef]
- Marchand, P.A.; Weller, D.M.; Bonsall, R.F. Convenient synthesis of 2,4-diacetylphloroglucinol a natural antibiotic involved in the control of take-all disease of wheat. J. Agric. Food Chem. 2000, 48, 1882–1887. [Google Scholar] [CrossRef] [PubMed]
- Suresh, P.; Varathraju, G.; Shanmugaiah, V.; Almaary, K.S.; Elbadawi, Y.B.; Mubarak, A. Partial purification and characterization of 2, 4-diacetylphloroglucinol producing Pseudomonas fluorescens VSMKU3054 against bacterial wilt disease of tomato. Saudi. J. Biol. Sci. 2021, 28, 2155–2167. [Google Scholar] [CrossRef] [PubMed]
- Findley, K.; Oh, J.; Yang, J.; Conlan, S.; Deming, C.; Meyer, J.A.; Schoenfeld, D.; Nomicos, E.; Park, M.; Kong, H.H.; et al. Topographic diversity of fungal and bacterial communities in human skin. Nature 2013, 498, 367–370. [Google Scholar] [CrossRef]
- Zipperer, A.; Konnerth, M.C.; Laux, C.; Berscheid, A.; Janek, D.; Weidenmaier, C.; Burian, M.; Schilling, N.A.; Slavetinsky, C.; Marschal, M.; et al. Human commensals producing a novel antibiotic impair pathogen colonization. Nature 2016, 535, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Feng, H.; Tian, Q.; Xiang, Y.; Wang, X.; He, Y.-X.; Zhu, K. Discovery of antibacterial diketones against gram-positive bacteria. Cell Chem. Biol. 2024, 31, 1874–1884.e6. [Google Scholar] [CrossRef]
- Maurya, S.; Thakur, R.; Vighnesh, R.; Suresh, S.; Dang, A.; Raj, D.; Srivastava, S. Eco-friendly management of plant pathogens through secondary metabolites released by Fluorescent Pseudomonads. J. Pure. Appl. Microbiol. 2024, 18, 1471–1488. [Google Scholar] [CrossRef]
- Dagenais, T.R.; Keller, N.P. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin. Microbiol. Rev. 2009, 22, 447–465. [Google Scholar] [CrossRef]
- Harris, F.M.; Best, K.B.; Bell, J.D. Use of laurdan fluorescence intensity and polarization to distinguish between changes in membrane fluidity and phospholipid order. Biochim. Biophys. Acta 2002, 1565, 123–128. [Google Scholar] [CrossRef]
- Gonzalez-Jimenez, I.; Perlin, D.S.; Shor, E. Reactive oxidant species induced by antifungal drugs: Identity, origins, functions, and connection to stress-induced cell death. Front. Cell. Infect. Microbiol. 2023, 13, 1276406. [Google Scholar] [CrossRef]
- Stepanov, A.A.; Shulaev, N.A.; Vasilchenko, A.S. The ecological strategy determines the response of fungi to stress: A study of the 2,4-diacetylphloroglucinol activity against Aspergillus and Fusarium species. J. Basic Microbiol. 2025, 65, e2400334. [Google Scholar] [CrossRef]
- Stepanov, A.A.; Vasilchenko, A.V.; Vasilchenko, A.S. Subinhibitory effects of 2,4-diacetylphloroglucinol on filamentous fungus Aspergillus fumigatus. J. Appl. Microbiol. 2023, 134, lxad294. [Google Scholar] [CrossRef]
- Han, P.; Liu, T.; Zheng, Y.; Song, R.; Nan, T.; Yang, X.; Huang, L.; Yuan, Y. A mycorrhizal bacteria strain isolated from Polyporus umbellatus exhibits broad-spectrum antifungal activity. Front. Plant. Sci. 2022, 13, 954160. [Google Scholar] [CrossRef]
- Janki, K.P.; Patel, G.A. Engineered production of 2,4-diacetylphloroglucinol in the diazotrophic endophytic bacterium Pseudomonas sp. WS5 and its beneficial effect in multiple plant-pathogen systems. Appl. Soil Ecol. 2018, 124, 34–44. [Google Scholar]
- Nowak-Thompson, B.; Gould, S.J.; Kraus, J.; Loper, J.E. Production of 2,4-diacetylphloroglucinol by the biocontrol agent Pseudomonas fluorescens Pf-5. Can. J. Microbiol. 1994, 40, 1064–1066. [Google Scholar] [CrossRef]
- Islam, M.T.; Fukushi, Y. Growth inhibition and excessive branching in Aphanomyces cochlioides induced by 2,4-diacetylphloroglucinol is linked to disruption of filamentous actin cytoskeleton in the hyphae. World J. Microbiol. Biotechnol. 2010, 26, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, O.; O’Gara, F.; Morrissey, J.P. The Pseudomonas fluorescens secondary metabolite 2,4-diacetylphloroglucinol impairs mitochondrial function in Saccharomyces cerevisiae. Antonie van Leeuwenhoek 2010, 97, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Kwak, Y.S.; Han, S.; Thomashow, L.S.; Rice, J.T.; Paulitz, T.C.; Kim, D.; Weller, D.M. Saccharomyces cerevisiae genome-wide mutant screen for sensitivity to 2,4-diacetylphloroglucinol, an antibiotic produced by Pseudomonas fluorescens. Appl. Environ. Microbiol. 2011, 77, 1770–1776. [Google Scholar] [CrossRef]
- Stepanov, A.A.; Poshvina, D.V.; Vasilchenko, A.S. 2,4-Diacetylphloroglucinol Modulates Candida albicans Virulence. J. Fungi 2022, 8, 1018. [Google Scholar] [CrossRef]
- Troppens, D.M.; Dmitriev, R.I.; Papkovsky, D.B.; O’Gara, F.; Morrissey, J.P. Genome-wide investigation of cellular targets and mode of action of the antifungal bacterial metabolite 2,4-diacetylphloroglucinol in Saccharomyces cerevisiae. FEMS Yeast Res. 2013, 13, 322–334. [Google Scholar] [CrossRef]
- Proctor, D.M.; Sansom, S.E.; Deming, C.; Conlan, S.; Blaustein, R.A.; Atkins, T.K.; Program, N.C.S.; Mullikin, J.; Thomas, J.; Young, A.; et al. Clonal Candida auris and ESKAPE pathogens on the skin of residents of nursing homes. Nature 2025, 639, 1019–1023. [Google Scholar] [CrossRef]
- Byrd, A.; Belkaid, Y.; Segre, J. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, B.; Hedayati, M.T.; Hedayati, N.; Ilkit, M.; Syedmousavi, S. Aspergillus species in indoor environments and their possible occupational and public health hazards. Curr. Med. Mycol. 2016, 2, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Brakhage, A.A.; Langfelder, K. Menacing mold: The molecular biology of Aspergillus fumigatus. Annu. Rev. Microbiol. 2002, 56, 433–455. [Google Scholar] [CrossRef]
- Jing, C.-X.; Hu, Y.-M.; Jin, Y.-R.; Li, A.-P.; Wang, R.; Zhang, S.-Y.; Wu, Z.; Yan, X.-Y.; Zhang, Z.-J.; Liang, H.-J.; et al. Antifungal activity of phloroglucinol derivatives against Botrytis cinerea and Monilinia fructicola. J. Agric. Food Chem. 2024, 72, 20882–20891. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.M.; Bradley, M.C.; Fernández-Del-Río, L.; Nag, A.; Tsui, H.S.; Clarke, C.F. Coenzyme Q10 deficiencies: Pathways in yeast and humans. Essays Biochem. 2018, 62, 361–376. [Google Scholar]
- Mesa-Arango, A.C.; Trevijano-Contador, N.; Román, E.; Sánchez-Fresneda, R.; Casas, C.; Herrero, E.; Argüelles, J.C.; Pla, J.; Cuenca-Estrella, M.; Zaragoza, O. The production of reactive oxygen species is a universal action mechanism of Amphotericin B against pathogenic yeasts and contributes to the fungicidal effect of this drug. Antimicrob. Agents Chemother. 2014, 58, 6627–6638. [Google Scholar] [CrossRef]
- Dar, D.; Thomashow, L.S.; Weller, D.M.; Newman, D.K. Global landscape of phenazine biosynthesis and biodegradation reveals species-specific colonization patterns in agricultural soils and crop microbiomes. Elife 2020, 9, e59726. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). M38-A2 Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi: Approved Standard, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Yu, M.; Yu, J.; Pan, X.; Cao, H.; Song, T.; Liu, Y. Farnesol inhibits growth and development of Ustilaginoidea virens. New Plant Prot. 2024, 1, e17. [Google Scholar] [CrossRef]
- Liu, Q.; Li, L.; Yang, Z.; Xiong, X.; Song, Q.; Li, B.; Zou, H.; Zhang, L.; Liu, T. Antifungal Effect of Oregano Essential Oil Against Penicillium expansum on Pyrus sinkiangensis. J. Fungi 2024, 10, 752. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Song, M.; Zhu, K.; Shen, J. A rhein-based derivative targets Staphylococcus aureus. Antibiotics 2024, 13, 882. [Google Scholar] [CrossRef]
- Ji, D.; Chen, T.; Ma, D.; Liu, J.; Xu, Y.; Tian, S. Inhibitory effects of methyl thujate on mycelial growth of Botrytis cinerea and possible mechanisms. Postharvest. Biol. Technol. 2018, 142, 46–54. [Google Scholar] [CrossRef]
- Marena, G.D.; Thomaz, L.; Nosanchuk, J.D.; Taborda, C.P. Galleria mellonella as an invertebrate model for studying fungal infections. J. Fungi 2025, 11, 157. [Google Scholar] [CrossRef] [PubMed]
- Cassilly, C.D.; Reynolds, T.B. PS, It’s complicated: The roles of phosphatidylserine and phosphatidylethanolamine in the pathogenesis of Candida albicans and other microbial pathogens. J. Fungi 2018, 4, 28. [Google Scholar] [CrossRef] [PubMed]


| Strains | Origin | MIC 1 (μg/mL) | |
|---|---|---|---|
| DAPG | DPPG | ||
| A. fumigatus ATCC 96918 | Standard strain | >128 | 32 |
| A. fumigatus CAUF136 | Human, respiratory infection | >128 | 32 |
| A. flavus ATCC 11492 | Standard strain | >128 | 64 |
| A. flavus CAUF135 | Human, respiratory infection | >128 | 32 |
| A. niger CAUF137 | Human, respiratory infection | >128 | 16 |
| A. niger CAUF140 | Human, respiratory infection | >128 | 32 |
| A. terrestris CAUF138 | Human, respiratory infection | >128 | 16 |
| A. terrestris CAUF139 | Human, respiratory infection | >128 | 16 |
| C. albicans ATCC 10231 | Standard strain | >128 | 64 |
| C. albicans CAUF1 | Human, urinary tract infection | >128 | 128 |
| C. albicans CAUF3 | Human, intestinal infection | >128 | 128 |
| C. tropicalis CAUF2 | Human, urinary tract infection | >128 | 64 |
| C. tropicalis CAUF5 | Human, intestinal infection | >128 | 64 |
| C. krusei ATCC 6258 | Standard strain | >128 | 16 |
| C. krusei CGMCC 2.3984 | Standard strain | >128 | 32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; He, J.; Feng, H.; Li, Q.; Song, M.; Gou, H.; He, Y.; Zhu, K. Antifungal Effects of the Phloroglucinol Derivative DPPG Against Pathogenic Aspergillus fumigatus. Antibiotics 2025, 14, 499. https://doi.org/10.3390/antibiotics14050499
Wang L, He J, Feng H, Li Q, Song M, Gou H, He Y, Zhu K. Antifungal Effects of the Phloroglucinol Derivative DPPG Against Pathogenic Aspergillus fumigatus. Antibiotics. 2025; 14(5):499. https://doi.org/10.3390/antibiotics14050499
Chicago/Turabian StyleWang, Liyang, Junying He, Hanzhong Feng, Qian Li, Meirong Song, Haoran Gou, Yongxing He, and Kui Zhu. 2025. "Antifungal Effects of the Phloroglucinol Derivative DPPG Against Pathogenic Aspergillus fumigatus" Antibiotics 14, no. 5: 499. https://doi.org/10.3390/antibiotics14050499
APA StyleWang, L., He, J., Feng, H., Li, Q., Song, M., Gou, H., He, Y., & Zhu, K. (2025). Antifungal Effects of the Phloroglucinol Derivative DPPG Against Pathogenic Aspergillus fumigatus. Antibiotics, 14(5), 499. https://doi.org/10.3390/antibiotics14050499







