Rapid Therapeutic Drug Monitoring of Voriconazole Based on High-Performance Liquid Chromatography: A Single-Center Pilot Study in Outpatients
Abstract
1. Introduction
2. Results
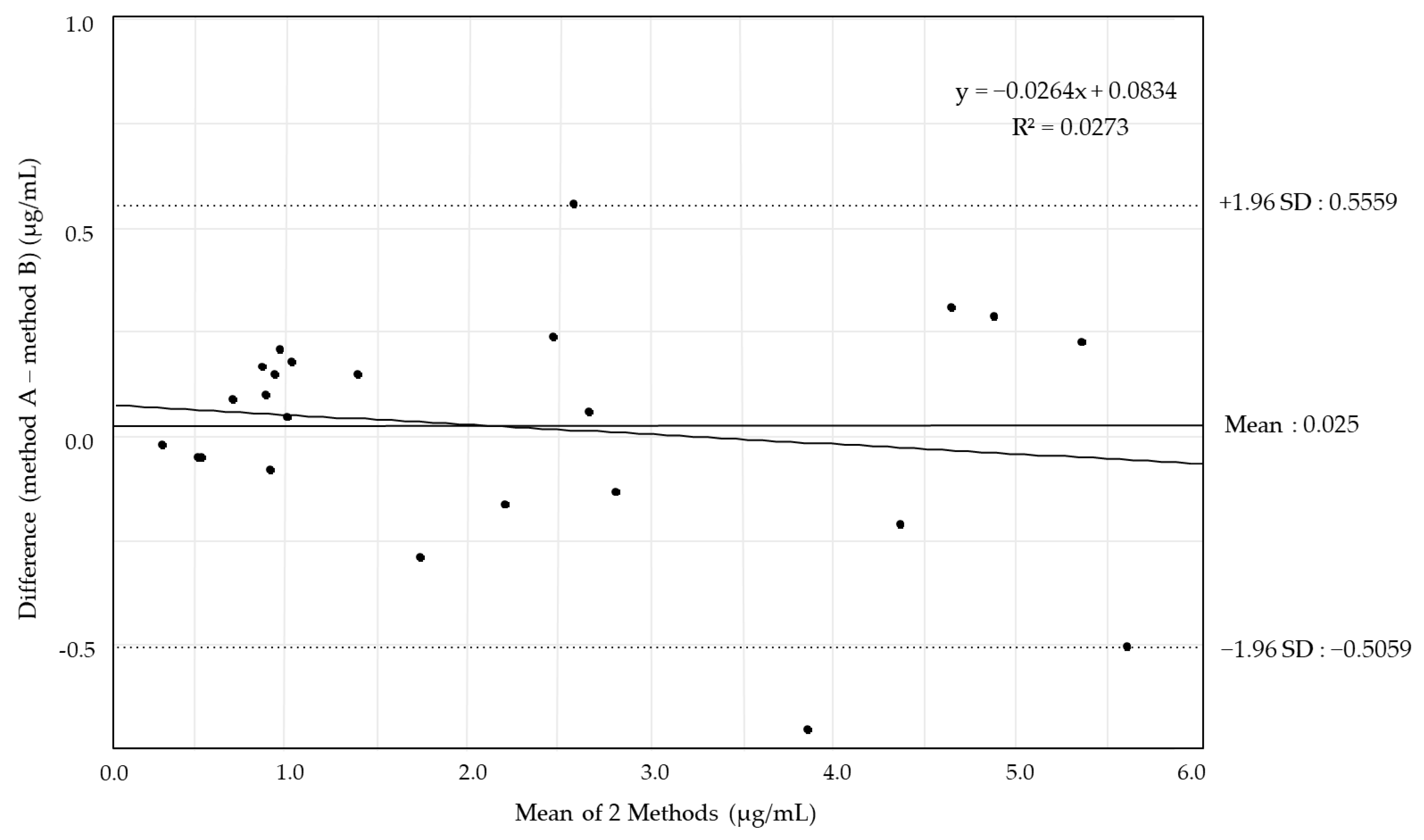
2.1. Analysis of VRCZ Blood Concentration
2.2. Validation of VRCZ Blood Concentration Analysis
2.3. Evaluation of Safety of VRCZ in Outpatients
3. Discussion
4. Materials and Methods
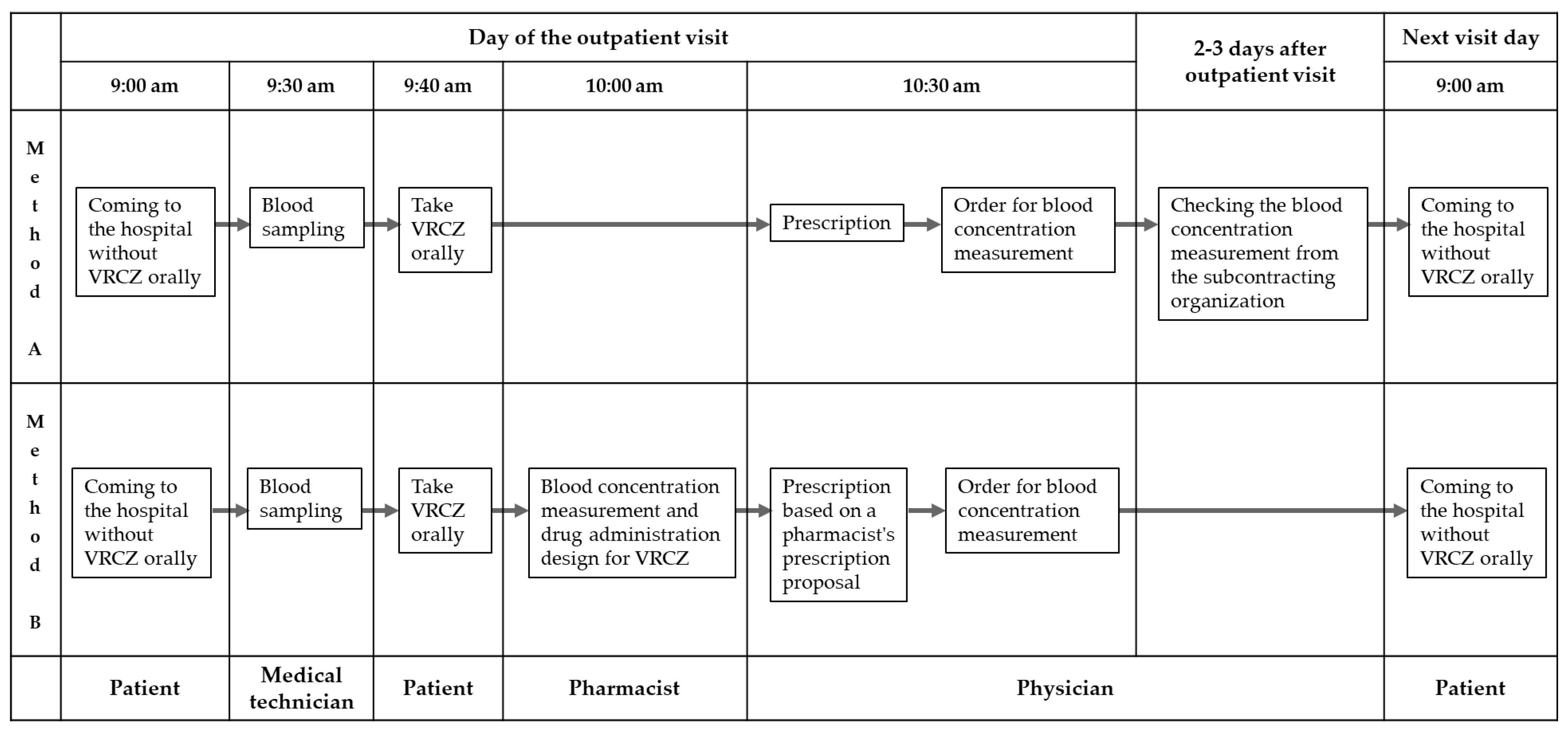
4.1. Analysis of VRCZ Blood Concentration
4.2. Validation of Analysis of VRCZ Blood Concentration
4.3. Evaluation of the Safety of VRCZ in Outpatients
4.4. Statistical Analysis
4.5. Ethical Approval
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VRCZ | Voriconazole |
| HPLC-UV | High-performance liquid chromatography with ultraviolet detection |
| LC-MS/MS | Liquid chromatography–tandem mass spectrometry |
| TDM | Therapeutic drug monitoring |
References
- Patterson, T.F.; Thompson, G.R., 3rd; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef]
- Denning, D.W.; Cadranel, J.; Beigelman-Aubry, C.; Ader, F.; Chakrabarti, A.; Blot, S.; Ullmann, A.J.; Dimopoulos, G.; Lange, C.; European Society for Clinical Microbiology and Infectious Diseases and European Respiratory Society. Chronic pulmonary aspergillosis: Rationale and clinical guidelines for diagnosis and management. Eur. Respir. J. 2016, 47, 45–68. [Google Scholar] [CrossRef]
- Bui, V.; Walker, S.A.; Elligsen, M.; Vyas, A.; Kiss, A.; Palmay, L. Voriconazole prophylaxis in leukemic patients: A retrospective single-center study. J. Oncol. Pharm. Pract. 2020, 26, 873–881. [Google Scholar] [CrossRef]
- Smith, J.; Safdar, N.; Knasinski, V.; Simmons, W.; Bhavnani, S.M.; Ambrose, P.G.; Andes, D. Voriconazole therapeutic drug monitoring. Antimicrob. Agents Chemother. 2006, 50, 1570–1572. [Google Scholar] [CrossRef]
- Tan, K.; Brayshaw, N.; Tomaszewski, K.; Troke, P.; Wood, N. Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse events or liver function test abnormalities. J. Clin. Pharmacol. 2006, 46, 235–243. [Google Scholar] [CrossRef]
- Imhof, A.; Schaer, D.J.; Schanz, U.; Schwarz, U. Neurological adverse events to voriconazole: Evidence for therapeutic drug monitoring. Swiss Med. Wkly. 2006, 136, 739–742. [Google Scholar] [CrossRef]
- Howard, S.J.; Pasqualotto, A.C.; Denning, D.W. Azole resistance in allergic bronchopulmonary aspergillosis and Aspergillus bronchitis. Clin. Microbiol. Infect. 2010, 16, 683–688. [Google Scholar] [CrossRef]
- Bellete, B.; Raberin, H.; Morel, J.; Flori, P.; Hafid, J.; Manhsung, R.T. Acquired resistance to voriconazole and itraconazole in a patient with pulmonary aspergilloma. Med. Mycol. 2010, 48, 197–200. [Google Scholar] [CrossRef]
- Purkins, L.; Wood, N.; Ghahramani, P.; Greenhalgh, K.; Allen, M.J.; Kleinermans, D. Pharmacokinetics and safety of voriconazole following intravenous- to oral-dose escalation regimens. Antimicrob. Agents Chemother. 2002, 46, 2546–2553. [Google Scholar] [CrossRef]
- Purkins, L.; Wood, N.; Kleinermans, D.; Greenhalgh, K.; Nichols, D. Effect of food on the pharmacokinetics of multiple-dose oral voriconazole. Br. J. Clin. Pharmacol. 2003, 56 (Suppl. S1), 17–23. [Google Scholar] [CrossRef]
- Takesue, Y.; Hanai, Y.; Oda, K.; Hamada, Y.; Ueda, T.; Mayumi, T.; Matsumoto, K.; Fujii, S.; Takahashi, Y.; Miyazaki, Y.; et al. Clinical practice guideline for the therapeutic drug monitoring of voriconazole in non-Asian and Asian adult patients: Consensus review by the Japanese society of chemotherapy and the Japanese society of therapeutic drug monitoring. Clin. Ther. 2022, 44, 1604–1623. [Google Scholar] [CrossRef]
- Kably, B.; Launay, M.; Derobertmasure, A.; Lefeuvre, S.; Dannaoui, E.; Billaud, E.M. Antifungal Drugs TDM: Trends and Update. Ther. Drug Monit. 2022, 44, 166–197. [Google Scholar] [CrossRef]
- Hamada, Y.; Ueda, T.; Miyazaki, Y.; Nakajima, K.; Fukunaga, K.; Miyazaki, T.; Nakada-Motokawa, N.; Nagao, M.; Kawamura, H.; Shigemi, A.; et al. Effects of antifungal stewardship using therapeutic drug monitoring in voriconazole therapy on the prevention and control of hepatotoxicity and visual symptoms: A multicentre study conducted in Japan. Mycoses 2020, 63, 779–786. [Google Scholar] [CrossRef]
- Jones, M.; Micallef, C.; Tyler, N.; Wong, V.K.; Enoch, D.A. The impact of an antifungal stewardship team on voriconazole therapeutic drug monitoring in a UK tertiary hospital. J. Infect. 2021, 83, e9–e11. [Google Scholar] [CrossRef]
- Yasu, T.; Nomura, Y.; Gando, Y.; Matsumoto, Y.; Sugita, T.; Kosugi, N.; Kobayashi, M. High-performance liquid chromatography for ultra-simple determination of plasma voriconazole concentration. J. Fungi 2022, 8, 1035. [Google Scholar] [CrossRef]
- Kato, H.; Umemura, T.; Hagihara, M.; Shiota, A.; Asai, N.; Hamada, Y.; Mikamo, H.; Iwamoto, T. Development of a therapeutic drug-monitoring algorithm for outpatients receiving voriconazole: A multicentre retrospective study. Br. J. Clin. Pharmacol. 2024, 90, 1222–1230. [Google Scholar] [CrossRef]
- Ebihara, F.; Hamada, Y.; Kato, H.; Maruyama, T.; Kimura, T. Importance and reality of TDM for antibiotics not covered by insurance in Japan. Int. J. Environ. Res. Public Health 2022, 19, 2516. [Google Scholar] [CrossRef]
- Muraki, Y.; Koizumi, R.; Kusama, Y.; Inose, R.; Ishikane, M.; Ohmagari, N. Necessity for a system implementing therapeutic drug monitoring in outpatient settings based on the actual use of voriconazole using the national database of health insurance claims and specific health checkups of Japan: A descriptive epidemiological study. Biol. Pharm. Bull. 2023, 46, 1490–1493. [Google Scholar] [CrossRef]
- Blanco-Dorado, S.; Maroñas, O.; Latorre-Pellicer, A.; Rodríguez Jato, M.T.; López-Vizcaíno, A.; Gómez Márquez, A.; Bardán García, B.; Belles Medall, D.; Barbeito Castiñeiras, G.; Pérez Del Molino Bernal, M.L.; et al. Impact of CYP2C19 Genotype and Drug Interactions on Voriconazole Plasma Concentrations: A Spain Pharmacogenetic-Pharmacokinetic Prospective Multicenter Study. Pharmacotherapy 2020, 40, 17–25. [Google Scholar] [CrossRef]
- Imamura, C.K.; Furihata, K.; Okamoto, S.; Tanigawara, Y. Impact of cytochrome P450 2C19 polymorphisms on the pharmacokinetics of tacrolimus when coadministered with voriconazole. J. Clin. Pharmacol. 2016, 56, 408–413. [Google Scholar] [CrossRef]
- Aiuchi, N.; Nakagawa, J.; Sakuraba, H.; Takahata, T.; Kamata, K.; Saito, N.; Ueno, K.; Ishiyama, M.; Yamagata, K.; Kayaba, H.; et al. Impact of polymorphisms of pharmacokinetics-related genes and the inflammatory response on the metabolism of voriconazole. Pharmacol. Res. Perspect. 2022, 10, e00935. [Google Scholar] [CrossRef] [PubMed]
- Chaudhri, K.; Stocker, S.L.; Williams, K.M.; McLeay, R.C.; Marriott, D.J.E.; Di Tanna, G.L.D.; Day, R.O.; Carland, J.E. Voriconazole: An audit of hospital-based dosing and monitoring and evaluation of the predictive performance of a dose-prediction software package. J. Antimicrob. Chemother. 2020, 75, 1981–1984. [Google Scholar] [CrossRef]
- Umemura, T.; Kakizaki, H.; Mutoh, Y.; Mizuno, T.; Ito, Y.; Hioki, T.; Kato, H.; Hagihara, M.; Yamada, T.; Ikeda, Y.; et al. Effectiveness and safety of the simulation-based first-dose design of voriconazole. J. Infect. Chemother. 2025, 31, 102453. [Google Scholar] [CrossRef] [PubMed]
- Farowski, F.; Cornely, O.A.; Vehreschild, J.J.; Hartmann, P.; Bauer, T.; Steinbach, A.; Rüping, M.J.G.T.; Müller, C. Quantitation of azoles and echinocandins in compartments of peripheral blood by liquid chromatography-tandem mass spectrometry. Antimicrob. Agents Chemother. 2010, 54, 1815–1819. [Google Scholar] [CrossRef]
- Mak, J.; Sujishi, K.K.; French, D. Development and validation of a liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay to quantify serum voriconazole. J. Chromatogr. B 2015, 986–987, 94–99. [Google Scholar] [CrossRef]
- Keevil, B.G.; Newman, S.; Lockhart, S.; Howard, S.J.; Moore, C.B.; Denning, D.W. Validation of an assay for voriconazole in serum samples using liquid chromatography-tandem mass spectrometry. Ther. Drug Monit. 2004, 26, 650–657. [Google Scholar] [CrossRef]
- Ashbee, H.R.; Barnes, R.A.; Johnson, E.M.; Richardson, M.D.; Gorton, R.; Hope, W.W. Therapeutic drug monitoring (TDM) of antifungal agents: Guidelines from the British Society for Medical Mycology. J. Antimicrob. Chemother. 2014, 69, 1162–1176. [Google Scholar] [CrossRef]
- Perea, S.; Pennick, G.J.; Modak, A.; Fothergill, A.W.; Sutton, D.A.; Sheehan, D.J.; Rinaldi, M.G. Comparison of high-performance liquid chromatographic and microbiological methods for determination of voriconazole levels in plasma. Antimicrob. Agents Chemother. 2000, 44, 1209–1213. [Google Scholar] [CrossRef]
- Jeon, Y.; Han, M.; Han, E.Y.; Lee, K.; Song, J.; Song, S.H. Performance evaluation of enzyme immunoassay for voriconazole therapeutic drug monitoring with automated clinical chemistry analyzers. Pract. Lab. Med. 2017, 8, 86–94. [Google Scholar] [CrossRef]
- Péhourcq, F.; Jarry, C.; Bannwarth, B. Direct injection HPLC micro method for the determination of voriconazole in plasma using an internal surface reversed-phase column. Biomed. Chromatogr. 2004, 18, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Suzuki, R.; Yamazaki, R.; Kusuhara, Y.; Mitsumoto, S.; Kobayashi, H.; Shimoeda, S.; Ohta, S.; Yamato, S. Determination of the antifungal agent voriconazole in human plasma using a simple column-switching high-performance liquid chromatography and its application to a pharmacokinetic study. Chem. Pharm. Bull. 2008, 56, 328–331. [Google Scholar] [CrossRef]
- Gordien, J.B.; Pigneux, A.; Vigouroux, S.; Tabrizi, R.; Accoceberry, I.; Bernadou, J.M.; Rouault, A.; Saux, M.C.; Breilh, D. Simultaneous determination of five systemic azoles in plasma by high-performance liquid chromatography with ultraviolet detection. J. Pharm. Biomed. Anal. 2009, 50, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Doby, E.H.; Benjamin, D.K., Jr.; Blaschke, A.J.; Ward, R.M.; Pavia, A.T.; Martin, P.L.; Driscoll, T.A.; Cohen-Wolkowiez, M.; Moran, C. Therapeutic monitoring of voriconazole in children less than three years of age: A case report and summary of voriconazole concentrations for ten children. Pediatr. Infect. Dis. J. 2012, 31, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Pascual, A.; Nieth, V.; Calandra, T.; Bille, J.; Bolay, S.; Decosterd, L.A.; Buclin, T.; Majcherczyk, P.A.; Sanglard, D.; Marchetti, O. Variability of voriconazole plasma levels measured by new high-performance liquid chromatography and bioassay methods. Antimicrob. Agents Chemother. 2007, 51, 137–143. [Google Scholar] [CrossRef]
- Yousefian, S.; Dastan, F.; Marjani, M.; Tabarsi, P.; Barati, S.; Shahsavari, N.; Kobarfard, F. Determination of voriconazole plasma concentration by HPLC technique and evaluating its association with clinical outcome and adverse effects in patients with invasive aspergillosis. Can. J. Infect. Dis. Med Microbiol. 2021, 2021, 5497427. [Google Scholar] [CrossRef] [PubMed]
- Brüggemann, R.J.M.; Alffenaar, J.W.C.; Blijlevens, N.M.A.; Billaud, E.M.; Kosterink, J.G.W.; Verweij, P.E.; Burger, D.M. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin. Infect. Dis. 2009, 48, 1441–1458. [Google Scholar] [CrossRef]
- Chau, M.M.; Kong, D.C.M.; van Hal, S.J.; Urbancic, K.; Trubiano, J.A.; Cassumbhoy, M.; Wilkes, J.; Cooper, C.M.; Roberts, J.A.; Marriott, D.J.; et al. Consensus guidelines for optimising antifungal drug delivery and monitoring to avoid toxicity and improve outcomes in patients with haematological malignancy, 2014. Intern. Med. J. 2014, 44, 1364–1388. [Google Scholar] [CrossRef]
- Mellinghoff, S.C.; Panse, J.; Alakel, N.; Behre, G.; Buchheidt, D.; Christopeit, M.; Hasenkamp, J.; Kiehl, M.; Koldehoff, M.; Krause, S.W.; et al. Primary prophylaxis of invasive fungal infections in patients with haematological malignancies: 2017 update of the recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society for Haematology and Medical Oncology (DGHO). Ann. Hematol. 2018, 97, 197–207. [Google Scholar] [CrossRef]
- Denning, D.W.; Ribaud, P.; Milpied, N.; Caillot, D.; Herbrecht, R.; Thiel, E.; Haas, A.; Ruhnke, M.; Lode, H. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin. Infect. Dis. 2002, 34, 563–571. [Google Scholar] [CrossRef]
- Koyama, K.; Ohshima, N.; Suzuki, J.; Kawashima, M.; Takeda, K.; Ando, T.; Sato, R.; Nagai, H.; Matsui, H.; Ohta, K. Recurrence of chronic pulmonary aspergillosis after discontinuation of maintenance treatment by antifungal triazoles. J. Infect. Chemother. 2014, 20, 375–379. [Google Scholar] [CrossRef]
- van der Linden, J.W.M.; Snelders, E.; Kampinga, G.A.; Rijnders, B.J.A.; Mattsson, E.; Debets-Ossenkopp, Y.J.; Kuijper, E.J.; Van Tiel, F.H.; Melchers, W.J.G.; Verweij, P.E. Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007–2009. Emerg. Infect. Dis. 2011, 17, 1846–1854. [Google Scholar] [CrossRef]
- Casalini, G.; Giacomelli, A.; Antinori, S. The WHO fungal priority pathogens list: A crucial reappraisal to review the prioritisation. Lancet Microbe 2024, 5, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, C.O.; Kim, H.Y.; Duong, T.N.; Moran, E.; Alastruey-Izquierdo, A.; Denning, D.W.; Perfect, J.R.; Nucci, M.; Chakrabarti, A.; Rickerts, V.; et al. Aspergillus fumigatus-a systematic review to inform the World Health Organization priority list of fungal pathogens. Med. Mycol. 2024, 62, myad129. [Google Scholar] [CrossRef] [PubMed]
- Gautier-Veyret, E.; Thiebaut-Bertrand, A.; Roustit, M.; Bolcato, L.; Depeisses, J.; Schacherer, M.; Schummer, G.; Fonrose, X.; Stanke-Labesque, F. Optimization of voriconazole therapy for treatment of invasive aspergillosis: Pharmacogenomics and inflammatory status need to be evaluated. Br. J. Clin. Pharmacol. 2021, 87, 2534–2541. [Google Scholar] [CrossRef]
- Scott, J.; Valero, C.; Mato-López, Á.; Donaldson, I.J.; Roldán, A.; Chown, H.; Van Rhijn, N.; Lobo-Vega, R.; Gago, S.; Furukawa, T.; et al. Aspergillus fumigatus Can Display Persistence to the Fungicidal Drug Voriconazole. Microbiol. Spectr. 2023, 11, e0477022. [Google Scholar] [CrossRef] [PubMed]
- Hammad, S.F.; Hamid, M.A.A.; Adly, L.; Elagamy, S.H. Comprehensive review of greenness, whiteness, and blueness assessments of analytical methods. Green Anal. Chem. 2025, 12, 100209. [Google Scholar] [CrossRef]
- Smith, D.S.; Pourfarzaneh, M.; Kamel, R.S. Linear regression analysis by Deming’s method. Clin. Chem. 1980, 26, 1105–1106. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Takikawa, H.; Onji, M. A proposal of the diagnostic scale of drug-induced liver injury. Hepatol. Res. 2005, 32, 250–251. [Google Scholar] [CrossRef]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A.; Acute Kidney Injury Network. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef]
- Imataki, O.; Ohnishi, H.; Kitanaka, A.; Kubota, Y.; Ishida, T.; Tanaka, T. Visual disturbance comorbid with hallucination caused by voriconazole in the Japanese population. Int. J. Hematol. 2008, 88, 3–6. [Google Scholar] [CrossRef] [PubMed]


| LM1010 (Method A) | |
|---|---|
| Instrument | LM1010 (certified medical device), Hitachi High-Tech Analytical Science (Tokyo, Japan) |
| Mobile phase | Mobile phase A, mobile phase B, Hitachi High-Tech Analytical Science |
| Column | LaChrome LM Type A, Hitachi High-Tech Analytical Science |
| Extraction method | Spin column set, Hitachi High-Tech Analytical Science |
| Plasma volume (μL) | 150 |
| Fresh plasma sample | 1 μg/mL spiked serum recovery: 99.6% |
| Frozen (−30 °C) plasma sample | 1 μg/mL spiked serum recovery: 97.3% |
| Lower limits of quantitation (μg/mL) | 0.276 |
| Calibration curve range (μg/mL) | 1–5 |
| CV (%) | 0.630–1.02 |
| Accuracy (%) | 99.6–104.2 |
| Retention time of VRCZ blood concentration (min) | 1.76 |
| p-Value | ||
|---|---|---|
| Number | 7 | N/A |
| Age | 64 (55–76) | N/A |
| Sex (male) (%) | 2/7 (28.6) | N/A |
| Weight (kg) | 52.0 (36.5–67.0) | N/A |
| ALT (U/L) | 17.0 (5.0–71.0) | N/A |
| AST (U/L) | 25.5 (13.0–69.0) | N/A |
| Scr (mg/dL) | 0.78 (0.36–1.02) | N/A |
| BUN (mg/dL) | 18.0 (6.0–40.9) | N/A |
| Total bilirubin (mg/dL) | 0.30 (0.20–1.4) | N/A |
| VRCZ initial dosage (mg/day) | 600 (300–600) | N/A |
| VRCZ maintenance dosage (mg/day) | 300 (150–400) | N/A |
| VRCZ blood concentration (μg/mL) | ||
| Method A | 1.78 (0.33–5.61) | 0.750 |
| Method B | 1.53 (0.31–5.47) | |
| Time required for measuring (h) | ||
| Method A | 0.433 (0.400–0.467) | <0.001 |
| Method B | 74.3 (71.2–94.2) |
| Patients No. | No.1 | No.2 | No.3 |
|---|---|---|---|
| Age (years) | 71 | 70 | 65 |
| Sex | Female | Female | Male |
| Weight (kg) | 36.5 | 63.9 | 50.5 |
| Diagnosis | Chronic necrotizing pulmonary aspergillosis | Fungal sinusitis | Chronic necrotizing pulmonary aspergillosis |
| Pathogen | Aspergillus niger | Acrophialophora spp. | Aspergillus fumigatus |
| Concomitant medication (dosage/day) | Levothyroxine Sodium Hydrate 62.5 μg | Butyric acid-producing bacillus 3 g | L-Carbocisteine 1500 mg |
| Ambroxol Hydrochloride 45 mg | Lansoprazole 30 mg | Dimemorfan Phosphate 30 mg | |
| Montelukast Sodium 10 mg | Amlodipine Besilate 5 mg | Entecavir Hydrate 0.5 mg | |
| Gefapixant Citrate 90 mg | Lemborexant 2.5 mg | Eszopiclone 1 mg | |
| Ramelteon 8 mg | |||
| VRCZ dosage (/day) | 400 mg → 280 mg → 200 mg | 600 mg → 300 mg | 600 mg → 400 mg → 300 mg |
| VRCZ blood concentration (μg/mL) | 4.52 → 2.40 → 1.99 → 2.74 | 2.29 → 2.51 | 5.26 → 4.37 → 2.17 |
| VRCZ treatment period | 9 months~ | 4.5 months | 5 months~ |
| Hepatic function (before treatment) | ALT: 9 U/L, AST: 30 U/L | ALT: 33 U/L, AST: 17 U/L | ALT: 16 U/L, AST: 19 U/L |
| Hepatic function (after treatment) | ALT: 10 U/L, AST: 22 U/L | ALT: 17 U/L, AST: 22 U/L | ALT: 15 U/L, AST: 26 U/L |
| Renal function (before treatment) | Scr: 0.39 mg/dL, BUN: 8.5 mg/dL | Scr: 0.43 mg/dL, BUN: 18.4 mg/dL | Scr: 0.68 mg/dL, BUN: 7.3 mg/dL |
| Renal function (after treatment) | Scr: 0.53 mg/dL, BUN: 15.6 mg/dL | Scr: 0.54 mg/dL, BUN: 18.0 mg/dL | Scr: 0.57 mg/dL, BUN: 7.1 mg/dL |
| Hepatic dysfunction | N/A | N/A | N/A |
| Renal dysfunction | N/A | N/A | N/A |
| Visual impairment | N/A | N/A | N/A |
| Treatment outcome | Remission | Death | Remission |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morikawa, S.; Yagi, Y.; Okazaki, M.; Yanagisawa, N.; Ishida, T.; Jobu, K.; Maruyama, T.; Kato, T.; Matsushita, M.; Arakawa, Y.; et al. Rapid Therapeutic Drug Monitoring of Voriconazole Based on High-Performance Liquid Chromatography: A Single-Center Pilot Study in Outpatients. Antibiotics 2025, 14, 474. https://doi.org/10.3390/antibiotics14050474
Morikawa S, Yagi Y, Okazaki M, Yanagisawa N, Ishida T, Jobu K, Maruyama T, Kato T, Matsushita M, Arakawa Y, et al. Rapid Therapeutic Drug Monitoring of Voriconazole Based on High-Performance Liquid Chromatography: A Single-Center Pilot Study in Outpatients. Antibiotics. 2025; 14(5):474. https://doi.org/10.3390/antibiotics14050474
Chicago/Turabian StyleMorikawa, Satoru, Yusuke Yagi, Moemi Okazaki, Narika Yanagisawa, Tomoaki Ishida, Kohei Jobu, Takumi Maruyama, Takahiro Kato, Miyuki Matsushita, Yu Arakawa, and et al. 2025. "Rapid Therapeutic Drug Monitoring of Voriconazole Based on High-Performance Liquid Chromatography: A Single-Center Pilot Study in Outpatients" Antibiotics 14, no. 5: 474. https://doi.org/10.3390/antibiotics14050474
APA StyleMorikawa, S., Yagi, Y., Okazaki, M., Yanagisawa, N., Ishida, T., Jobu, K., Maruyama, T., Kato, T., Matsushita, M., Arakawa, Y., Yamagishi, Y., & Hamada, Y. (2025). Rapid Therapeutic Drug Monitoring of Voriconazole Based on High-Performance Liquid Chromatography: A Single-Center Pilot Study in Outpatients. Antibiotics, 14(5), 474. https://doi.org/10.3390/antibiotics14050474






