High Prevalence of Cefiderocol Resistance Among New Delhi Metallo-β-Lactamase Producing Klebsiella pneumoniae High-Risk Clones in Hungary
Abstract
1. Introduction
2. Results
2.1. Cefiderocol Susceptibility of CPKP Strains by Carbapenemase Types
2.1.1. Prevalence of Carbapenemase Genes (by Polymerase Chain Reaction (PCR)) and Cefiderocol Resistance Patterns
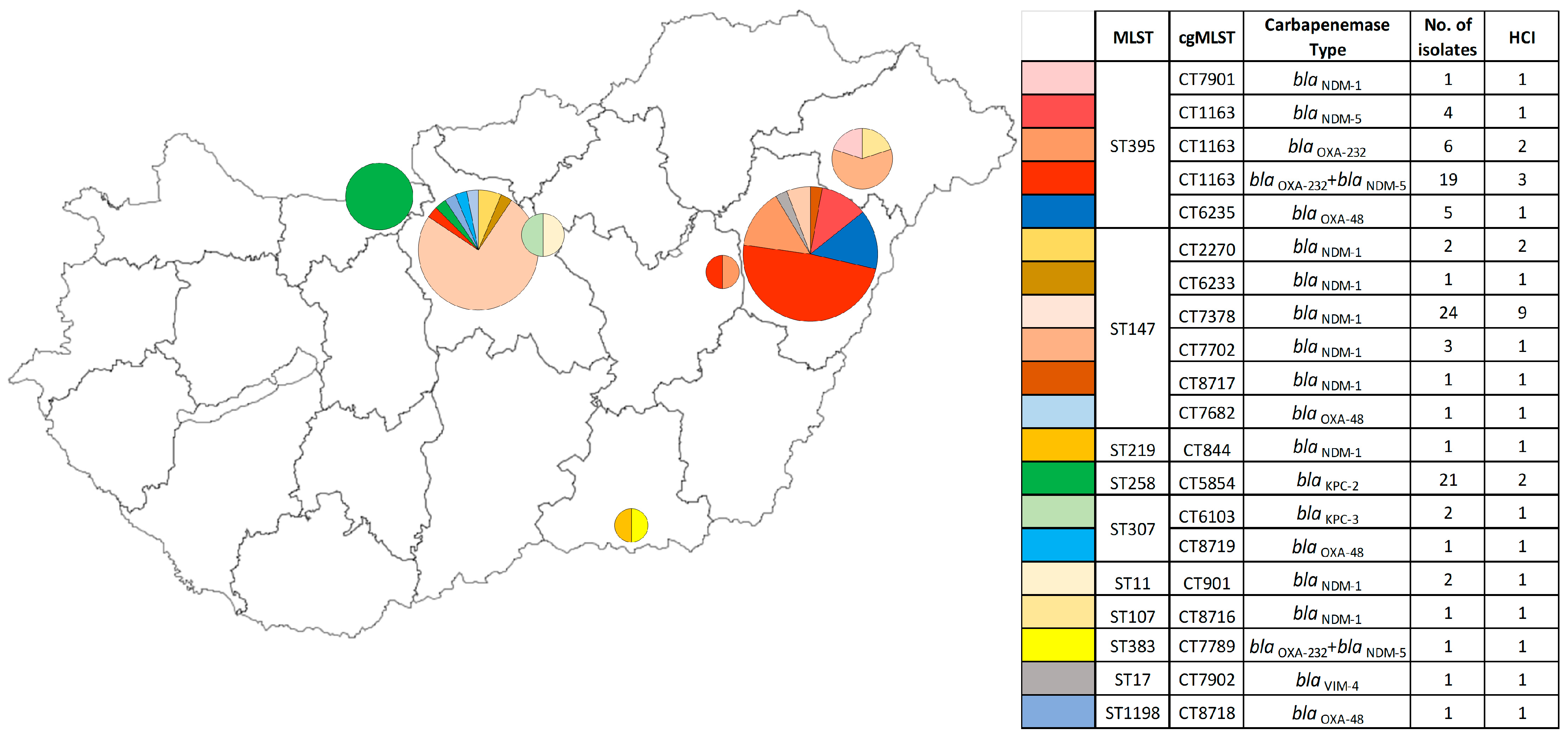
2.1.2. Prevalence of Cefiderocol Resistance Among PFGE Pulsotypes and Their Related Carbapenemase Genes
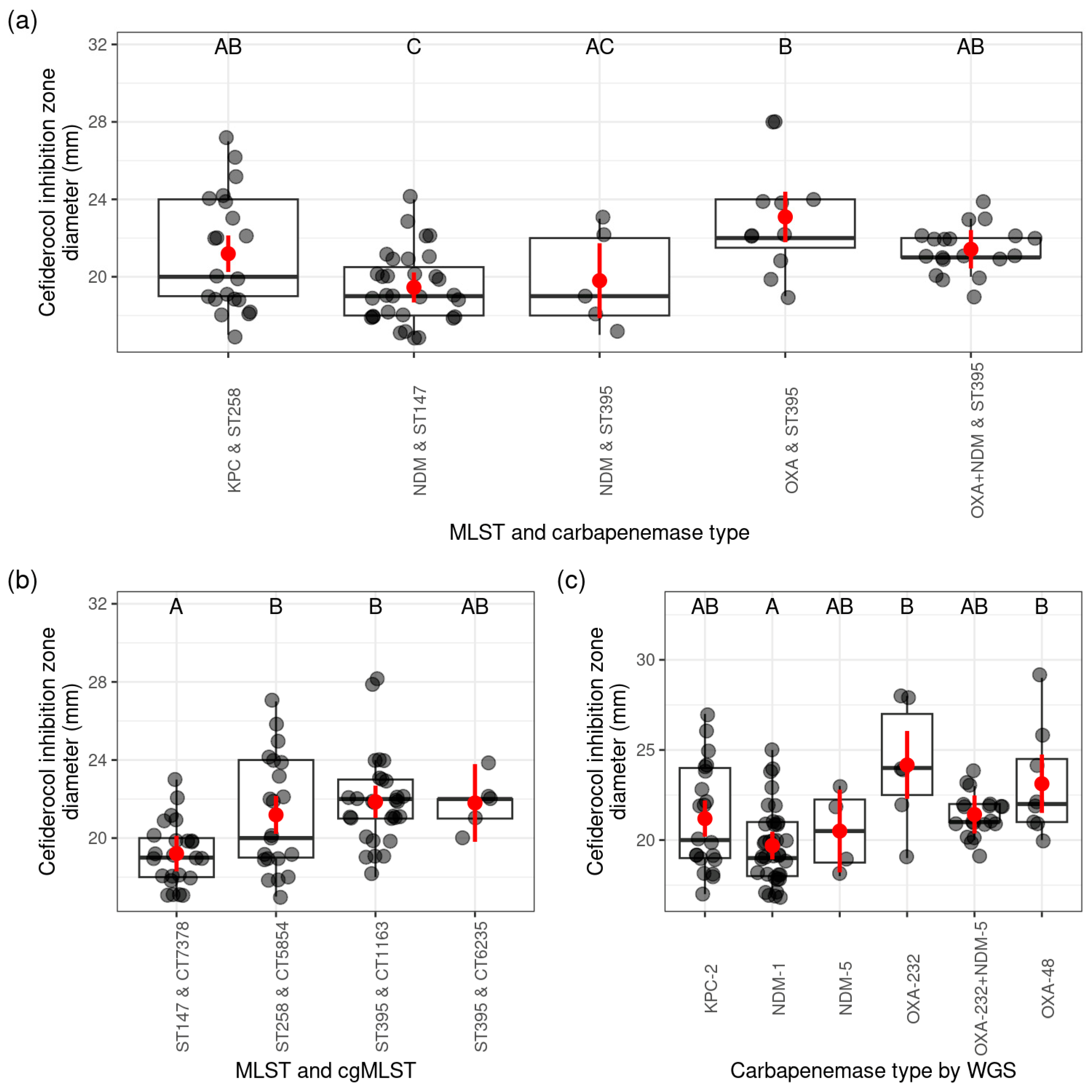
2.1.3. Occurrence of Cefiderocol Resistance Between ST (Sequence Type) Based on PFGE Pulsotypes
2.1.4. Overview of the Frequency of Cefiderocol Resistance Based on the cgMLST (Core-Genome MLST) Results
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates
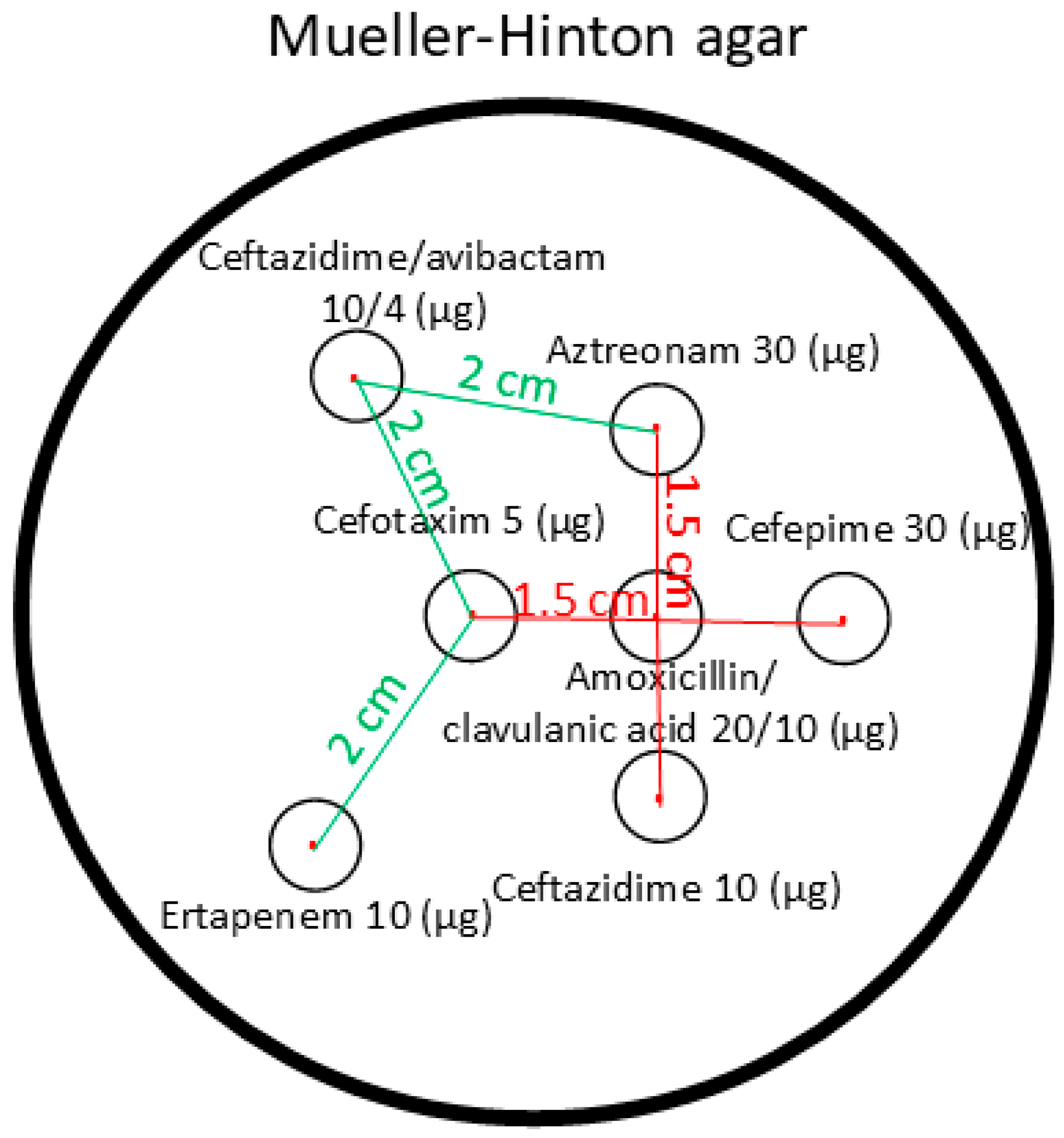
4.2. Bacterial Identification and In Vitro Susceptibility Testing for Cefiderocol
4.3. PCR Analysis for Carbapenemase Genes
4.4. Genotyping with PFGE
4.5. Genome-Based Typing
4.6. Statistical Analysis of the Cefiderocol Inhibition Zone Diameters and Carbapenemase and Clone Types of the CPKP Strains
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esteban-Cantos, A.; Aracil, B.; Bautista, V.; Ortega, A.; Lara, N.; Saez, D.; Fernández-Romero, S.; Pérez-Vázquez, M.; Navarro, F.; Grundmann, H.; et al. The Carbapenemase-Producing Klebsiella Pneumoniae Population Is Distinct and More Clonal than the Carbapenem-Susceptible Population. Antimicrob. Agents Chemother. 2017, 61, e02520-16. [Google Scholar] [CrossRef] [PubMed]
- Arcari, G.; Carattoli, A. Global Spread and Evolutionary Convergence of Multidrug-Resistant and Hypervirulent Klebsiella Pneumoniae High-Risk Clones. Pathog. Glob. Health 2023, 117, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022. Available online: https://iris.who.int/bitstream/handle/10665/364996/9789240062702-eng.pdf?sequence=1 (accessed on 28 October 2024).
- Peirano, G.; Chen, L.; Kreiswirth, B.N.; Pitout, J.D.D. Emerging Antimicrobial-Resistant High-Risk Klebsiella Pneumoniae Clones ST307 and ST147. Antimicrob. Agents Chemother. 2020, 64, e01148-20. [Google Scholar] [CrossRef]
- Mathers, A.J.; Peirano, G.; Pitout, J.D.D. The Role of Epidemic Resistance Plasmids and International High- Risk Clones in the Spread of Multidrug-Resistant Enterobacteriaceae. Clin. Microbiol. Rev. 2015, 28, 565–591. [Google Scholar] [CrossRef]
- Karaiskos, I.; Galani, I.; Papoutsaki, V.; Galani, L.; Giamarellou, H. Carbapenemase Producing Klebsiella Pneumoniae: Implication on Future Therapeutic Strategies. Expert Rev. Anti Infect. Ther. 2022, 20, 53–69. [Google Scholar] [CrossRef]
- Wyres, K.L.; Lam, M.M.C.; Holt, K.E. Population Genomics of Klebsiella Pneumoniae. Nat. Rev. Microbiol. 2020, 18, 344–359. [Google Scholar] [CrossRef]
- Surveillance Atlas of Infectious Diseases. Available online: https://atlas.ecdc.europa.eu/public/index.aspx (accessed on 29 July 2024).
- Karampatakis, T.; Tsergouli, K.; Behzadi, P. Carbapenem-Resistant Klebsiella Pneumoniae: Virulence Factors, Molecular Epidemiology and Latest Updates in Treatment Options. Antibiotics 2023, 12, 234. [Google Scholar] [CrossRef]
- WHO Updates List of Drug-Resistant Bacteria Most Threatening to Human Health. Available online: https://www.who.int/news/item/17-05-2024-who-updates-list-of-drug-resistant-bacteria-most-threatening-to-human-health (accessed on 29 July 2024).
- ECDC. Carbapenem-Resistant Enterobacterales—Third Update, 3 February 2025; ECDC: Solna, Sweden, 2025. [Google Scholar] [CrossRef]
- Bassetti, M.; Magnè, F.; Giacobbe, D.R.; Bini, L.; Vena, A. New Antibiotics for Gram-Negative Pneumonia. Eur. Respir. Rev. 2022, 31, 220119. [Google Scholar] [CrossRef]
- Jean, S.-S.; Hsueh, S.-C.; Lee, W.-S.; Hsueh, P.-R. Expert Review of Anti-Infective Therapy Cefiderocol: A Promising Antibiotic against Multidrug-Resistant Gram-Negative Bacteria Cefiderocol: A Promising Antibiotic against Multidrug-Resistant Gram-Negative Bacteria. Expert Rev. Anti-Infect. Ther. 2019, 17, 307–309. [Google Scholar] [CrossRef]
- Kanj, S.S.; Bassetti, M.; Kiratisin, P.; Rodrigues, C.; Villegas, M.V.; Yu, Y.; van Duin, D. Clinical Data from Studies Involving Novel Antibiotics to Treat Multidrug-Resistant Gram-Negative Bacterial Infections. Int. J. Antimicrob. Agents 2022, 60, 106633. [Google Scholar] [CrossRef]
- Losito, A.R.; Raffaelli, F.; Del Giacomo, P.; Tumbarello, M. New Drugs for the Treatment of Pseudomonas Aeruginosa Infections with Limited Treatment Options: A Narrative Review. Antibiotics 2022, 11, 579. [Google Scholar] [CrossRef] [PubMed]
- Simner, P.J.; Patel, R. Cefiderocol Antimicrobial Susceptibility Testing Considerations: The Achilles’ Heel of the Trojan Horse? J. Clin. Microbiol. 2021, 59, e00951-20. [Google Scholar] [CrossRef] [PubMed]
- Tilahun, M.; Kassa, Y.; Gedefie, A.; Ashagire, M. Emerging Carbapenem-Resistant Enterobacteriaceae Infection, Its Epidemiology and Novel Treatment Options: A Review. Infect. Drug Resist. 2021, 14, 4363–4374. [Google Scholar] [CrossRef]
- Highlights of Prescribing Information. Available online: https://www.shionogi.com/content/dam/shionogi/si/products/pdf/fetroja.pdf (accessed on 28 October 2024).
- European Medicines Agency (EMA). Fetcroja: EPAR–Product Information. Available online: https://www.ema.europa.eu/en/documents/product-information/fetcroja-epar-product-information_en.pdf (accessed on 2 May 2025).
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 14.0, 2024. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Consultation/2023/v_14.0_Breakpoint_Tables_preliminary_231205.xlsx (accessed on 1 January 2024).
- Eucast: Warnings! Available online: https://www.eucast.org/ast-of-bacteria/warnings (accessed on 29 July 2024).
- Tóth, Á.; Buzgó, L.; Lesinszki, V.; Tóth, K.; Ungvári, E.; Kiss, Z.; Göbhardter, D.; Hajdu, Á.; Székelyi-Szabó, E.; Kollár, R.; et al. Emergence of NDM-5 and OXA-232 Co-Producing Klebsiella Pneumoniae ST395 in a Tertiary-Level Hospital in Hungary. ECCMID 2023 Accepted Abstract (Poster Number: P0296). Available online: https://elibrary.escmid.org/?search%5Bquery%5D=%22P0296%22&tab=docs (accessed on 26 March 2023).
- Guducuoglu, H.; Gursoy, N.C.; Yakupogullari, Y.; Parlak, M.; Karasin, G.; Sunnetcioglu, M.; Otlu, B. Hospital Outbreak of a Colistin-Resistant, NDM-1- A Nd OXA-48-Producing Klebsiella Pneumoniae: High Mortality from Pandrug Resistance. Microb. Drug Resist. 2018, 24, 966–972. [Google Scholar] [CrossRef]
- Remya, P.; Shanthi, M.; Sekar, U. Prevalence and Clonal Relatedness of NDM and OXA-48-Producing Klebsiella Pneumoniae in a Tertiary Care Hospital in South India. J. Lab. Physicians 2019, 11, 312–316. [Google Scholar] [CrossRef]
- Damjanova, I.; Tóth, Á.; Pászti, J.; Hajbel-Vékony, G.; Jakab, M.; Berta, J.; Milch, H.; Füzi, M. Expansion and Countrywide Dissemination of ST11, ST15 and ST147 Ciprofloxacin-Resistant CTX-M-15-Type β-Lactamase-Producing Klebsiella Pneumoniae Epidemic Clones in Hungary in 2005—The New “MRSAs”? J. Antimicrob. Chemother. 2008, 62, 978–985. [Google Scholar] [CrossRef]
- Kovács, K.; Nyul, A.; Mestyán, G.; Melegh, S.; Fenyvesi, H.; Jakab, G.; Szabó, H.; Jánvári, L.; Damjanova, I.; Tóth, Á. Emergence and Interhospital Spread of OXA-48-Producing Klebsiella Pneumoniae ST395 Clone in Western Hungary. Infect. Dis. 2017, 49, 231–233. [Google Scholar] [CrossRef]
- Tóth, Á.; Damjanova, I.; Puskás, E.; Jánvári, L.; Farkas, M.; Dobák, A.; Böröcz, K.; Pászti, J. Emergence of a Colistin-Resistant KPC-2-Producing Klebsiella Pneumoniae ST258 Clone in Hungary. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 765–769. [Google Scholar] [CrossRef]
- David, S.; Cohen, V.; Reuter, S.; Sheppard, A.E.; Giani, T.; Parkhill, J.; Maria Rossolini, G.; Feil, E.J.; Grundmann, H.; Aanensen, D.M. Integrated Chromosomal and Plasmid Sequence Analyses Reveal Diverse Modes of Carbapenemase Gene Spread among Klebsiella pneumoniae. Proc. Natl. Acad. Sci. USA 2020, 117, 25043–25054. [Google Scholar] [CrossRef]
- Sandfort, M.; Hans, J.B.; Fischer, M.A.; Reichert, F.; Cremanns, M.; Eisfeld, J.; Pfeifer, Y.; Heck, A.; Eckmanns, T.; Werner, G.; et al. Increase in NDM-1 and NDM-1/OXA-48-Producing Klebsiella Pneumoniae in Germany Associated with the War in Ukraine, 2022. Eurosurveillance 2022, 27, 2200926. [Google Scholar] [CrossRef]
- Aqel, A.A.; Giakkoupi, P.; Alzoubi, H.; Masalha, I.; Ellington, M.J.; Vatopoulos, A. Detection of OXA-48-like and NDM Carbapenemases Producing Klebsiella Pneumoniae in Jordan: A Pilot Study. J. Infect. Public Health 2017, 10, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Barguigua, A.; Zerouali, K.; Katfy, K.; El Otmani, F.; Timinouni, M.; Elmdaghri, N. Occurrence of OXA-48 and NDM-1 Carbapenemase-Producing Klebsiella Pneumoniae in a Moroccan University Hospital in Casablanca, Morocco. Infect. Genet. Evol. 2015, 31, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Shibl, A.; Al-Agamy, M.; Memish, Z.; Senok, A.; Khader, S.A.; Assiri, A. The Emergence of OXA-48- and NDM-1-Positive Klebsiella Pneumoniae in Riyadh, Saudi Arabia. Int. J. Infect. Dis. 2013, 17, e1130–e1133. [Google Scholar] [CrossRef]
- Solgi, H.; Badmasti, F.; Giske, C.G.; Aghamohammad, S.; Shahcheraghi, F. Molecular Epidemiology of NDM-1- and OXA-48-Producing Klebsiella Pneumoniae in an Iranian Hospital: Clonal Dissemination of ST11 and ST893. J. Antimicrob. Chemother. 2018, 73, 1517–1524. [Google Scholar] [CrossRef]
- Kohira, N.; Hackel, M.A.; Ishioka, Y.; Kuroiwa, M.; Sahm, D.F.; Sato, T.; Maki, H.; Yamano, Y. Reduced Susceptibility Mechanism to Cefiderocol, a Siderophore Cephalosporin, among Clinical Isolates from a Global Surveillance Programme (SIDERO-WT-2014). J. Glob. Antimicrob. Resist. 2020, 22, 738–741. [Google Scholar] [CrossRef]
- Nurjadi, D.; Kocer, K.; Chanthalangsy, Q.; Klein, S.; Heeg, K.; Boutin, S. New Delhi Metallo-Beta-Lactamase Facilitates the Emergence of Cefiderocol Resistance in Enterobacter Cloacae. Antimicrob. Agents Chemother. 2022, 66, e02011-21. [Google Scholar] [CrossRef]
- Longshaw, C.; Manissero, D.; Tsuji, M.; Echols, R.; Yamano, Y. In Vitro Activity of the Siderophore Cephalosporin, Cefiderocol, against Molecularly Characterized, Carbapenem-Non-Susceptible Gram-Negative Bacteria from Europe. JAC Antimicrob. Resist. 2020, 2, dlaa060. [Google Scholar] [CrossRef]
- Mushtaq, S.; Meunier, D.; Vickers, A.; Woodford, N.; Livermore, D.M. Activity of Imipenem/Relebactam against Pseudomonas Aeruginosa Producing ESBLs and Carbapenemases. J. Antimicrob. Chemother. 2021, 76, 434–442. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Rousaki, M.; Kritsotakis, E.I. Cefiderocol: Systematic Review of Mechanisms of Resistance, Heteroresistance and In Vivo Emergence of Resistance. Antibiotics 2022, 11, 723. [Google Scholar] [CrossRef]
- Kazmierczak, K.M.; Tsuji, M.; Wise, M.G.; Hackel, M.; Yamano, Y.; Echols, R.; Sahm, D.F. In Vitro Activity of Cefiderocol, a Siderophore Cephalosporin, against a Recent Collection of Clinically Relevant Carbapenem-Non-Susceptible Gram-Negative Bacilli, Including Serine Carbapenemase- and Metallo-β-Lactamase-Producing Isolates (SIDERO-WT-2014 Study). Int. J. Antimicrob. Agents 2019, 53, 177–184. [Google Scholar] [CrossRef]
- Lan, P.; Lu, Y.; Chen, Z.; Wu, X.; Hua, X.; Jiang, Y.; Zhou, J.; Yu, Y. Emergence of High-Level Cefiderocol Resistance in Carbapenem-Resistant Klebsiella Pneumoniae from Bloodstream Infections in Patients with Hematologic Malignancies in China. Microbiol. Spectr. 2022, 10, e00084-22. [Google Scholar] [CrossRef] [PubMed]
- Saladini, F.; Giannini, A.; Giammarino, F.; Boccuto, A.; Dragoni, F.; Vicenti, I.; Zazzi, M. In Vitro Susceptibility of HIV-1 CRF02_AG to Temsavir, the Active Compound of the Attachment Inhibitor Fostemsavir. J. Antimicrob. Chemother. 2021, 76, 3310–3312. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 13.1, 2023. Available online: http://www.eucast.org (accessed on 28 October 2024).
- Martinez-Martinez, L.; Cantón Spain, R.; Stefani, S.; Skov, R.; Glupczynski, Y.; Nordmann, P.; Wootton, M.; Miriagou, V.; Skov Simonsen, G. EUCAST Guidelines for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance. 2017. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_170711.pdf (accessed on 11 July 2017).
- Eucast: Breakpoint Table 14.0 (2024). Available for Consultation (5–19 December, 2023). Available online: https://www.eucast.org/eucast_news/news_singleview?tx_ttnews%5Btt_news%5D=566&cHash=db55f3a8829726044512a1fe74cce41b (accessed on 29 July 2024).
- Ellington, M.J.; Kistler, J.; Livermore, D.M.; Woodford, N. Multiplex PCR for Rapid Detection of Genes Encoding Acquired Metallo-β-Lactamases. J. Antimicrob. Chemother. 2007, 59, 321–322. [Google Scholar] [CrossRef]
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for Detection of Acquired Carbapenemase Genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef]
- Tóth, Á.; Kocsis, B.; Damjanova, I.; Kristóf, K.; Jánvári, L.; Pászti, J.; Csercsik, R.; Topf, J.; Szabó, D.; Hamar, P.; et al. Fitness Cost Associated with Resistance to Fluoroquinolones Is Diverse across Clones of Klebsiella Pneumoniae and May Select for CTX-M-15 Type Extended-Spectrum β-Lactamase. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 837–843. [Google Scholar] [CrossRef]
- Fida, M.; Cunningham, S.A.; Murphy, M.P.; Bonomo, R.A.; Hujer, K.M.; Hujer, A.M.; Kreiswirth, B.N.; Chia, N.; Jeraldo, P.R.; Nelson, H.; et al. Core Genome MLST and Resistome Analysis of Klebsiella Pneumoniae Using a Clinically Amenable Workflow. Diagn. Microbiol. Infect. Dis. 2020, 97, 114996. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, K.; Jin, D.; Shen, W.; Liu, C.; Zhou, H.; Zhang, R. Evaluation of IR Biotyper for Carbapenem-Resistant Pseudomonas Aeruginosa Typing and Its Application Potential for the Investigation of Nosocomial Infection. Front. Microbiol. 2023, 14, 1068872. [Google Scholar] [CrossRef]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for Predictions of Phenotypes from Genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and Applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Zankari, E.; Allesøe, R.; Joensen, K.G.; Cavaco, L.M.; Lund, O.; Aarestrup, F.M. PointFinder: A Novel Web Tool for WGS-Based Detection of Antimicrobial Resistance Associated with Chromosomal Point Mutations in Bacterial Pathogens. J. Antimicrob. Chemother. 2017, 72, 2764–2768. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans Estimated Marginal Means, Aka Least-Squares Means.—References—Scientific Research Publishing. 2021. Available online: https://www.scirp.org/reference/referencespapers?referenceid=3437485 (accessed on 19 April 2025).
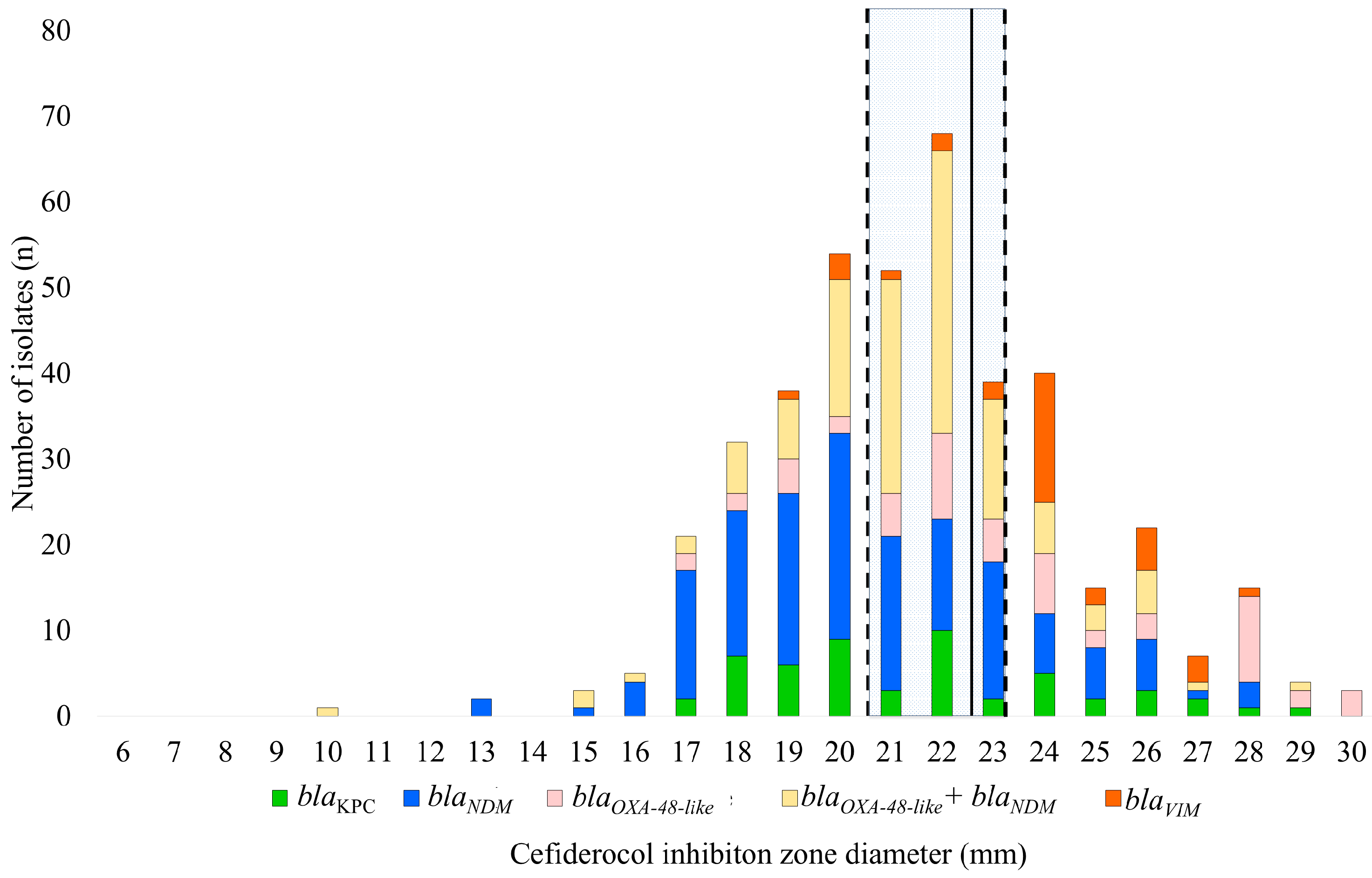
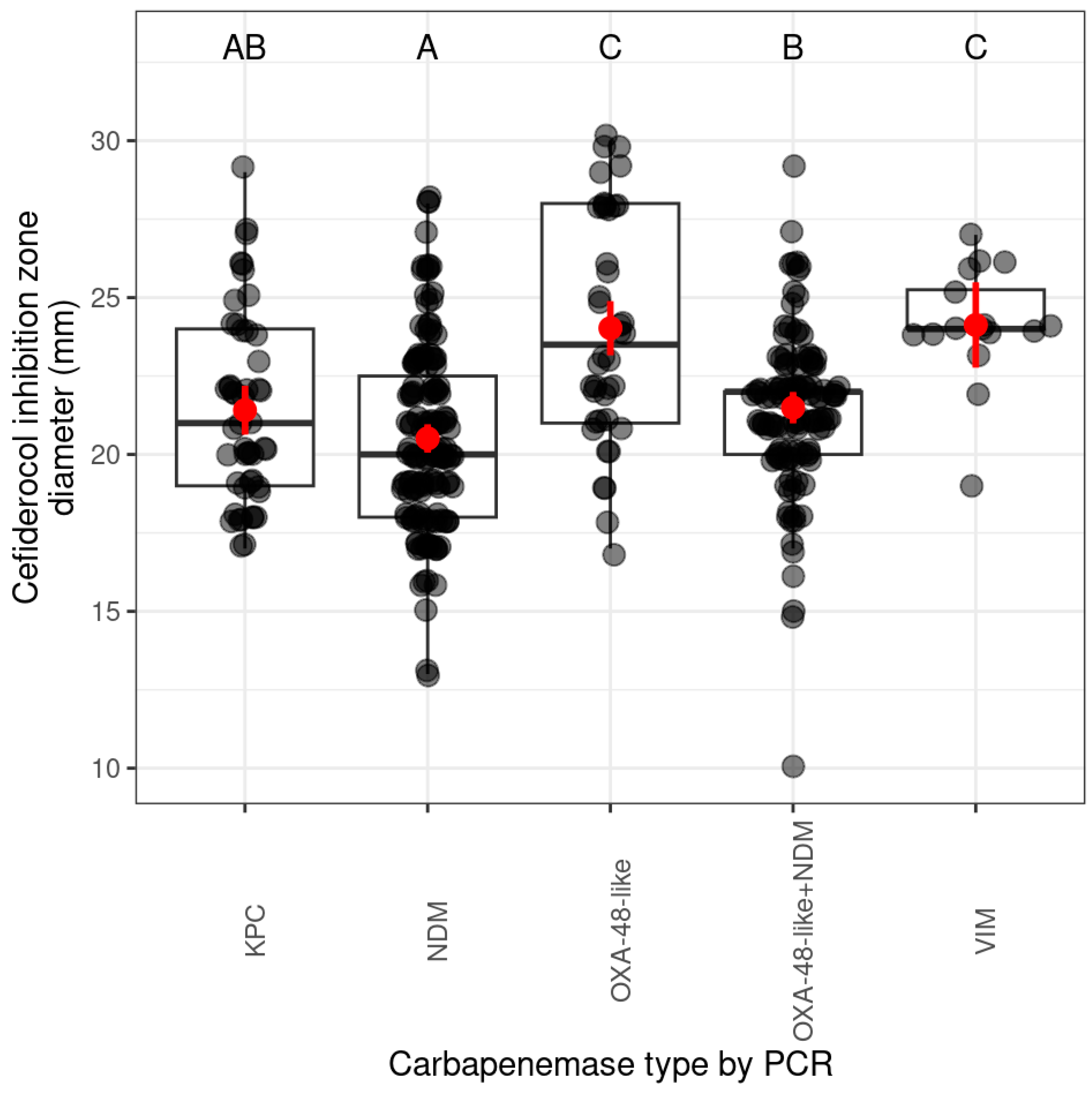
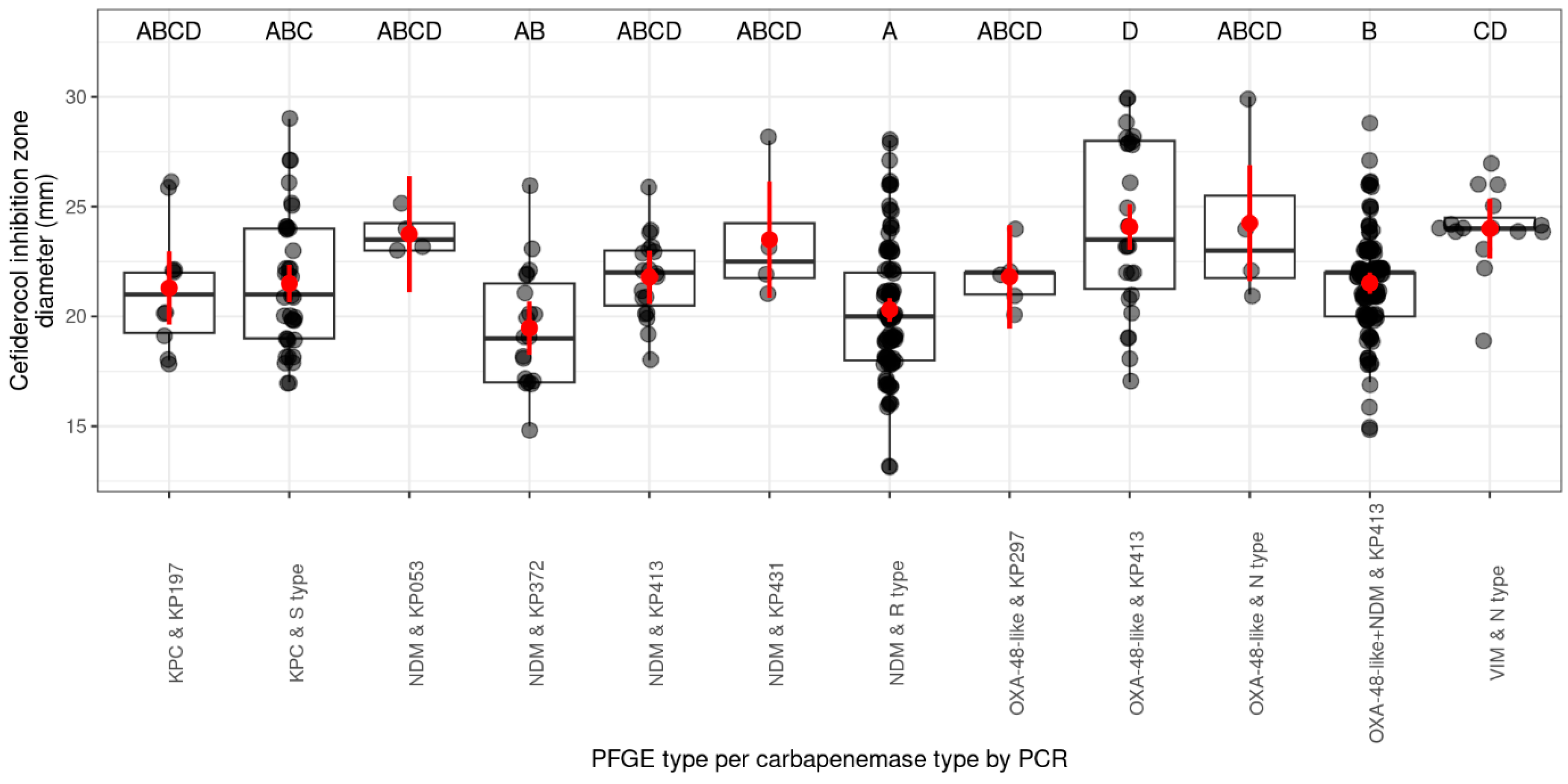
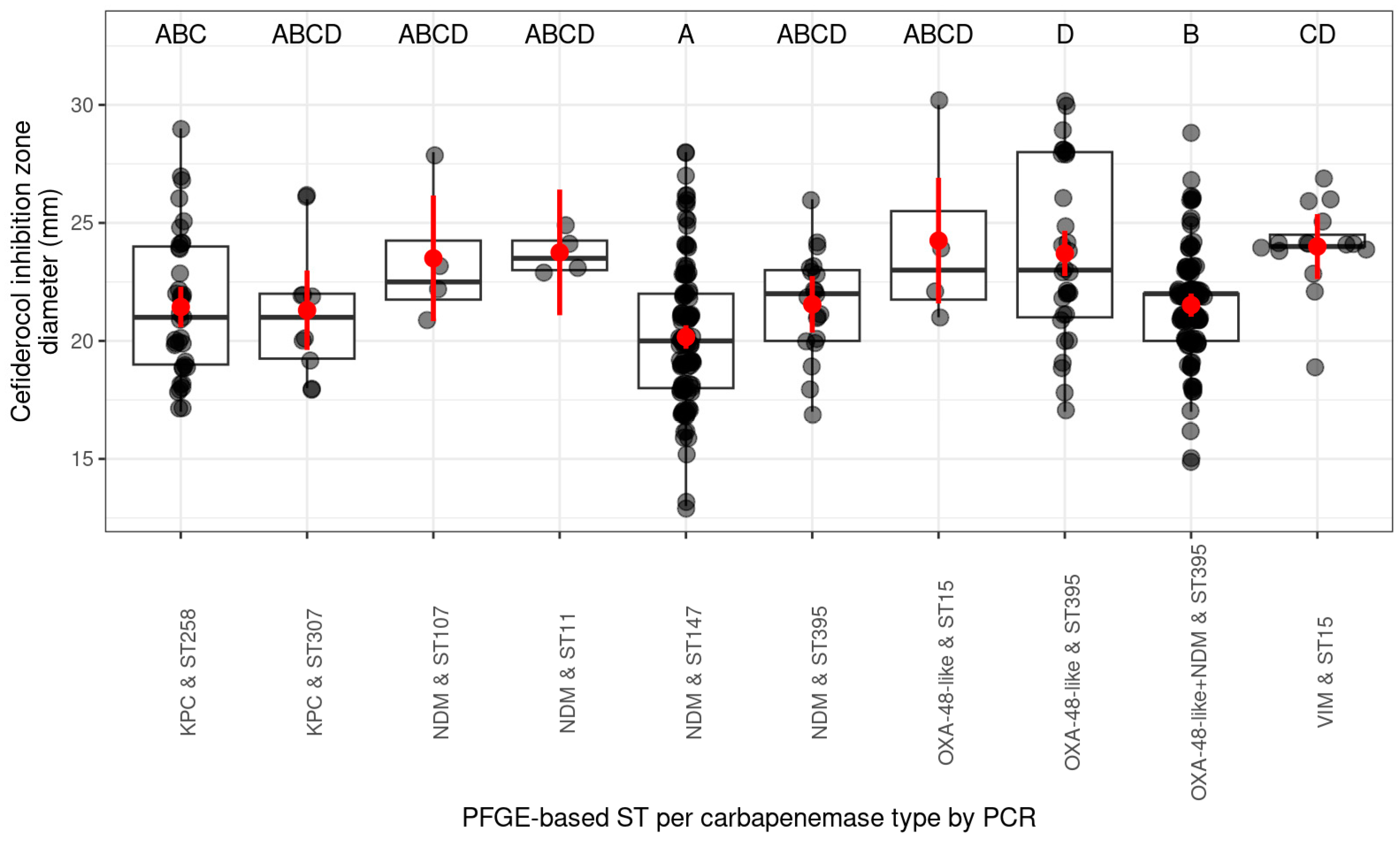
| Carbapenemase Gene | n | S without ATU% | S with ATU% * | R without ATU% | R with ATU% * | ATU% |
|---|---|---|---|---|---|---|
| blaVIM | 35 | 86 | 80 | 13 | 20 | 14 |
| blaOXA-48-like | 57 | 73 | 56 | 27 | 44 | 35 |
| blaKPC | 53 | 63 | 30 | 27 | 70 | 28 |
| blaNDM | 153 | 22 | 30 | 78 | 70 | 31 |
| blaOXA-48-like and blaNDM | 123 | 32 | 25 | 68 | 75 | 59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buzgó, L.; Kiss, Z.; Göbhardter, D.; Lesinszki, V.; Ungvári, E.; Rádai, Z.; Laczkó, L.; Damjanova, I.; Kardos, G.; Tóth, Á. High Prevalence of Cefiderocol Resistance Among New Delhi Metallo-β-Lactamase Producing Klebsiella pneumoniae High-Risk Clones in Hungary. Antibiotics 2025, 14, 475. https://doi.org/10.3390/antibiotics14050475
Buzgó L, Kiss Z, Göbhardter D, Lesinszki V, Ungvári E, Rádai Z, Laczkó L, Damjanova I, Kardos G, Tóth Á. High Prevalence of Cefiderocol Resistance Among New Delhi Metallo-β-Lactamase Producing Klebsiella pneumoniae High-Risk Clones in Hungary. Antibiotics. 2025; 14(5):475. https://doi.org/10.3390/antibiotics14050475
Chicago/Turabian StyleBuzgó, Lilla, Zsanett Kiss, Dániel Göbhardter, Virág Lesinszki, Erika Ungvári, Zoltán Rádai, Levente Laczkó, Ivelina Damjanova, Gábor Kardos, and Ákos Tóth. 2025. "High Prevalence of Cefiderocol Resistance Among New Delhi Metallo-β-Lactamase Producing Klebsiella pneumoniae High-Risk Clones in Hungary" Antibiotics 14, no. 5: 475. https://doi.org/10.3390/antibiotics14050475
APA StyleBuzgó, L., Kiss, Z., Göbhardter, D., Lesinszki, V., Ungvári, E., Rádai, Z., Laczkó, L., Damjanova, I., Kardos, G., & Tóth, Á. (2025). High Prevalence of Cefiderocol Resistance Among New Delhi Metallo-β-Lactamase Producing Klebsiella pneumoniae High-Risk Clones in Hungary. Antibiotics, 14(5), 475. https://doi.org/10.3390/antibiotics14050475






