The α-Helical Amphipathic Peptide Alleviates Colistin-Induced Nephrotoxicity by Maintaining Mitochondrial Function in Both In Vitro and In Vivo Infection Models
Abstract
1. Introduction
2. Results
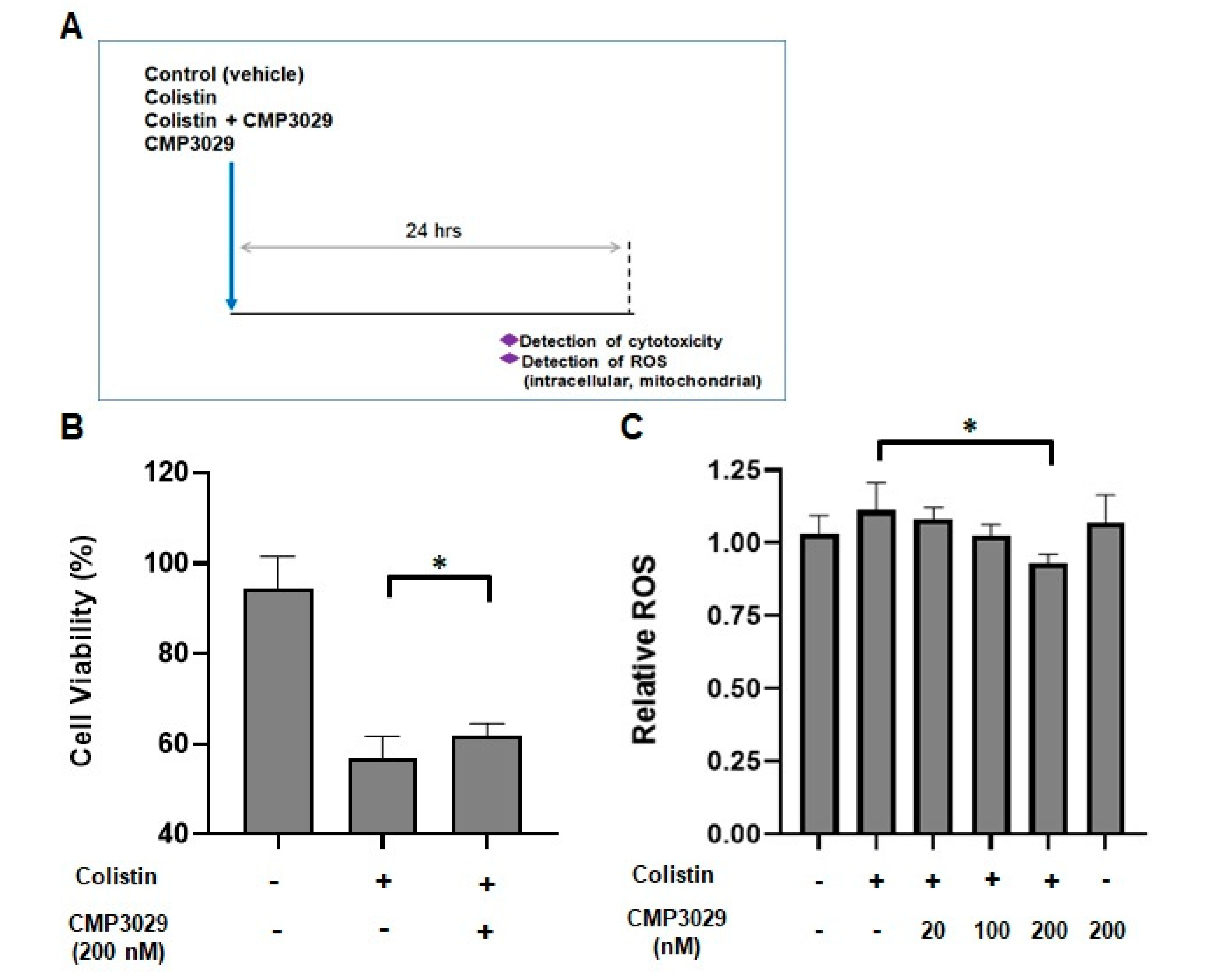
2.1. CMP3029 Improved the Cell Viability of the HK-2 Cell Line When Exposed to Colistin
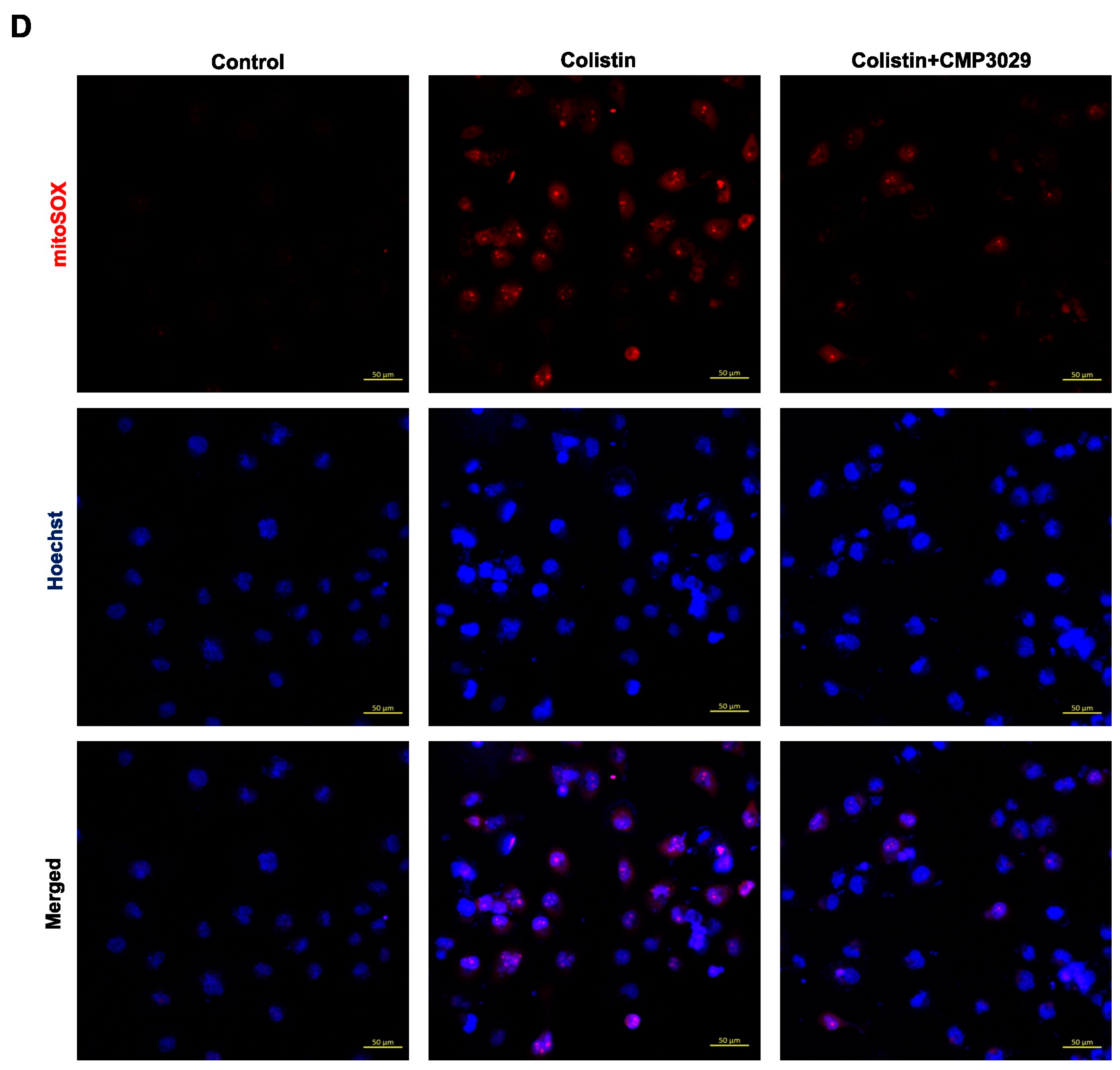
2.2. CMP3029 Attenuated Colistin-Induced Intracellular and Mitochondrial ROS Overproduction of HK-2 Cells
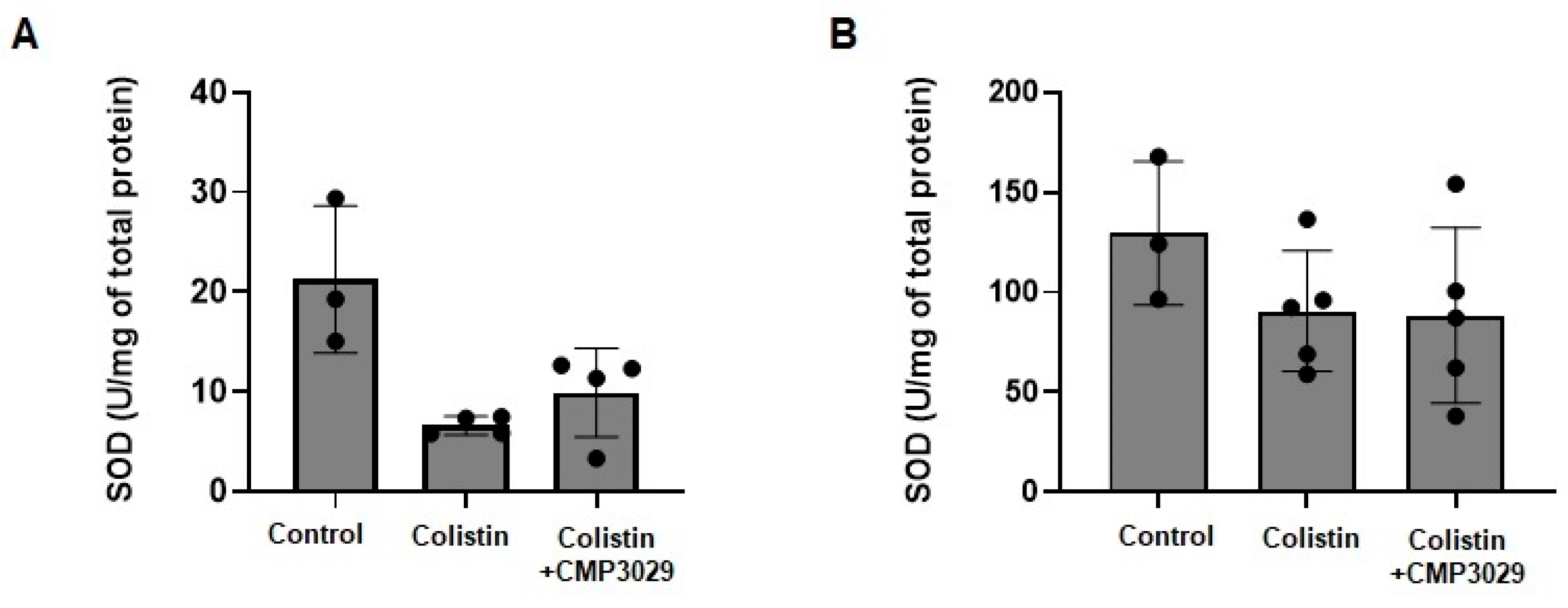
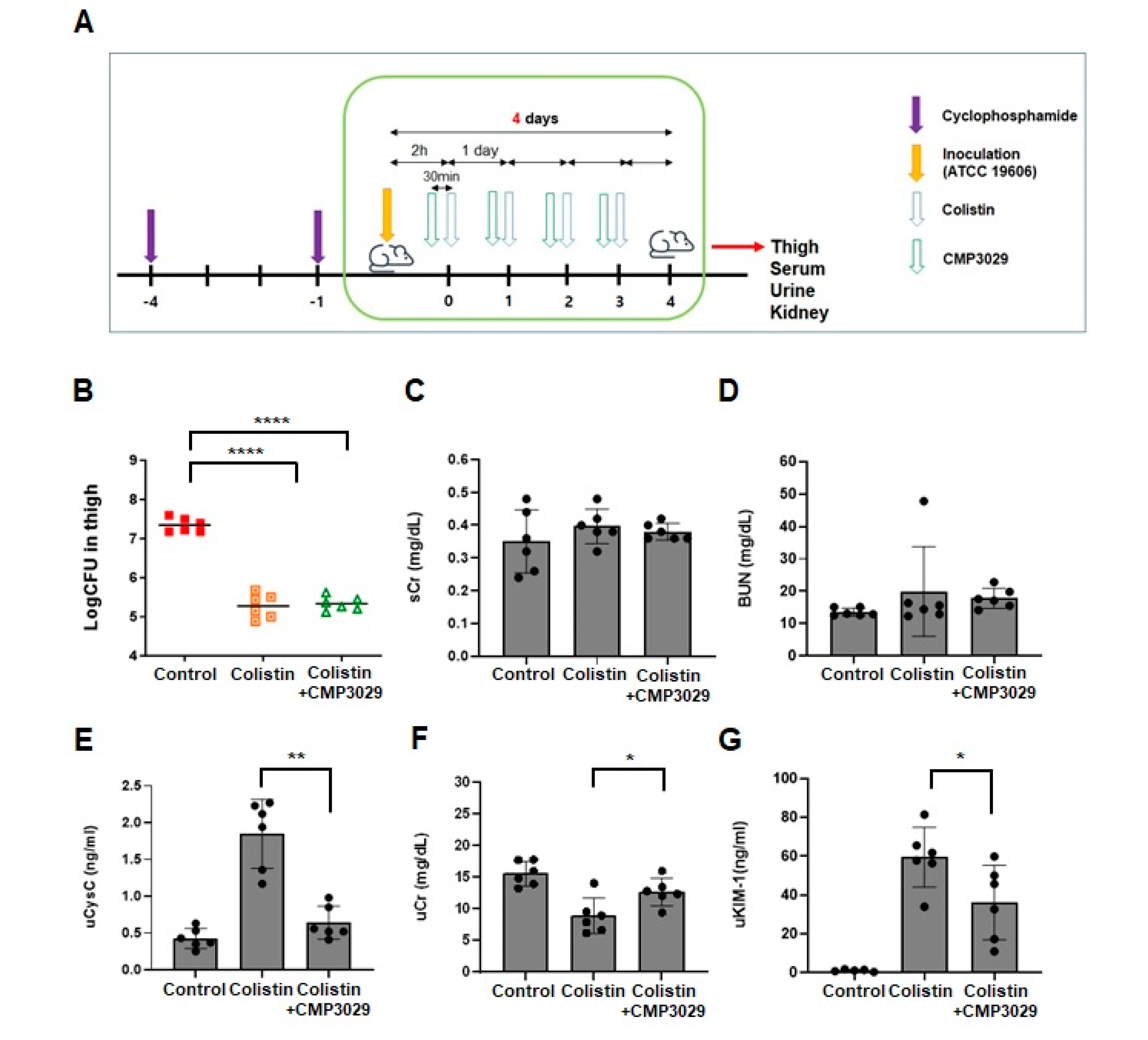
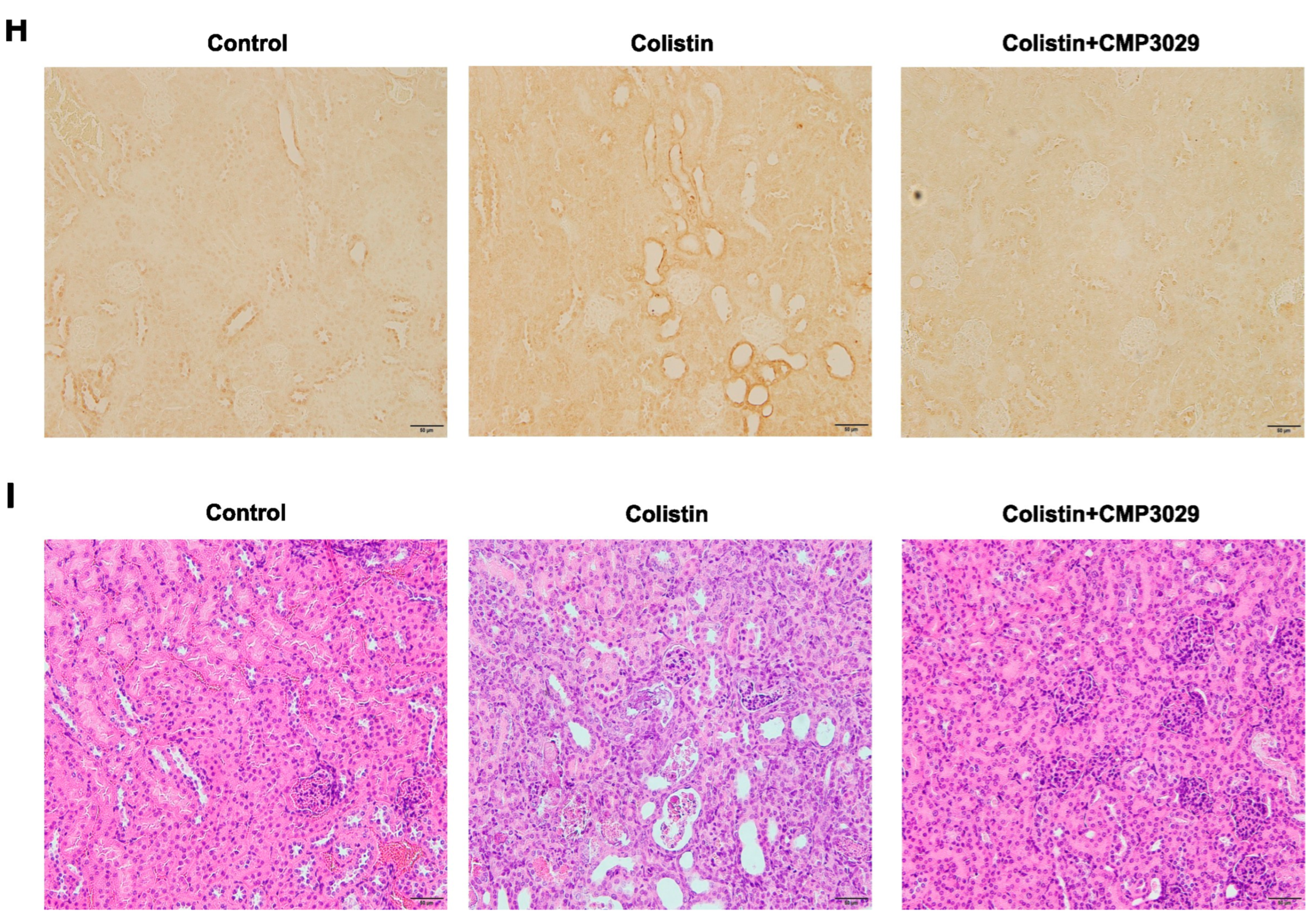
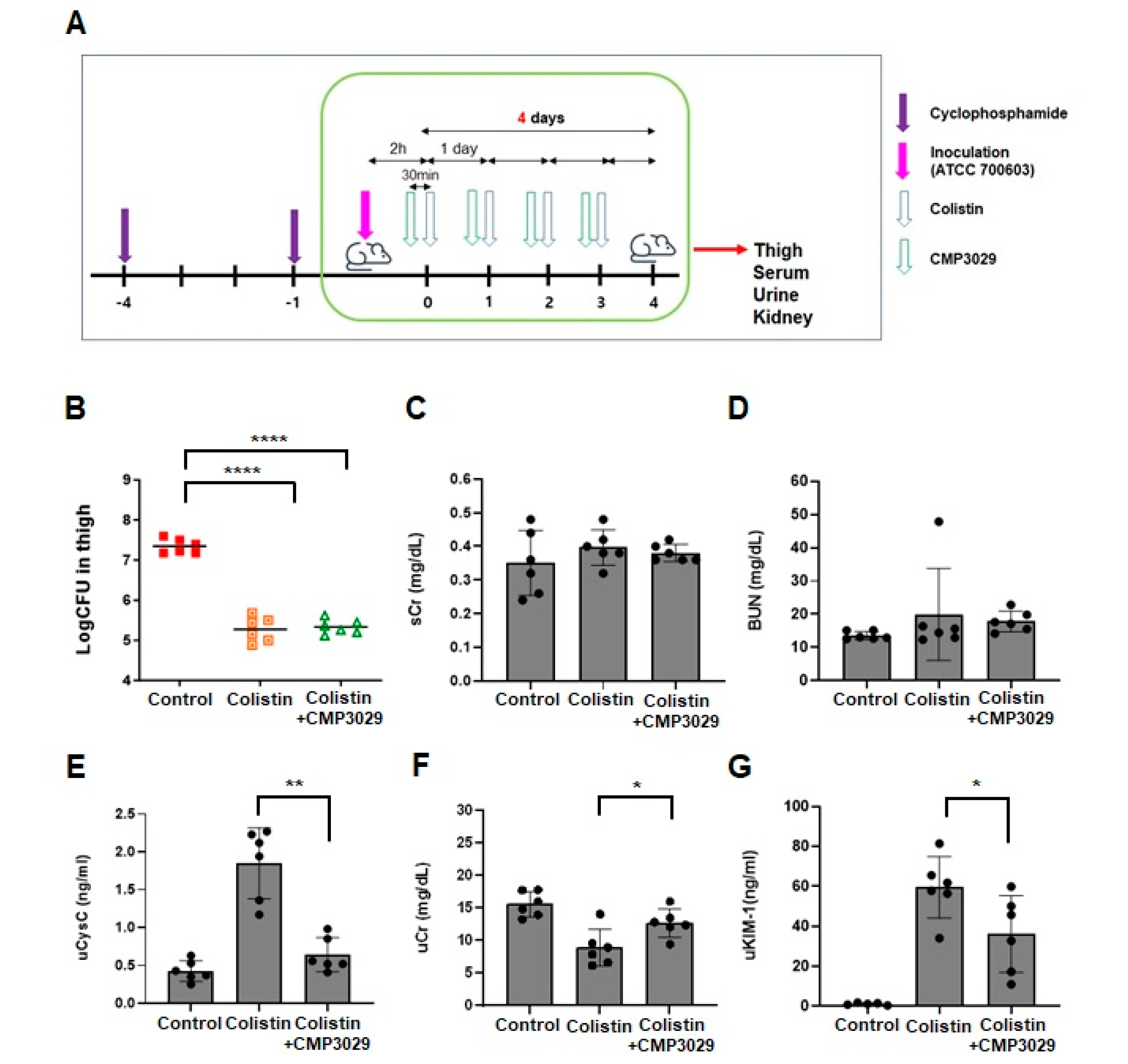
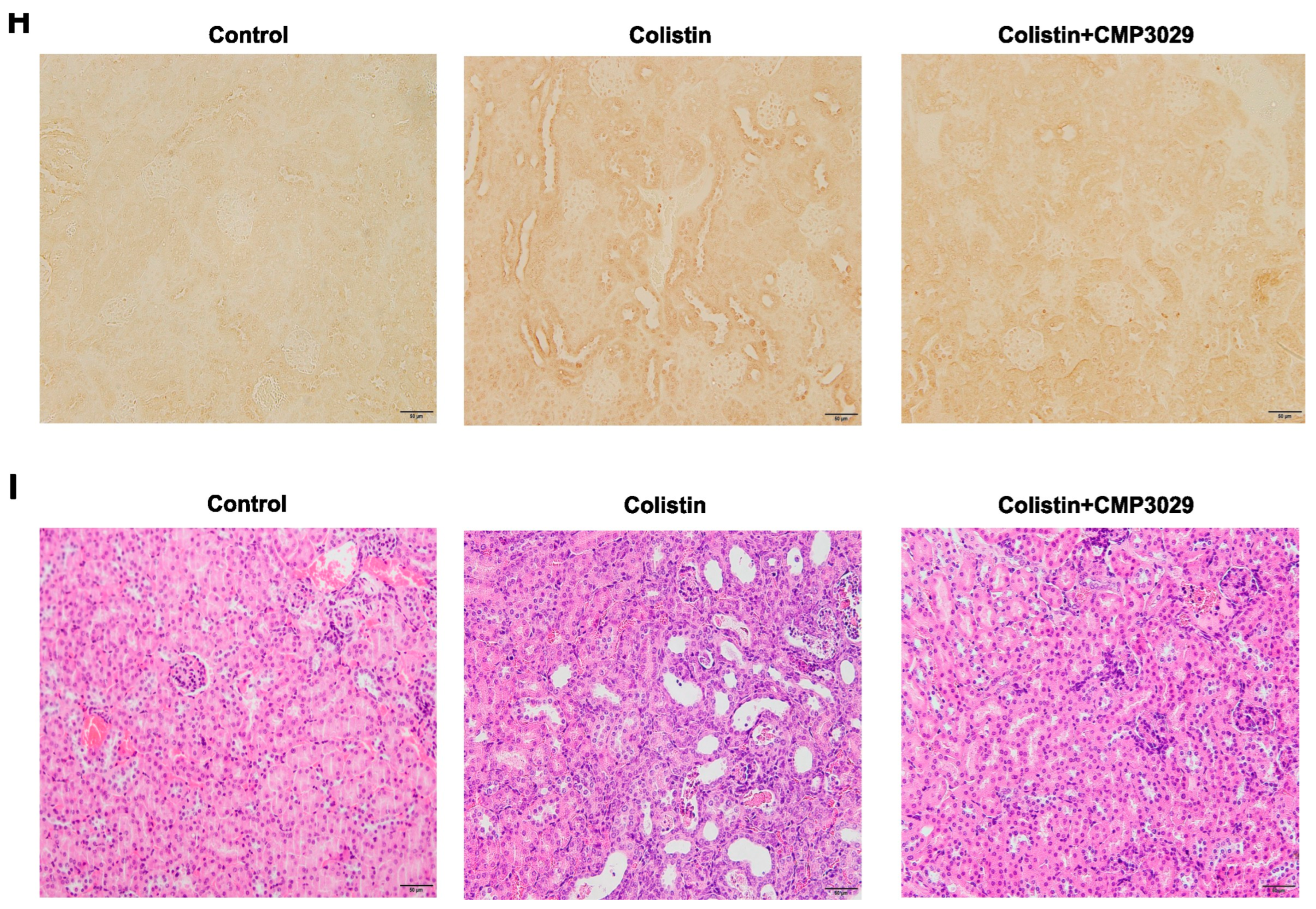
2.3. CMP3029 Mitigated Colistin-Induced Nephrotoxicity in an In Vivo Infection Model
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Mice
4.4. Bacterial Strains
4.5. Analysis of Cell Viability
4.6. Measurement of Intracellular ROS Levels
4.7. Measurement of Mitochondrial ROS Levels
4.8. Neutropenic Mouse Thigh Infection Model
4.9. Urine Collection
4.10. Determination of Serum and Urinary Biomarkers for Kidney Injury
4.11. Immunohistochemistry
4.12. Histopathological Examination
4.13. Measurement of Markers of Oxidative Stress in Kidney Tissues
4.14. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CR-GNB | Carbapenem-resistant Gram-negative bacteria |
| ROS | Reactive oxygen species |
| KIM-1 | Kidney injury molecule-1 |
| NGAL | Neutrophil gelatinase-associated lipocalin |
| CPPs | Cell-penetrating peptides |
| IMM | Inner mitochondrial membrane |
| HK-2 | Human renal proximal tubular epithelial cells |
| LCD2 | Lipocalin-2 |
| SOD | Superoxide dismutase |
| BUN | Blood urea nitrogen |
| sCr | Serum creatinine |
| uCr | Urinary creatinine |
| ELISA | Enzyme-linked immunosorbent assay |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| DCFDA | 2′,7′-Dichlorodihydrofluorescein diacetate |
| SQS | Semi-quantitative score |
| CFU | Colony-forming unit |
Appendix A
| Percentage of the Kidney Slice Affected in Individual Mice of Each Group (n = 5 or 6) | ||||
| Abnormality grade | control | colistin | CMP3029 | Colistin/CMP3029 |
| 1 | 0, 0, 0, 0, 0 | 3, 4, 3, 3, 3, 2 | 0, 0, 0, 0, 0, 0 | 1, 3, 1, 1, 1, 2 |
| 2 | 0, 0, 0, 0, 0 | 0, 0, 0, 0, 0, 0 | 0, 0, 0, 0, 0, 0 | 0, 0, 0, 0, 0, 0 |
| 3 | 0, 0, 0, 0, 0 | 0, 0, 0, 0, 0, 0 | 0, 0, 0, 0, 0, 0 | 0, 0, 0, 0, 0, 0 |
| SQS for individual mice * | 0, 0, 0, 0, 0 | 1, 1, 1, 1, 1, 1 | 0, 0, 0, 0, 0, 0 | 1, 1, 1, 1, 1, 1 |
| Percentage of the Kidney Slice Affected in Individual Mice of Each Group (n = 3 or 5) | ||||
| Abnormality grade | control | colistin | CMP3029 | Colistin/CMP3029 |
| 1 | 0, 0, 0 | 3, 3, 3, 4, 2 | 0, 0, 0, 0, 0, 0 | 2, 2, 3, 1, 1 |
| 2 | 0, 0, 0 | 0, 0, 0, 0, 0 | 0, 0, 0, 0, 0, 0 | 0, 0, 0, 0, 0 |
| 3 | 0, 0, 0 | 0, 0, 0, 0, 0 | 0, 0, 0, 0, 0, 0 | 0, 0, 0, 0, 0 |
| SQS for individual mice * | 0, 0, 0 | 1, 1, 1, 1, 1 | 0, 0, 0, 0, 0, 0 | 1, 1, 1, 1, 1 |

References
- Moja, P.L. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Li, J.; Nation, R.L.; Milne, R.W.; Turnidge, J.D.; Coulthard, K. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int. J. Antimicrob. Agents 2005, 25, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, M.; Mirzaei, E.; Vazin, A. Pharmacological agents for the prevention of colistin-induced nephrotoxicity. Eur. J. Med. Res. 2022, 27, 64. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Liu, Y.; Chen, C.; Velkov, T.; Tang, S.; Shen, J.; Dai, C. Colistin Induces Oxidative Stress and Apoptotic Cell Death through the Activation of the AhR/CYP1A1 Pathway in PC12 Cells. Antioxidants 2024, 13, 827. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.Y.; Park, S.R.; Cho, S.; Yu, S.L.; Lee, H.Y.; Park, C.G.; Kang, J.; Jung, D.Y.; Park, M.H.; Hwang, W.M.; et al. TGF-β-mediated NADPH oxidase 4-dependent oxidative stress promotes colistin-induced acute kidney injury. J. Antimicrob. Chemother. 2018, 73, 962–972. [Google Scholar] [CrossRef]
- Zhu, Y.; Luo, M.; Bai, X.; Li, J.; Nie, P.; Li, B.; Luo, P. SS-31, a Mitochondria-Targeting Peptide, Ameliorates Kidney Disease. Oxidative Med. Cell. Longev. 2022, 2022, 1295509. [Google Scholar] [CrossRef]
- Xie, J.; Bi, Y.; Zhang, H.; Dong, S.; Teng, L.; Lee, R.J.; Yang, Z. Cell-Penetrating Peptides in Diagnosis and Treatment of Human Diseases: From Preclinical Research to Clinical Application. Front. Pharmacol. 2020, 11, 697. [Google Scholar] [CrossRef]
- Shin, G.; Hyun, S.; Kim, D.; Choi, Y.; Kim, K.H.; Kim, D.; Kwon, S.; Kim, Y.S.; Yang, S.H.; Yu, J. Cyclohexylalanine-Containing α-Helical Amphipathic Peptide Targets Cardiolipin, Rescuing Mitochondrial Dysfunction in Kidney Injury. J. Med. Chem. 2024, 67, 3385–3399. [Google Scholar] [CrossRef]
- Dai, C.; Li, J.; Tang, S.; Li, J.; Xiao, X. Colistin-induced nephrotoxicity in mice involves the mitochondrial, death receptor, and endoplasmic reticulum pathways. Antimicrob. Agents Chemother. 2014, 58, 4075–4085. [Google Scholar] [CrossRef]
- Yousef, J.M.; Chen, G.; Hill, P.A.; Nation, R.L.; Li, J. Ascorbic acid protects against the nephrotoxicity and apoptosis caused by colistin and affects its pharmacokinetics. J. Antimicrob. Chemother. 2012, 67, 452–459. [Google Scholar] [CrossRef]
- Sirijatuphat, R.; Limmahakhun, S.; Sirivatanauksorn, V.; Nation, R.L.; Li, J.; Thamlikitkul, V. Preliminary Clinical Study of the Effect of Ascorbic Acid on Colistin-Associated Nephrotoxicity. Antimicrob. Agents Chemother. 2015, 59, 3224–3232. [Google Scholar] [CrossRef]
- Dai, C.; Tang, S.; Wang, Y.; Velkov, T.; Xiao, X. Baicalein acts as a nephroprotectant that ameliorates colistin-induced nephrotoxicity by activating the antioxidant defence mechanism of the kidneys and down-regulating the inflammatory response. J. Antimicrob. Chemother. 2017, 72, 2562–2569. [Google Scholar] [CrossRef] [PubMed]
- Yousef, J.M.; Chen, G.; Hill, P.A.; Nation, R.L.; Li, J. Melatonin Attenuates Colistin-Induced Nephrotoxicity in Rats. Antimicrob. Agents Chemother. 2011, 55, 4044–4049. [Google Scholar] [CrossRef] [PubMed]
- van Meer, L.; Moerland, M.; Cohen, A.F.; Burggraaf, J. Urinary kidney biomarkers for early detection of nephrotoxicity in clinical drug development. Br. J. Clin. Pharmacol. 2014, 77, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Keirstead, N.D.; Wagoner, M.P.; Bentley, P.; Blais, M.; Brown, C.; Cheatham, L.; Ciaccio, P.; Dragan, Y.; Ferguson, D.; Fikes, J.; et al. Early Prediction of Polymyxin-Induced Nephrotoxicity with Next-Generation Urinary Kidney Injury Biomarkers. Toxicol. Sci. 2014, 137, 278–291. [Google Scholar] [CrossRef]
- Luo, Q.H.; Chen, M.L.; Sun, F.J.; Chen, Z.L.; Li, M.Y.; Zeng, W.; Gong, L.; Cheng, A.C.; Peng, X.; Fang, J.; et al. KIM-1 and NGAL as biomarkers of nephrotoxicity induced by gentamicin in rats. Mol. Cell. Biochem. 2014, 397, 53–60. [Google Scholar] [CrossRef]
- Sahre, M.D.K.; Rogers, H. Biomarker Qualification Program Office of Clinical Pharmacology Full Qualification Package Review; Food and Drug Administration: Silver Spring, MD, USA, 2015; pp. 1–14.
- Mehrab, H.; Sharifi, M.; Akhavan, A.; Aarabi, M.-H.; Mansourian, M.; Mosavi, E.; Moghaddas, A. Curcumin supplementation prevents cisplatin-induced nephrotoxicity: A randomized, double-blinded, and placebo-controlled trial. Res. Pharm. Sci. 2023, 18, 648–662. [Google Scholar] [CrossRef]
- Adhikari, A.; Mondal, S.; Chatterjee, T.; Das, M.; Biswas, P.; Ghosh, R.; Darbar, S.; Alessa, H.; Althakafy, J.T.; Sayqal, A.; et al. Redox nanomedicine ameliorates chronic kidney disease (CKD) by mitochondrial reconditioning in mice. Commun. Biol. 2021, 4, 1013. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Lu, M.; Yin, N.; Liu, W.; Cui, X.; Chen, S.; Wang, E. Curcumin Ameliorates Diabetic Nephropathy by Suppressing NLRP3 Inflammasome Signaling. BioMed Res. Int. 2017, 2017, 1516985. [Google Scholar] [CrossRef]
- Sun, L.-N.; Liu, X.-C.; Chen, X.-J.; Guan, G.-J.; Liu, G. Curcumin attenuates high glucose-induced podocyte apoptosis by regulating functional connections between caveolin-1 phosphorylation and ROS. Acta Pharmacol. Sin. 2016, 37, 645–655. [Google Scholar] [CrossRef]
- Schneider, M.P.; Sullivan, J.C.; Wach, P.F.; Boesen, E.I.; Yamamoto, T.; Fukai, T.; Harrison, D.G.; Pollock, D.M.; Pollock, J.S. Protective role of extracellular superoxide dismutase in renal ischemia/reperfusion injury. Kidney Int. 2010, 78, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-Y.; Han, S.; Zhang, L.; Zhu, Y.-H.; Wang, L.-M.; Zeng, L. Mitochondria-Targeted Antioxidant Peptide SS31 Prevents Hypoxia/Reoxygenation-Induced Apoptosis by Down-Regulating p66Shc in Renal Tubular Epithelial Cells. Cell. Physiol. Biochem. 2013, 32, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Dudhani, R.V.; Turnidge, J.D.; Coulthard, K.; Milne, R.W.; Rayner, C.R.; Li, J.; Nation, R.L. Elucidation of the Pharmacokinetic/Pharmacodynamic Determinant of Colistin Activity against Pseudomonas aeruginosa in Murine Thigh and Lung Infection Models. Antimicrob. Agents Chemother. 2010, 54, 1117–1124. [Google Scholar] [CrossRef]
- Kurien, B.T.; Everds, N.E.; Scofield, R.H. Experimental animal urine collection: A review. Lab. Anim. 2004, 38, 333–361. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kook, M.S.; Kim, H.; Choi, Y.; Bae, S.M.; Yu, J.; Kim, Y.S. The α-Helical Amphipathic Peptide Alleviates Colistin-Induced Nephrotoxicity by Maintaining Mitochondrial Function in Both In Vitro and In Vivo Infection Models. Antibiotics 2025, 14, 445. https://doi.org/10.3390/antibiotics14050445
Kook MS, Kim H, Choi Y, Bae SM, Yu J, Kim YS. The α-Helical Amphipathic Peptide Alleviates Colistin-Induced Nephrotoxicity by Maintaining Mitochondrial Function in Both In Vitro and In Vivo Infection Models. Antibiotics. 2025; 14(5):445. https://doi.org/10.3390/antibiotics14050445
Chicago/Turabian StyleKook, Min Soo, Heeseung Kim, Yoonhwa Choi, Seong Man Bae, Jaehoon Yu, and Yang Soo Kim. 2025. "The α-Helical Amphipathic Peptide Alleviates Colistin-Induced Nephrotoxicity by Maintaining Mitochondrial Function in Both In Vitro and In Vivo Infection Models" Antibiotics 14, no. 5: 445. https://doi.org/10.3390/antibiotics14050445
APA StyleKook, M. S., Kim, H., Choi, Y., Bae, S. M., Yu, J., & Kim, Y. S. (2025). The α-Helical Amphipathic Peptide Alleviates Colistin-Induced Nephrotoxicity by Maintaining Mitochondrial Function in Both In Vitro and In Vivo Infection Models. Antibiotics, 14(5), 445. https://doi.org/10.3390/antibiotics14050445






