In Vitro Activity of Imipenem/Relebactam Alone and in Combination Against Cystic Fibrosis Isolates of Mycobacterium abscessus
Abstract
1. Introduction
2. Results
2.1. In Vitro Susceptibility and Synergy Testing of Antibiotics Against M. abscessus ATCC 19977
2.2. In Vitro Susceptibility Testing and Screening of Antibiotics for Synergy with Imipenem/Relebactam Against M. abscessus CF Clinical Isolates
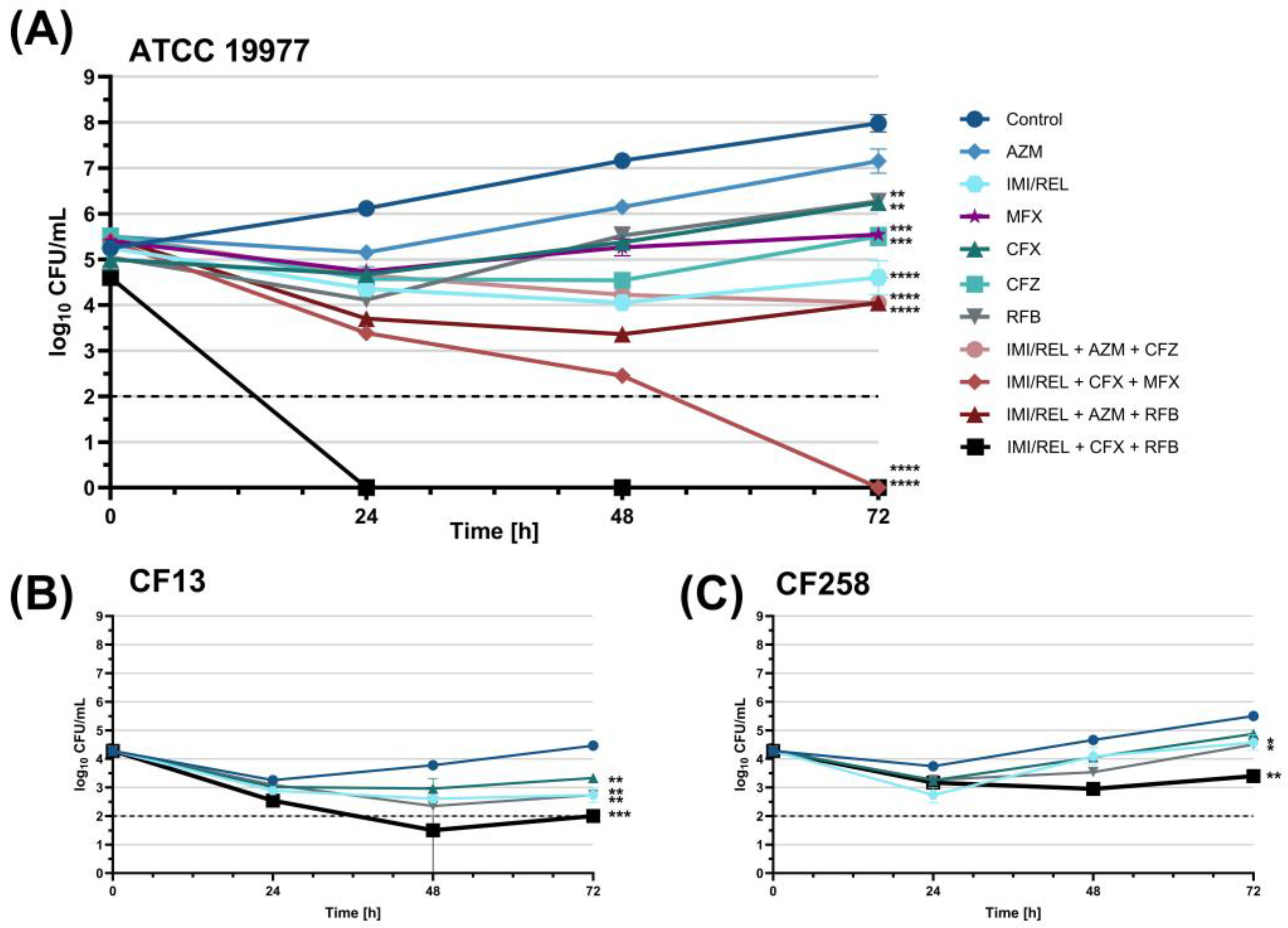
2.3. Evaluation of Three-Drug Antibiotic Combinations with Imipenem/Relebactam Using Time-Kill Assay
2.3.1. Initial Screening of Antibiotic Combinations Against M. abscessus ATCC 19977
2.3.2. Kinetic Time-Kill Assay with Antibiotic Combinations Against M. abscessus ATCC 19977 and CF Clinical Isolates CF13 and CF258
3. Discussion
4. Materials and Methods
4.1. Preparation of Antibiotics and Growth Media
4.2. Bacterial Strains and Culture Conditions
4.3. Broth Microdilution Assay and Minimum Inhibitory Concentration (MIC) Determination for Susceptibility Testing
4.4. Checkerboard Assay and Fractional Inhibitory Concentration (FIC) Index Determination for Synergy Testing
4.5. Time-Kill Assays
4.6. Data and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MABS | Mycobacterium abscessus; |
| PwCF | People with cystic fibrosis; |
| CFTR | Cystic fibrosis transmembrane conductance regulator; |
| NTM-PD | Nontuberculous mycobacteria pulmonary disease; |
| MIC50/90 | Minimum inhibitory concentration that inhibited 50% and 90%, respectively, of the tested microorganisms; |
| FIC | Fractional inhibitory concentration. |
References
- De Boeck, K.; Zolin, A.; Cuppens, H.; Olesen, H.V.; Viviani, L. The relative frequency of CFTR mutation classes in European patients with cystic fibrosis. J. Cyst. Fibros. 2014, 13, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Elborn, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Floto, R.A.; Olivier, K.N.; Saiman, L.; Daley, C.L.; Herrmann, J.L.; Nick, J.A.; Noone, P.G.; Bilton, D.; Corris, P.; Gibson, R.L.; et al. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax 2016, 71 (Suppl. S1), i1–i22. [Google Scholar] [CrossRef] [PubMed]
- Adjemian, J.; Olivier, K.N.; Prevots, D.R. Epidemiology of pulmonary nontuberculous mycobacterial sputum positivity in patients with cystic fibrosis in the United States, 2010–2014. Ann. Am. Thorac. Soc. 2018, 15, 817–826. [Google Scholar] [CrossRef]
- Cystic Fibrosis Foundation. 2023 Patient Registry: Annual Data Report. 2024. Available online: https://www.cff.org/media/34491/download (accessed on 9 May 2025).
- Marshall, J.E.; Mercaldo, R.A.; Lipner, E.M.; Prevots, D.R. Incidence of nontuberculous mycobacteria infections among persons with cystic fibrosis in the United States (2010–2019). BMC Infect. Dis. 2023, 23, 489. [Google Scholar] [CrossRef] [PubMed]
- Bernut, A.; Dupont, C.; Ogryzko, N.V.; Neyret, A.; Herrmann, J.L.; Floto, R.A.; Renshaw, S.A.; Kremer, L. CFTR Protects against Mycobacterium abscessus Infection by Fine-Tuning Host Oxidative Defenses. Cell Rep. 2019, 26, 1828–1840.e4. [Google Scholar] [CrossRef]
- Shamaei, M.; Mirsaeidi, M. Nontuberculous mycobacteria, macrophages, and host innate immune response. Infect. Immun. 2021, 89, e0081220. [Google Scholar] [CrossRef]
- Touré, H.; Galindo, L.A.; Lagune, M.; Glatigny, S.; Waterhouse, R.M.; Guénal, I.; Herrmann, J.L.; Girard-Misguich, F.; Szuplewski, S. Mycobacterium abscessus resists the innate cellular response by surviving cell lysis of infected phagocytes. PLoS Pathog. 2023, 19, e1011257. [Google Scholar] [CrossRef]
- Catherinot, E.; Roux, A.L.; Vibet, M.A.; Bellis, G.; Ravilly, S.; Lemonnier, L.; Le Roux, E.; Bernède-Bauduin, C.; Le Bourgeois, M.; Herrmann, J.L.; et al. Mycobacterium avium and Mycobacterium abscessus complex target distinct cystic fibrosis patient subpopulations. J. Cyst. Fibros. 2013, 12, 74–80. [Google Scholar] [CrossRef]
- Qvist, T.; Taylor-Robinson, D.; Waldmann, E.; Olesen, H.V.; Hansen, C.R.; Mathiesen, I.H.; Høiby, N.; Katzenstein, T.L.; Smyth, R.L.; Diggle, P.J.; et al. Comparing the harmful effects of nontuberculous mycobacteria and Gram-negative bacteria on lung function in patients with cystic fibrosis. J. Cyst. Fibros. 2016, 15, 380–385. [Google Scholar] [CrossRef]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef]
- Barto, T.L.; Olivier, K.N. Nontuberculous mycobacteria clinical care guidelines. Cystic Fibrosis Foundation. 2016. Available online: https://www.cff.org/medical-professionals/nontuberculous-mycobacteria-clinical-care-guidelines (accessed on 9 May 2025).
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J., Jr.; Andrejak, C.; Böttger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of nontuberculous mycobacterial pulmonary disease: An official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur. Respir. J. 2020, 56, 2000535. [Google Scholar] [CrossRef]
- Kwak, N.; Dalcolmo, M.P.; Daley, C.L.; Eather, G.; Gayoso, R.; Hasegawa, N.; Jhun, B.W.; Koh, W.J.; Namkoong, H.; Park, J.; et al. Mycobacterium abscessus pulmonary disease: Individual patient data meta-analysis. Eur. Respir. J. 2019, 54, 1801991. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, L.; Mao, Y.; Ye, M.; Guo, Q.; Zhang, Y.; Xu, L.; Zhang, Z.; Li, B.; Chu, H. Clinical efficacy and adverse effects of antibiotics used to treat Mycobacterium abscessus pulmonary disease. Front. Microbiol. 2019, 10, 1977. [Google Scholar] [CrossRef]
- Nessar, R.; Cambau, E.; Reyrat, J.M.; Murray, A.; Gicquel, B. Mycobacterium abscessus: A new antibiotic nightmare. J. Antimicrob. Chemother. 2012, 67, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Luthra, S.; Rominski, A.; Sander, P. The role of antibiotic-target-modifying and antibiotic-modifying enzymes in Mycobacterium abscessus drug resistance. Front. Microbiol. 2018, 9, 2179. [Google Scholar] [CrossRef]
- Soroka, D.; Dubée, V.; Soulier-Escrihuela, O.; Cuinet, G.; Hugonnet, J.E.; Gutmann, L.; Mainardi, J.-L.; Arthur, M. Characterization of broad-spectrum Mycobacterium abscessus class A β-lactamase. J. Antimicrob. Chemother. 2014, 69, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Sheng, W.H.; Hung, C.C.; Yu, C.J.; Lee, L.N.; Hsueh, P.R. Mycobacterium abscessus complex infections in humans. Emerg. Infect. Dis. 2015, 21, 1638–1646. [Google Scholar] [CrossRef]
- Lopeman, R.C.; Harrison, J.; Rathbone, D.L.; Desai, M.; Lambert, P.A.; Cox, J.A.G. Effect of amoxicillin in combination with imipenem-relebactam against Mycobacterium abscessus. Sci. Rep. 2020, 10, 928. [Google Scholar] [CrossRef]
- Le Run, E.; Atze, H.; Arthur, M.; Mainardi, J.L. Impact of relebactam-mediated inhibition of Mycobacterium abscessus BlaMab β-lactamase on the in vitro and intracellular efficacy of imipenem. J. Antimicrob. Chemother. 2020, 75, 379–383. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, Y.; He, R.; Yu, B.; Zhong, Y.; Lin, F. Efficacy and safety of novel carbapenem-β-lactamase inhibitor combinations: Imipenem-cilastatin/relebactam results from randomized controlled trials. Front. Med. 2023, 10, 1304369. [Google Scholar] [CrossRef]
- Kaushik, A.; Ammerman, N.C.; Lee, J.; Martins, O.; Kreiswirth, B.N.; Lamichhane, G.; Parrish, N.M.; Nuermberger, E.L. In vitro activity of the new β-lactamase inhibitors relebactam and vaborbactam in combination with β-lactams against Mycobacterium abscessus complex clinical isolates. Antimicrob. Agents Chemother. 2019, 63, e02623-18. [Google Scholar] [CrossRef]
- Burke, A.; Carter, R.; Tolson, C.; Congdon, J.; Duplancic, C.; Bursle, E.; Bell, S.C.; Roberts, J.A.; Thomson, R. In vitro susceptibility testing of imipenem-relebactam and tedizolid against 102 Mycobacterium abscessus isolates. Int. J. Antimicrob. Agents 2023, 62, 106938. [Google Scholar] [CrossRef] [PubMed]
- van Hasselt, J.G.; Rizk, M.L.; Lala, M.; Chavez-Eng, C.; Visser, S.A.; Kerbusch, T.; Danhof, M.; Rao, G.; van der Graaf, P.H. Pooled population pharmacokinetic model of imipenem in plasma and the lung epithelial lining fluid. Br. J. Clin. Pharmacol. 2016, 81, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Rizk, M.L.; Rhee, E.G.; Jumes, P.A.; Gotfried, M.H.; Zhao, T.; Mangin, E.; Bi, S.; Chavez-Eng, C.M.; Zhang, Z.; Butterton, J.R. Intrapulmonary pharmacokinetics of relebactam, a novel β-lactamase inhibitor, dosed in combination with imipenem-cilastatin in healthy subjects. Antimicrob. Agents Chemother. 2018, 62, e01411-17. [Google Scholar] [CrossRef]
- Miyasaka, T.; Kunishima, H.; Komatsu, M.; Tamai, K.; Mitsutake, K.; Kanemitsu, K.; Ohisa, Y.; Yanagisawa, H.; Kaku, M. In vitro efficacy of imipenem in combination with six antimicrobial agents against Mycobacterium abscessus. Int. J. Antimicrob. Agents 2007, 30, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Takei, S.; Ihara, H.; Togo, S.; Nakamura, A.; Fujimoto, Y.; Watanabe, J.; Kurokawa, K.; Shibayama, K.; Sumiyoshi, I.; Ochi, Y.; et al. The synergetic effect of imipenem-clarithromycin combination in the Mycobacteroides abscessus complex. BMC Microbiol. 2020, 20, 316. [Google Scholar] [CrossRef]
- Story-Roller, E.; Maggioncalda, E.C.; Lamichhane, G. Select β-lactam combinations exhibit synergy against Mycobacterium abscessus in vitro. Antimicrobial Agents and Chemotherapy 2019, 63, e02613-18. [Google Scholar] [CrossRef]
- Pandey, R.; Chen, L.; Manca, C.; Jenkins, S.; Glaser, L.; Vinnard, C.; Stone, G.; Lee, J.; Mathema, B.; Nuermberger, E.L.; et al. Dual β-lactam combinations highly active against Mycobacterium abscessus complex in vitro. mBio 2019, 10, e02895-18. [Google Scholar] [CrossRef]
- Yamatani, I.; Aono, A.; Fujiwara, K.; Asami, T.; Kamada, K.; Morishige, Y.; Igarashi, Y.; Chikamatsu, K.; Murase, Y.; Yamada, H.; et al. In vitro effects of the new oral β-lactamase inhibitor xeruborbactam in combination with oral β-lactams against clinical Mycobacterium abscessus isolates. Microbiol. Spectr. 2024, 12, e00084-24. [Google Scholar] [CrossRef]
- Wallace, R.J., Jr.; Brown-Elliott, B.A.; Crist, C.J.; Mann, L.; Wilson, R.W. Comparison of the in vitro activity of the glycylcycline tigecycline (formerly GAR-936) with those of tetracycline, minocycline, and doxycycline against isolates of nontuberculous mycobacteria. Antimicrob. Agents Chemother. 2002, 46, 3164–3167. [Google Scholar] [CrossRef] [PubMed]
- Mudde, S.E.; Meliefste, H.M.; Ammerman, N.C.; de Steenwinkel, J.E.M.; Bax, H.I. Mycobacterium abscessus strain variability in preclinical drug development: Does it really matter? J. Antimicrob. Chemother. 2024, 79, 3169–3173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hua, W.; Lin, S.; Zhang, Y.; Chen, X.; Wang, S.; Chen, J.; Zhang, W. In vitro susceptibility of nontuberculous mycobacteria to tedizolid. Infect. Drug Resist. 2022, 15, 4845–4852. [Google Scholar] [CrossRef]
- Aziz, D.B.; Low, J.L.; Wu, M.L.; Gengenbacher, M.; Teo, J.W.P.; Dartois, V.; Dick, T. Rifabutin is active against Mycobacterium abscessus complex. Antimicrob. Agents Chemother. 2017, 61, e00155-17. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; He, S.; Li, J.; Zhang, Z.; Li, B.; Chu, H. Omadacycline, eravacycline, and tigecycline express anti-Mycobacterium abscessus activity in vitro. Microbiol. Spectr. 2023, 11, e00718-23. [Google Scholar] [CrossRef]
- Schulthess, B.; Akdoğan Kittana, F.N.; Hömke, R.; Sander, P. In vitro bedaquiline and clofazimine susceptibility testing in Mycobacterium abscessus. Antimicrob. Agents Chemother. 2022, 66, e0234621. [Google Scholar] [CrossRef]
- Woods, G.L.; Brown-Elliott, B.A.; Conville, P.S.; Desmond, E.P.; Hall, G.S.; Lin, G.; Pfyffer, G.E.; Ridderhof, J.C.; Siddiqi, S.H.; Wallace, R.J., Jr.; et al. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2011; Available online: https://www.ncbi.nlm.nih.gov/books/NBK544374/ (accessed on 9 May 2025).
- Kreutzfeldt, K.M.; McAdam, P.R.; Claxton, P.; Holmes, A.; Seagar, A.L.; Laurenson, I.F.; Fitzgerald, J.R. Molecular longitudinal tracking of Mycobacterium abscessus spp. during chronic infection of the human lung. PLoS ONE 2013, 8, e63237. [Google Scholar] [CrossRef]
- Choo, S.W.; Wee, W.Y.; Ngeow, Y.F.; Mitchell, W.; Tan, J.L.; Wong, G.J.; Zhao, Y.; Xiao, J. Genomic reconnaissance of clinical isolates of emerging human pathogen Mycobacterium abscessus reveals high evolutionary potential. Sci. Rep. 2014, 4, 4061. [Google Scholar] [CrossRef]
- Bryant, J.M.; Brown, K.P.; Burbaud, S.; Everall, I.; Belardinelli, J.M.; Rodriguez-Rincon, D.; Grogono, D.M.; Peterson, C.M.; Verma, D.; Evans, I.E.; et al. Stepwise pathogenic evolution of Mycobacterium abscessus. Science 2021, 372, eabb8699. [Google Scholar] [CrossRef]
- Lewin, A.; Kamal, E.; Semmler, T.; Winter, K.; Kaiser, S.; Schäfer, H.; Mao, L.; Eschenhagen, P.; Grehn, C.; Bender, J.; et al. Genetic diversification of persistent Mycobacterium abscessus within cystic fibrosis patients. Virulence 2021, 12, 2415–2429. [Google Scholar] [CrossRef]
- Shallom, S.J.; Tettelin, H.; Chandrasekaran, P.; Park, I.K.; Agrawal, S.; Arora, K.; Sadzewicz, L.; Milstone, A.M.; Aitken, M.L.; Brown-Elliott, B.A.; et al. Evolution of Mycobacterium abscessus in the human lung: Cumulative mutations and genomic rearrangement of porin genes in patient isolates. Virulence 2023, 14, 2215602. [Google Scholar] [CrossRef] [PubMed]
- Story-Roller, E.; Maggioncalda, E.C.; Lamichhane, G. Synergistic efficacy of β-lactam combinations against Mycobacterium abscessus pulmonary infection in mice. Antimicrob. Agents Chemother. 2019, 63, e00614-19. [Google Scholar] [CrossRef]
- Van, N.; Degefu, Y.N.; Leus, P.A.; Larkins-Ford, J.; Klickstein, J.; Maurer, F.P.; Stone, D.; Poonawala, H.; Thorpe, C.M.; Smith, T.C., 2nd; et al. Novel synergies and isolate specificities in the drug interaction landscape of Mycobacterium abscessus. Antimicrob. Agents Chemother. 2023, 67, e0009023. [Google Scholar] [CrossRef]
- Conte, J.E., Jr.; Golden, J.; Duncan, S.; McKenna, E.; Lin, E.; Zurlinden, E. Single-dose intrapulmonary pharmacokinetics of azithromycin, clarithromycin, ciprofloxacin, and cefuroxime in volunteer subjects. Antimicrob. Agents Chemother. 1996, 40, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Cook, P.J.; Andrews, J.M.; Wise, R.; Honeybourne, D. Distribution of cefdinir, a third generation cephalosporin antibiotic, in serum and pulmonary compartments. J. Antimicrob. Chemother. 1996, 37, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Cook, P.J.; Andrews, J.M.; Woodcock, J.; Wise, R.; Honeybourne, D. Concentration of amoxycillin and clavulanate in lung compartments in adults without pulmonary infection. Thorax 1994, 49, 1134–1138. [Google Scholar] [CrossRef]
- Saivin, S.; Houin, G. Clinical pharmacokinetics of doxycycline and minocycline. Clin. Pharmacokinet. 1988, 15, 355–366. [Google Scholar] [CrossRef]
- Lerat, I.; Cambau, E.; Roth dit Bettoni, R.; Gaillard, J.-L.; Jarlier, V.; Truffot, C.; Veziris, N. In vivo evaluation of antibiotic activity against Mycobacterium abscessus. J. Infect. Dis. 2014, 209, 905–912. [Google Scholar] [CrossRef]
- Samson, C.; Tamalet, A.; Thien, H.V.; Taytard, J.; Perisson, C.; Nathan, N.; Clement, A.; Boelle, P.Y.; Corvol, H. Long-term effects of azithromycin in patients with cystic fibrosis. Respir. Med. 2016, 117, 1–6. [Google Scholar] [CrossRef]
- Southern, K.W.; Solis-Moya, A.; Kurz, D.; Smith, S. Macrolide antibiotics (including azithromycin) for cystic fibrosis. Cochrane Database Syst. Rev. 2024, 2024, CD002203. [Google Scholar] [CrossRef]
- Blau, H.; Klein, K.; Shalit, I.; Halperin, D.; Fabian, I. Moxifloxacin but not ciprofloxacin or azithromycin selectively inhibits IL-8, IL-6, ERK1/2, JNK, and NF-kappaB activation in a cystic fibrosis epithelial cell line. Am. J. Physiol. -Lung Cell. Mol. Physiol. 2007, 292, L343–L352. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Jhun, B.W.; Moon, S.M.; Lee, H.; Park, H.Y.; Jeon, K.; Kim, D.H.; Kim, S.Y.; Shin, S.J.; Daley, C.L.; et al. Clofazimine-containing regimen for the treatment of Mycobacterium abscessus lung disease. Antimicrob. Agents Chemother. 2017, 61, e02052-16. [Google Scholar] [CrossRef]
- Carey, G.B.; Tebes, P.; Vinnard, C.; Kim, D.; Hadjiliadis, D.; Hansen-Flaschen, J.; Dorgan, D.; Glaser, L.; Barton, G.; Hamilton, K.W. Clinical outcomes of clofazimine use for rapidly growing mycobacteria infections. Open Forum Infect. Dis. 2019, 6, ofz456. [Google Scholar] [CrossRef]
- Dick, T. Rifabutin: A repurposing candidate for Mycobacterium abscessus lung disease. Front. Microbiol. 2020, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Johansen, M.D.; Daher, W.; Roquet-Banères, F.; Raynaud, C.; Alcaraz, M.; Maurer, F.P.; Kremer, L. Rifabutin is bactericidal against intracellular and extracellular forms of Mycobacterium abscessus. Antimicrob. Agents Chemother. 2020, 64, e00363-20. [Google Scholar] [CrossRef]
- Aziz, D.B.; Go, M.L.; Dick, T. Rifabutin suppresses inducible clarithromycin resistance in Mycobacterium abscessus by blocking induction of whiB7 and erm41. Antibiotics 2020, 9, 72. [Google Scholar] [CrossRef]
- Nash, K.A.; Brown-Elliott, B.A.; Wallace, R.J., Jr. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob. Agents Chemother. 2009, 53, 1367–1376. [Google Scholar] [CrossRef]
- Zhou, J.; Qian, Y.; Lang, Y.; Zhang, Y.; Tao, X.; Moya, B.; Sayed, A.R.M.; Landersdorfer, C.B.; Shin, E.; Werkman, C.; et al. Comprehensive stability analysis of 13 β-lactams and β-lactamase inhibitors in in vitro media, and novel supplement dosing strategy to mitigate thermal drug degradation. Antimicrob. Agents Chemother. 2024, 68, e0139923. [Google Scholar] [CrossRef]
- Philley, J.V.; Griffith, D.E. Management of nontuberculous mycobacterial (NTM) lung disease. Semin. Respir. Crit. Care Med. 2013, 34, 135–142. [Google Scholar] [CrossRef]
- Ferro, B.E.; Srivastava, S.; Deshpande, D.; Sherman, C.M.; Pasipanodya, J.G.; van Soolingen, D.; Mouton, J.W.; van Ingen, J.; Gumbo, T. Amikacin pharmacokinetics/pharmacodynamics in a novel hollow-fiber Mycobacterium abscessus disease model. Antimicrob. Agents Chemother. 2015, 60, 1242–1248. [Google Scholar] [CrossRef]
- Maggioncalda, E.C.; Story-Roller, E.; Mylius, J.; Illei, P.; Basaraba, R.J.; Lamichhane, G. A mouse model of pulmonary Mycobacteroides abscessus infection. Sci. Rep. 2020, 10, 3690. [Google Scholar] [CrossRef] [PubMed]
- Riva, C.; Tortoli, E.; Cugnata, F.; Sanvito, F.; Esposito, A.; Rossi, M.; Colarieti, A.; Canu, T.; Cigana, C.; Bragonzi, A.; et al. A new model of chronic Mycobacterium abscessus lung infection in immunocompetent mice. Int. J. Mol. Sci. 2020, 21, 6590. [Google Scholar] [CrossRef]
- Davidson, R.M.; Hasan, N.A.; Epperson, L.E.; Benoit, J.B.; Kammlade, S.M.; Levin, A.R.; de Moura, V.C.; Hunkins, J.; Weakly, N.; Beagle, S.; et al. Population genomics of Mycobacterium abscessus from U.S. cystic fibrosis care centers. Ann. Am. Thorac. Soc. 2021, 18, 1960–1969. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, M.C. What is synergy? Pharmacol. Rev. 1989, 41, 93–141, Erratum in Pharmacol. Rev. 1990, 41, 422. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bouzinbi, N.; Chaturvedi, V.; Godreuil, S.; Kremer, L. In vitro evaluation of a new drug combination against clinical isolates belonging to the Mycobacterium abscessus complex. Clin. Microbiol. Infect. 2014, 20, O1124–O1127. [Google Scholar] [CrossRef]
- Ferro, B.E.; Meletiadis, J.; Wattenberg, M.; de Jong, A.; van Soolingen, D.; Mouton, J.W.; van Ingen, J. Clofazimine prevents the regrowth of Mycobacterium abscessus and Mycobacterium avium type strains exposed to amikacin and clarithromycin. Antimicrob. Agents Chemother. 2015, 60, 1097–1105. [Google Scholar] [CrossRef]
- Burgess, D.S.; Hastings, R.W.; Horan, J.L. A time-kill evaluation of clarithromycin and azithromycin against two extracellular pathogens and the development of resistance. Ann. Pharmacother. 1999, 33, 1262–1265. [Google Scholar] [CrossRef]
- Barry, A.L.; Craig, W.A.; Nadler, H.; Reller, L.B.; Sanders, C.C.; Swenson, J.M. Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline (NCCLS Document M26-A); Clinical and Laboratory Standards Institute: Wayne, PA, USA, 1999. [Google Scholar]
- Leonard, S.N.; Cheung, C.M.; Rybak, M.J. Activities of ceftobiprole, linezolid, vancomycin, and daptomycin against community-associated and hospital-associated methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2008, 52, 2974–2976. [Google Scholar] [CrossRef]
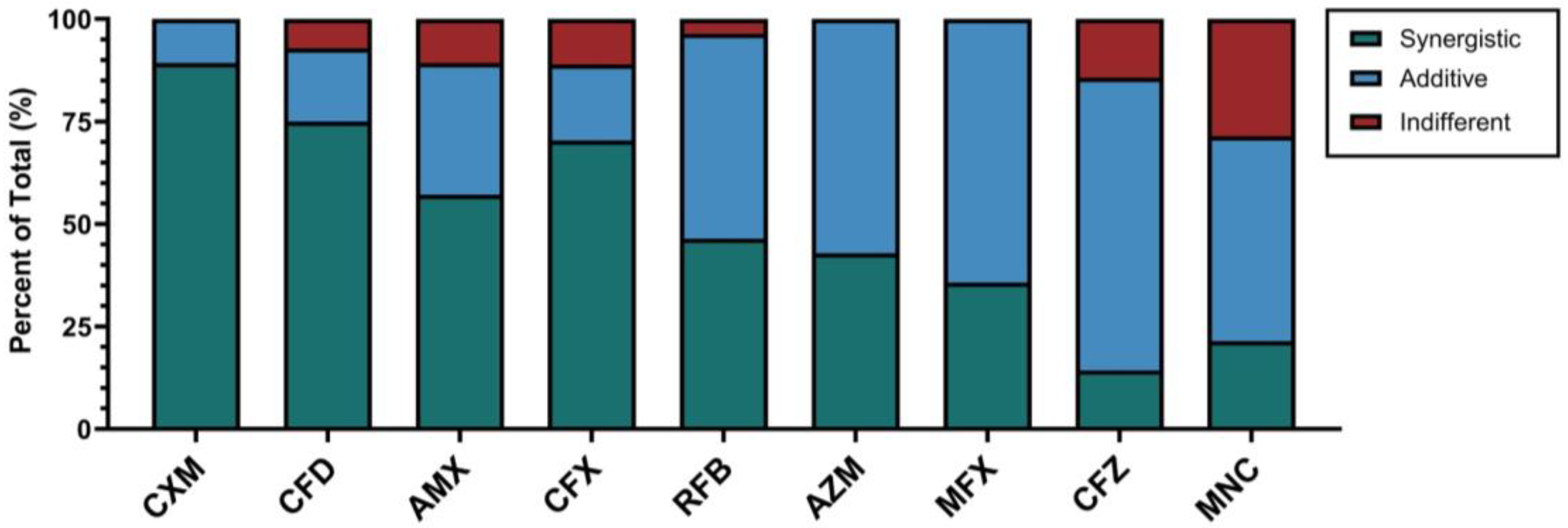

| Antibacterial Agent | MIC Value (µg/mL) | FIC Index | Interaction | |||
|---|---|---|---|---|---|---|
| Antibiotic Alone | Antibiotic with IMI/REL | IMI/REL Alone | IMI/REL with Antibiotic | |||
| Cefuroxime | 512 | 64 | 4 | 0.25 | 0.188 | Synergistic |
| Cefdinir | 256 | 16 | 4 | 1 | 0.313 | Synergistic |
| Cefoxitin | 32 | 8 | 4 | 0.5 | 0.375 | Synergistic |
| Moxifloxacin | 16 | 4 | 4 | 1 | 0.5 | Synergistic |
| Rifabutin | 16 | 4 | 4 | 1 | 0.5 | Synergistic |
| Minocycline | 256 | 64 | 4 | 1 | 0.5 | Synergistic |
| Amoxicillin | 2048 | 16 | 4 | 2 | 0.508 | Additive |
| Azithromycin | 8 | 1 | 4 | 2 | 0.625 | Additive |
| Tigecycline | 4 | 2 | 4 | 0.5 | 0.625 | Additive |
| Tedizolid | 8 | 8 | 4 | 2 | 1.5 | Indifferent |
| Antibacterial Agent | MIC50 Value (µg/mL) 1 | FIC Index | Interaction | |||
|---|---|---|---|---|---|---|
| Drug Alone | Drug with IMI/REL | IMI/REL Alone | IMI/REL with Drug | |||
| Cefuroxime | 512 (256, 512) | 64 (32, 128) | 4 (4, 8) | 0.38 (0.13, 0.50) | 0.250 (0.180, 0.328) | Synergistic |
| Cefdinir | 192 (112, 256) | 24 (14, 40) | 4 (4, 8) | 1 (0.50, 1) | 0.375 (0.313, 0.502) | Synergistic |
| Amoxicillin | 2048 (1024, 2048) | 128 (32, 256) | 8 (4, 8) | 2 (1, 2.50) | 0.438 (0.352, 0.625) | Synergistic |
| Cefoxitin | 48 (32, 64) | 8 (4, 8) | 8 (4, 16) | 2 (1, 8) | 0.500 (0.375, 0.563) | Synergistic |
| Rifabutin | 8 (7, 16) | 2 (1, 4) | 4 (4, 8) | 1 (0.88, 2) | 0.563 (0.500, 0.625) | Additive |
| Azithromycin | 8 (4, 16) | 2 (1, 4) | 8 (8, 16) | 2 (1, 4) | 0.625 (0.500, 0.750) | Additive |
| Moxifloxacin | 16 (8, 16) | 4 (4, 4) | 6 (4, 8) | 2 (2, 2) | 0.750 (0.500, 0.750) | Additive |
| Clofazimine | 1 (0.88, 2) | 0.50 (0.25, 0.63) | 8 (4, 8) | 2 (1, 4) | 0.750 (0.625, 1.000) | Additive |
| Minocycline | 256 (256, 256) | 96 (64, 128) | 4 (2, 5) | 2 (1, 2) | 0.750 (0.609, 1.039) | Additive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanders, M.; Kim, S.W.; Shinde, A.; Fletcher-Williams, D.; Quach, E.; Beringer, P. In Vitro Activity of Imipenem/Relebactam Alone and in Combination Against Cystic Fibrosis Isolates of Mycobacterium abscessus. Antibiotics 2025, 14, 486. https://doi.org/10.3390/antibiotics14050486
Sanders M, Kim SW, Shinde A, Fletcher-Williams D, Quach E, Beringer P. In Vitro Activity of Imipenem/Relebactam Alone and in Combination Against Cystic Fibrosis Isolates of Mycobacterium abscessus. Antibiotics. 2025; 14(5):486. https://doi.org/10.3390/antibiotics14050486
Chicago/Turabian StyleSanders, Madeline, Sun Woo Kim, Aditi Shinde, Danielle Fletcher-Williams, Eric Quach, and Paul Beringer. 2025. "In Vitro Activity of Imipenem/Relebactam Alone and in Combination Against Cystic Fibrosis Isolates of Mycobacterium abscessus" Antibiotics 14, no. 5: 486. https://doi.org/10.3390/antibiotics14050486
APA StyleSanders, M., Kim, S. W., Shinde, A., Fletcher-Williams, D., Quach, E., & Beringer, P. (2025). In Vitro Activity of Imipenem/Relebactam Alone and in Combination Against Cystic Fibrosis Isolates of Mycobacterium abscessus. Antibiotics, 14(5), 486. https://doi.org/10.3390/antibiotics14050486






