Dissemination of Tylosin Residues in the Poultry Environment: Evaluating Litter and Droppings as Sources of Risk
Abstract
1. Introduction
2. Results
2.1. Validation and Optimization of Chemical Analytical Method
2.2. Detection and Quantification of Tylosin Residues in Litter and Droppings in Poultry
2.3. Determination of the Dissemination of Tylosin Residues in the Poultry Environment
3. Discussion
4. Materials and Methods
4.1. Description of Experimental Animals
Sampling of Litter and Droppings
4.2. Chemical Analysis
4.2.1. Tylosin Standards
4.2.2. Chemicals and Solvents
4.2.3. Sample Preparation
4.2.4. LC-MS/MS Analysis
4.2.5. Analytical Method Validation/Verification
- Limit of detection (LOD): Twenty fortified samples were analyzed at the selected LODs, with a signal-to-noise ratio of ≥3:1. A coefficient of variation (CV%) of <25% was accepted.
- Limit of quantification (LOQ): Twenty fortified samples at the LOD were analyzed, and the standard deviation was determined, which was then multiplied by 1.64 and added to the LOD. The signal-to-noise ratio for this parameter must be ≥10:1 to be accepted.
- Retention time (TR): Six samples with a tylosin standard were analyzed to evaluate the TR of each, with an accepted coefficient of variation of <1%.
- Specificity: Twenty blank samples were evaluated to determine if there were any interferents in the TR.
- Linearity: Three calibration curves were created using five different concentrations, with the lowest concentration at LOD. A determination coefficient (R2) ≥ 0.95 and a p-value >0.05 using the Mandel test was required for acceptance.
- Precision and recovery: To achieve precision, two parameters were evaluated: intra-laboratory reproducibility and repeatability. Six calibration curves at 25, 50, and 75 µg kg−1 were analyzed for each parameter on three days. Repeatability was assessed by performing all analyses under identical conditions by the same analyst, while intra-laboratory reproducibility involved analyses conducted by different analysts under varying conditions. Recovery was evaluated based on these samples, and this was achieved when the mean recovery percentage was between −20 and 20% on each level.
- Matrix effect: A chemical extraction was performed on 20 blank samples fortified at the end of the extraction process. The signal obtained from these samples was compared to pure standard injections.
- Ruggedness: This was evaluated by modifying three conditions: the amount of EDTA/McIlvaine buffer added to the sample initially (8 mL to 4 mL of buffer), the centrifugation time at the beginning of the extraction process (10 to 5 min), and the volume used to wash the extraction column (5 mL to 2 mL of HPLC water). The method was considered robust when the standard deviation of the modified samples was equal to or lower than intra-laboratory reproducibility.
- Stability: fifteen chicken litter and dropping samples were fortified at 50 µg kg−1 and stored at −20 °C. Five samples were analyzed after 1 week, another five samples were analyzed after 2 weeks, and the remaining five were analyzed after 3 weeks.
4.2.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baralla, E.; Demontis, M.P.; Dessi, F.; Varoni, M.V. An Overview of Antibiotics as Emerging Contaminants: Occurrence in Bivalves as Biomonitoring Organisms. Animals 2021, 11, 3239. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xiang, L.; Leung, K.S.; Elsner, M.; Zhang, Y.; Guo, Y.; Pan, B.; Sun, H.; An, T.; Ying, G.; et al. Emerging contaminants: A One Health perspective. Innovation 2024, 5, 100612. [Google Scholar] [CrossRef]
- Nguyen, M.; Lin, C.; Nguyen, H.; Hung, N.T.Q.; La, D.D.; Nguyen, X.H.; Chang, S.W.; Chung, W.J.; Nguyen, D.D. Occurrence, fate, and potential risk of pharmaceutical pollutants in agriculture: Challenges and environmentally friendly solutions. Sci. Total Environ. 2023, 899, 165323. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Bose, P.; Rahman, M.Z.; Muktaruzzaman, M.; Sultana, P.; Ahamed, T.; Khatun, M.M. A review of antimicrobial usage practice in livestock and poultry production and its consequences on human and animal health. J. Adv. Vet. Anim. Res. 2024, 11, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ying, G.-G.; Deng, W.-J. Antibiotic Residues in Food: Extraction, Analysis, and Human Health Concerns. J. Agric. Food Chem. 2019, 67, 7569–7586. [Google Scholar] [CrossRef]
- Ghimpețeanu, O.M.; Pogurschi, E.N.; Popa, D.C.; Dragomir, N.; Drăgotoiu, T.; Mihai, O.D.; Petcu, C.D. Antibiotic Use in Livestock and Residues in Food—A Public Health Threat: A Review. Foods 2022, 11, 1430. [Google Scholar] [CrossRef]
- Ashworth, A.J.; Chastain, J.P.; Moore, P.A. Nutrient Characteristics of Poultry Manure and Litter. In Animal Manure: Production, Characteristics, Environmental Concerns, and Management; ASA Special Publications; John Wiley & Sons: Hoboken, NJ, USA, 2020; Volume 67, pp. 63–87. [Google Scholar] [CrossRef]
- Gržinić, G.; Piotrowicz-Cieślak, A.; Klimkowicz-Pawlas, A.; Górny, R.L.; Ławniczek-Wałczyk, A.; Piechowicz, L.; Olkowska, E.; Potrykus, M.; Tankiewicz, M.; Krupka, M.; et al. Intensive poultry farming: A review of the impact on the environment and human health. Sci. Total Environ. 2023, 858, 160014. [Google Scholar] [CrossRef]
- Paranhos, A.G.O.; Pereira, A.R.; da Fonseca, I.C.; Sanson, A.L.; Afonso, R.J.C.F.; Aquino, S.F. Analysis of tylosin in poultry litter by HPLC-UV and HPLC-MS/MS after LTPE. Int. J. Environ. Anal. Chem. 2020, 101, 2568–2585. [Google Scholar] [CrossRef]
- Monir, H.H.; Fayez, Y.M.; Nessim, C.K.; Michael, A.M. When is it safe to eat different broiler chicken tissues after administration of doxycycline and Tylosin mixture? J. Food Sci. 2021, 86, 1162–1171. [Google Scholar] [CrossRef]
- Alonso, L.L.; Podio, N.S.; Marino, D.J.G.; Almada, N.S.; Gange, J.M.; Bernigaud, I.; Mórtola, N.; Wunderlin, D.A. Evaluating antibiotic occurrence, degradation, and environmental risks in poultry litter within Argentina’s agricultural hub. Sci. Total Environ. 2024, 920, 170993. [Google Scholar] [CrossRef]
- Pokrant, E.; Trincado, L.; Yévenes, K.; Terraza, G.; Maddaleno, A.; Martín, B.S.; Zavala, S.; Hidalgo, H.; Lapierre, L.; Cornejo, J. Determination of five antimicrobial families in droppings of therapeutically treated broiler chicken by high-performance liquid chromatography-tandem mass spectrometry. Poult. Sci. 2021, 100, 101313. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Li, Z.; Zhu, R.; Wang, J.; Liu, X.; Liu, X. The Antibiotics Degradation and Its Mechanisms during the Livestock Manure Anaerobic Digestion. Molecules 2023, 28, 4090. [Google Scholar] [CrossRef] [PubMed]
- Bos, B.; Groot Koerkamp, P.W.G.; Groenestein, K. A novel design approach for livestock housing based on recursive control—With examples to reduce environmental pollution. Livest. Prod. Sci. 2003, 84, 157–170. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazard (BIOHAZ). Role played by the environment in the emergence and spread of antimicrobial resistance (AMR) through the food chain. EFSA J. 2021, 19, e06651. [Google Scholar] [CrossRef]
- Ghirardini, A.; Grillini, V.; Verlicchi, P. A review of the occurrence of selected micropollutants and microorganisms in different raw and treated manure—Environmental risk due to antibiotics after application to soil. Sci. Total Environ. 2020, 707, 136118. [Google Scholar] [CrossRef]
- Marutescu, L.G.; Jaga, M.; Postolache, C.; Barbuceanu, F.; Milita, N.M.; Romascu, L.M.; Schmitt, H.; de Roda Husman, A.M.; Sefeedpari, P.; Glaeser, S.; et al. Insights into the impact of manure on the environmental antibiotic residues and resistance pool. Front. Microbiol. 2022, 13, 965132. [Google Scholar] [CrossRef]
- McEachran, A.D.; Blackwell, B.R.; Hanson, J.D.; Wooten, K.J.; Mayer, G.D.; Cox, S.B.; Smith, P.N. Antibiotics, Bacteria, and Antibiotic Resistance Genes: Aerial Transport from Cattle Feed Yards via Particulate Matter. Environ. Health Perspect. 2015, 123, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Pokrant, E.; Yévenes, K.; Trincado, L.; Terraza, G.; Galarce, N.; Maddaleno, A.; Martín, B.S.; Lapierre, L.; Cornejo, J. Evaluation of Antibiotic Dissemination into the Environment and Untreated Animals, by Analysis of Oxytetracycline in Poultry Droppings and Litter. Animals 2021, 11, 853. [Google Scholar] [CrossRef]
- Vargas, M.B.; Pokrant, E.; García, I.; Cadena, R.; Mena, F.; Yévenes, K.; Fuentes, C.; Zavala, S.; Flores, A.; Maturana, M.; et al. Assessing the spread of sulfachloropyridazine in poultry environment and its impact on Escherichia coli resistance. Prev. Vet. Med. 2024, 233, 106362. [Google Scholar] [CrossRef]
- Bolan, N.S.; Szogi, A.A.; Chuasavathi, T.; Seshadri, B.; Rothrock, M.J.; Panneerselvam, P. Uses and management of poultrylitter. World’s Poult. Sci. J. 2010, 66, 673–698. [Google Scholar] [CrossRef]
- Kyakuwaire, M.; Olupot, G.; Amoding, A.; Nkedi-Kizza, P.; Basamba, T.A. How Safe is Chicken Litter for Land Application as an Organic Fertilizer?: A Review. Int. J. Environ. Res. Public Health 2019, 16, 3521. [Google Scholar] [CrossRef]
- Rashid, A.; Muhammad, J.; Khan, S.; Kanwal, A.; Sun, Q. Poultry manure gleaned antibiotic residues in soil environment: A perspective of spatial variability and influencing factors. Chemosphere 2023, 317, 137907. [Google Scholar] [CrossRef] [PubMed]
- Albero, B.; Tadeo, J.L.; Escario, M.; Miguel, E.; Pérez, R.A. Persistence and availability of veterinary antibiotics in soil and soil-manure systems. Sci. Total Environ. 2018, 643, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, B.; Lahr, J.; Nibbeling, C.; Jansen, L.; Bongers, I.; Wipfler, E.; Van de Schans, M. The persistence of a broad range of antibiotics during calve, pig and broiler manure storage. Chemosphere 2018, 204, 267–276. [Google Scholar] [CrossRef]
- Cortés, P.; Pokrant, E.; Yévenes, K.; Maddaleno, A.; Flores, A.; Vargas, M.B.; Trincado, L.; Maturana, M.; Lapierre, L.; Cornejo, J. Antimicrobial Residues in Poultry Litter: Assessing the Association of Antimicrobial Persistence with Resistant Escherichia coli Strains. Antibiotics 2025, 14, 89. [Google Scholar] [CrossRef]
- European Commission. Council Regulation (EC) No 2021/808 of 22 March 2021 on the Performance of Analytical Methods for Residues of Pharmacologically Active Substances Used in Food-Producing Animals and on the Interpretation of Results as well as on the Methods to Be Used for Sampling and Repealing Decisions 2002/657/EC and 98/179/EC. Available online: https://eur-lex.europa.eu/eli/reg_impl/2021/808/oj/eng (accessed on 23 March 2025).
- European Medicines Agency (EMA). VICH GL2: Validation of Analytical Procedures: Methodology-Scientific Guideline. 1998. Available online: https://www.ema.europa.eu/en/vich-gl2-validation-analytical-procedures-methodology-scientific-guideline (accessed on 23 March 2025).
- Ji, L.; Dong, L.; Ji, H.; Feng, X.; Li, D.; Ding, R.; Jiang, S. Comparative pharmacokinetics and bioavailability of tylosin tartrate and tylosin phosphate after a single oral and i.v. administration in chickens. J. Vet. Pharmacol. Ther. 2013, 37, 312–315. [Google Scholar] [CrossRef]
- Ho, Y.B.; Zakaria, M.P.; Latif, P.A.; Saari, N. Simultaneous determination of veterinary antibiotics and hormone in broiler manure, soil and manure compost by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2012, 1262, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Chu, L.M. Fate of antibiotics in soil and their uptake by edible crops. Sci. Total Environ. 2017, 599–600, 500–512. [Google Scholar] [CrossRef]
- Cornejo, J.; Pokrant, E.; Carvallo, C.; Maddaleno, A.; San Martín, B. Depletion of tylosin residues in feathers, muscle and liver from broiler chickens after completion of antimicrobial therapy. Food Addit. Contam. Part A 2018, 35, 448–457. [Google Scholar] [CrossRef]
- Derakhshani, S.M.; Ogink, N.W.M.; Bos, B.A.P.; Groot Koerkamp, P.W.G. Sensitivity analysis of fine dust spreading from litter in poultry houses. Biosyst. Eng. 2021, 208, 272–286. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, X. Microbiological Safety of Chicken Litter or Chicken Litter-Based Organic Fertilizers: A Review. Agriculture 2014, 4, 1–29. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the Soil Environment—Degradation and Their Impact on Microbial Activity and Diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.G.J.; Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K. (Ed.) Tylosin. In Meyler’s Side Effects of Drugs (Sixteenth Edition); Elsevier: Amsterdam, The Netherlands, 2016; p. 233. [Google Scholar] [CrossRef]
- CONICYT. Manual de Normas de Bioseguridad y Riesgos Asociados-Fondecyt-CONICYT; Versión 2018; CONICYT: Santiago, Chile, 2018. [Google Scholar]
- Ministerio de Salud Subsecretaría de Salud Pública Ley Núm. 20.380 Sobre Protección de Animales. 2009. Available online: https://www.bcn.cl/leychile/navegar?idNorma=1006858 (accessed on 10 March 2025).
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific PurposesText with EEA Relevance. 47p. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF (accessed on 10 March 2025).
- Council Regulation (EC) No 1099/2009 of 24 September 2009 on the Protection of Animals at the Time of KillingText with EEA Relevance. 30p. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:303:0001:0030:EN:PDF (accessed on 10 March 2025).
- US Department of Agriculture (USDA). Poultry Industry Manual; US Department of Agriculture (USDA): Washington, DC, USA, 2013. [Google Scholar]
- IAEA International Atomic Energy Agency. Soil Sampling for Environmental Contaminants; IAEA International Atomic Energy Agency: Vienna, Austria, 2004. [Google Scholar]
- Yévenes, K.; Pokrant, E.; Trincado, L.; Lapierre, L.; Galarce, N.; Martín, B.S.; Maddaleno, A.; Hidalgo, H.; Cornejo, J. Detection of Antimicrobial Residues in Poultry Litter: Monitoring a Risk through a Selective and Sensitive HPLC–MS/MS Method. Animals 2021, 11, 1399. [Google Scholar] [CrossRef]
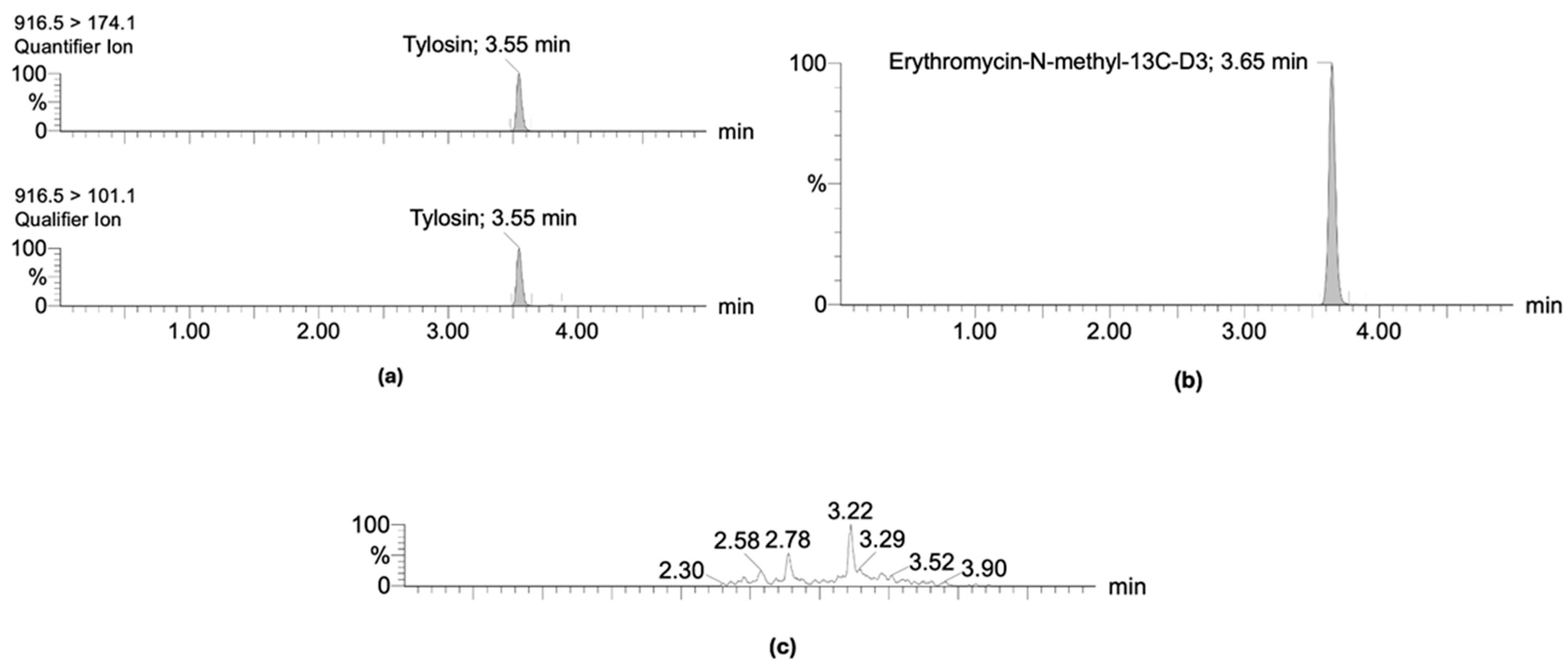


| Matrix | Fortified Concentration (µg kg−1) | Repeatability (RSD %) * | Reproducibility (RSD %) | Recovery (RSD%) |
|---|---|---|---|---|
| Droppings | 25 | 3.01 | 4.67 | 98.34 |
| 50 | 2.88 | 4.52 | 101.66 | |
| 75 | 0.99 | 1.54 | 99.45 | |
| Litter | 25 | 4.17 | 4.61 | 97.11 |
| 50 | 3.96 | 4.35 | 102.89 | |
| 75 | 1.37 | 1.51 | 99.04 |
| Tylosin Concentrations (µg·kg−1 ww) | |||||||
|---|---|---|---|---|---|---|---|
| Group | Matrix | Day 3 | Day 6 | Day 9 | Day 12 | Day 15 | Day 18 |
| A | Litter | 1313.88 | 1284.94 | 1015.09 | 955.42 | 351.61 | 290.16 |
| Droppings | 8.67 | <LOQ ** | <LOQ | <LOD * | <LOD | <LOD | |
| B.1 | Litter | 25.89 | 24.95 | 21.38 | 9.71 | 9.51 | 9.35 |
| Droppings | <LOQ | <LOD | <LOD | <LOD | <LOD | <LOD | |
| B.2 | Litter | 11.41 | <LOQ | <LOQ | <LOQ | <LOD | <LOD |
| Droppings | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | |
| Time (Minutes) | Solution A (%) | Solution B (%) |
|---|---|---|
| 0.00 | 98 | 2 |
| 1.00 | 98 | 2 |
| 2.00 | 55 | 45 |
| 3.10 | 25 | 75 |
| 3.30 | 98 | 2 |
| 5.50 | 98 | 2 |
| 7.00 | 98 | 2 |
| Analyte | Precursor Ion (m/z) | Productor Ion (m/z) | Dwell Time (s) | Cone Voltage | Collision Energy |
|---|---|---|---|---|---|
| Tylosin | 916.50 | 174.10 1 | 0.009 | 45.00 | 40.00 |
| 101.10 2 | 0.009 | 45.00 | 45.00 | ||
| Erythromycin-N-methyl-13C-D3 | 738.40 | 162.10 | 0.009 | 20.00 | 30.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas, M.B.; Soto, I.; Mena, F.; Cortés, P.; Pokrant, E.; Trincado, L.; Maturana, M.; Flores, A.; Maddaleno, A.; Lapierre, L.; et al. Dissemination of Tylosin Residues in the Poultry Environment: Evaluating Litter and Droppings as Sources of Risk. Antibiotics 2025, 14, 477. https://doi.org/10.3390/antibiotics14050477
Vargas MB, Soto I, Mena F, Cortés P, Pokrant E, Trincado L, Maturana M, Flores A, Maddaleno A, Lapierre L, et al. Dissemination of Tylosin Residues in the Poultry Environment: Evaluating Litter and Droppings as Sources of Risk. Antibiotics. 2025; 14(5):477. https://doi.org/10.3390/antibiotics14050477
Chicago/Turabian StyleVargas, María Belén, Ignacia Soto, Francisco Mena, Paula Cortés, Ekaterina Pokrant, Lina Trincado, Matías Maturana, Andrés Flores, Aldo Maddaleno, Lisette Lapierre, and et al. 2025. "Dissemination of Tylosin Residues in the Poultry Environment: Evaluating Litter and Droppings as Sources of Risk" Antibiotics 14, no. 5: 477. https://doi.org/10.3390/antibiotics14050477
APA StyleVargas, M. B., Soto, I., Mena, F., Cortés, P., Pokrant, E., Trincado, L., Maturana, M., Flores, A., Maddaleno, A., Lapierre, L., & Cornejo, J. (2025). Dissemination of Tylosin Residues in the Poultry Environment: Evaluating Litter and Droppings as Sources of Risk. Antibiotics, 14(5), 477. https://doi.org/10.3390/antibiotics14050477







