In Vivo Antimicrobial Activity of Nisin Z Against S. aureus and Polyurea Pharmadendrimer PUREG4OEI48 Against P. aeruginosa from Diabetic Foot Infections
Abstract
1. Introduction
2. Results
2.1. Minimum Inhibitory Concentration (MIC)
2.2. Galleria Mellonella Killing Assay
2.2.1. Health Index Score
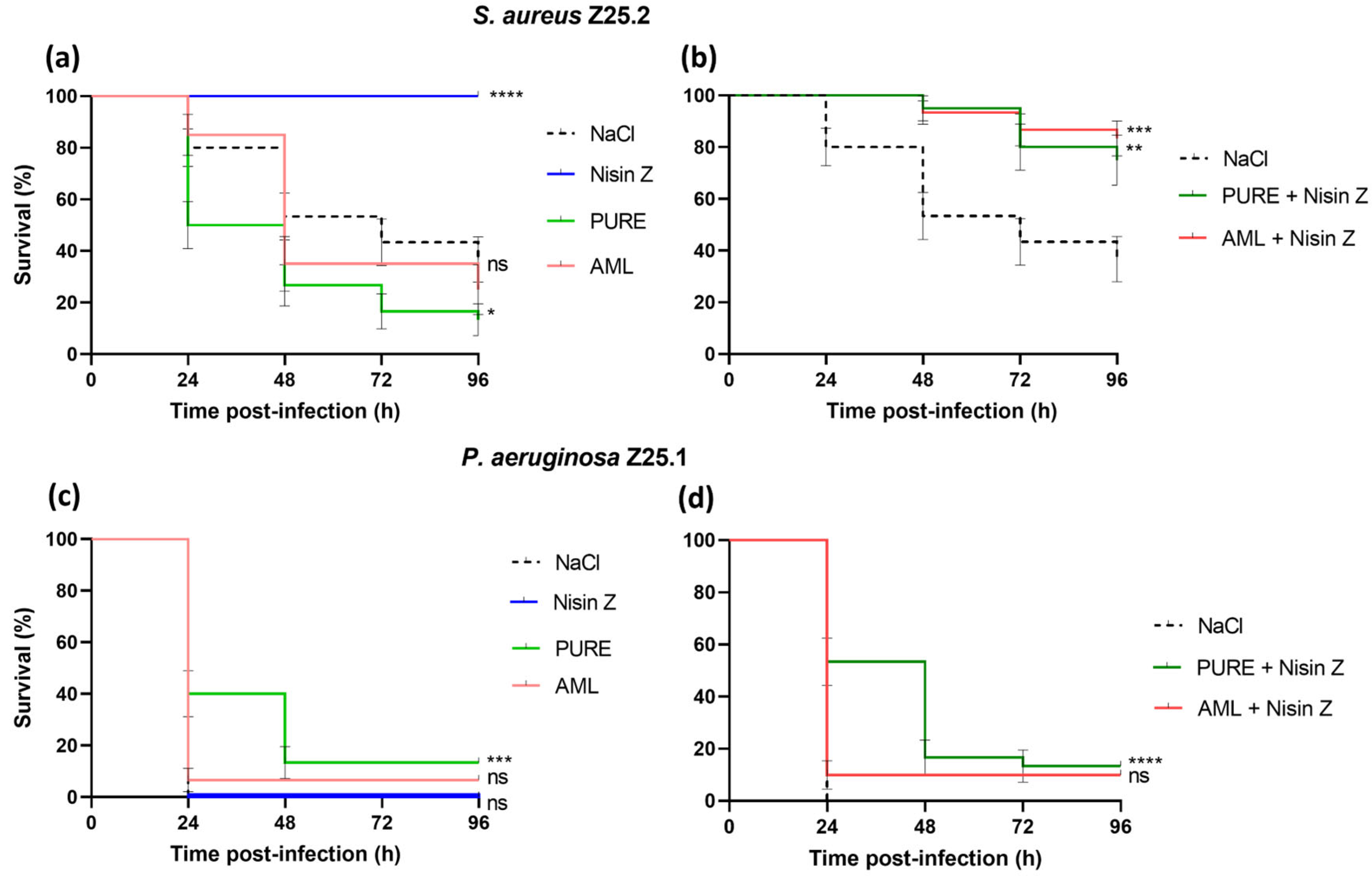
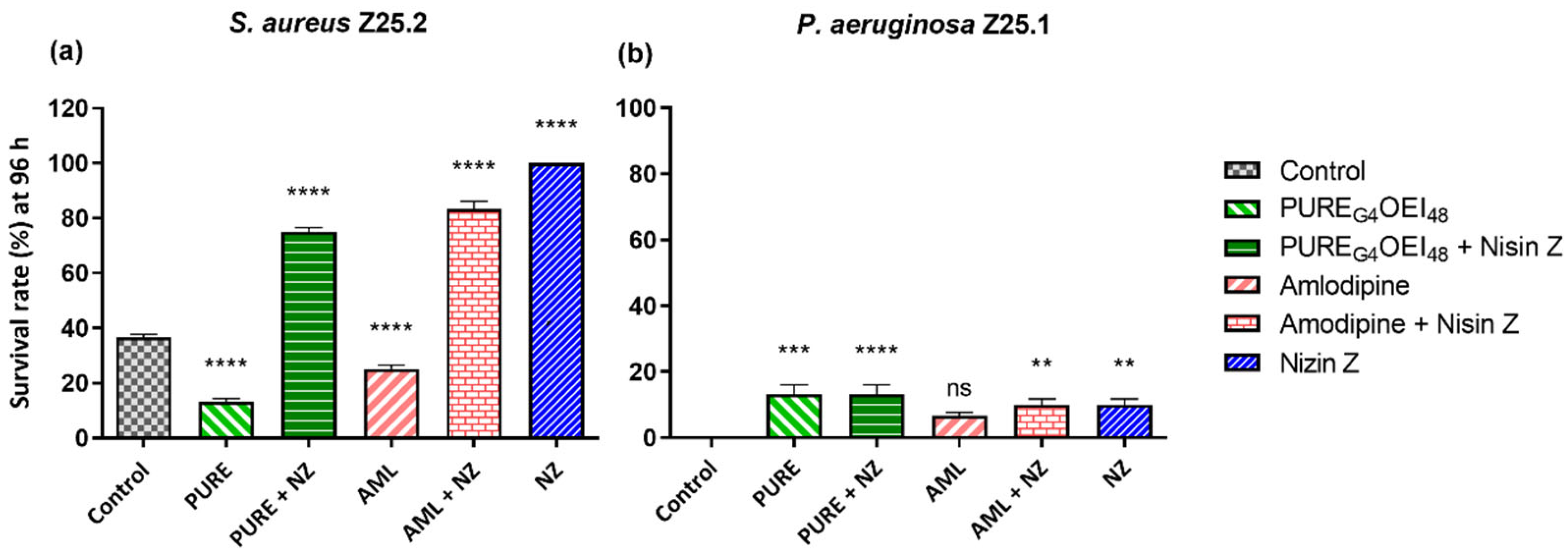
2.2.2. Survival Curves
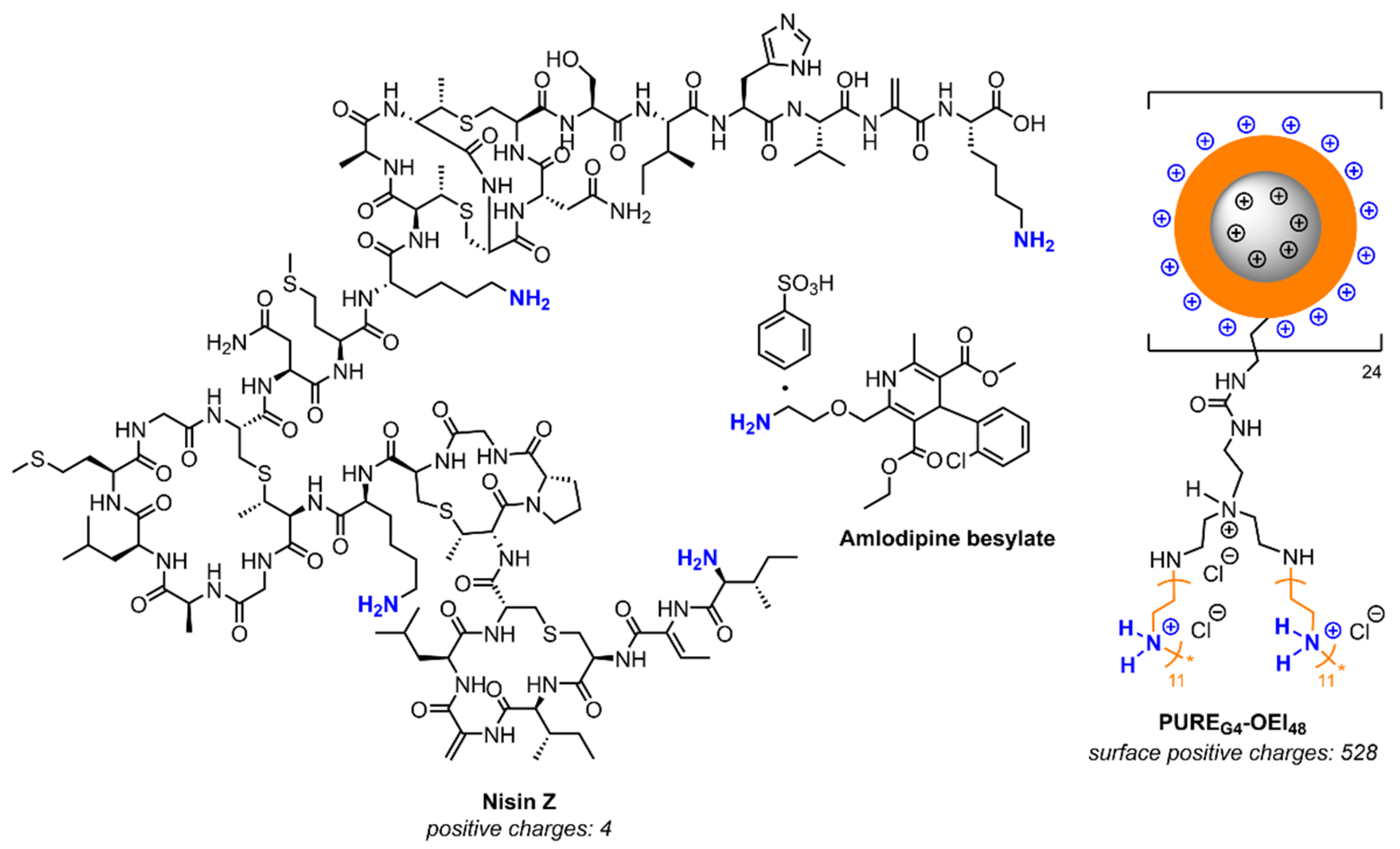
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Cultural Conditions
4.2. Preparation of Solutions
4.3. Minimum Inhibitory Concentration (MIC) of PUREG4OEI48 and Amlodipine
4.4. Galleria Mellonella Killing Assay and Quantification of S. aureus and P. aeruginosa CFU
4.5. Galleria Mellonella Toxicity Assay
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPs | Antimicrobial peptides |
| BHI | Brain heart infusion |
| CFU | Colony forming units |
| DFIs | Diabetic foot infections |
| DFUs | Diabetic foot ulcers |
| MIC | Minimum inhibitory concentration |
| SMAMPs | Synthetic mimics of antimicrobial peptides |
References
- Magliano, D.J.; Boyko, E.J.; IDF Diabetes Atlas 10th Edition Scientific Committee. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of Diabetes: Estimates for the Year 2000 and Projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Cohen, K.; Courric, S.; Bharara, M.; Marston, W. Diabetic Foot Ulcers and Vascular Insufficiency: Our Population Has Changed, but Our Methods Have Not. J. Diabetes Sci. Technol. 2011, 5, 1591–1595. [Google Scholar] [CrossRef] [PubMed]
- Chastain, C.A.; Klopfenstein, N.; Serezani, C.H.; Aronoff, D.M. A Clinical Review of Diabetic Foot Infections. Clin. Podiatr. Med. Surg. 2019, 36, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Schaper, N.C.; Van Netten, J.J.; Apelqvist, J.; Bus, S.A.; Hinchliffe, R.J.; Lipsky, B.A. IWGDF Editorial Board Practical Guidelines on the Prevention and Management of Diabetic Foot Disease (IWGDF 2019 Update). Diabetes Metab. Res. 2020, 36, e3266. [Google Scholar] [CrossRef]
- Vatan, A.; Saltoglu, N.; Yemisen, M.; Balkan, I.I.; Surme, S.; Demiray, T.; Mete, B.; Tabak, F.; Study Group, Cerrahpasa Diabetic Foot. Association between Biofilm and Multi/Extensive Drug Resistance in Diabetic Foot Infection. Int. J. Clin. Pract. 2018, 72, e13060. [Google Scholar] [CrossRef]
- Noor, S.; Khan, R.U.; Ahmad, J. Understanding Diabetic Foot Infection and Its Management. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, 149–156. [Google Scholar] [CrossRef]
- Johani, K.; Malone, M.; Jensen, S.; Gosbell, I.; Dickson, H.; Hu, H.; Vickery, K. Microscopy Visualisation Confirms Multi-species Biofilms Are Ubiquitous in Diabetic Foot Ulcers. Int. Wound J. 2017, 14, 1160–1169. [Google Scholar] [CrossRef]
- Tawre, M.S.; Kamble, E.E.; Kumkar, S.N.; Mulani, M.S.; Pardesi, K.R. Antibiofilm and Antipersister Activity of Acetic Acid against Extensively Drug Resistant Pseudomonas aeruginosa PAW1. PLoS ONE 2021, 16, e0246020. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Boulton, A.; Armstrong, D.; Hardman, M.; Malone, M.; Embil, J.; Attinger, C.; Lipsky, B.; Aragon-Sanchez, J.; Kwong, H.; Schultz, G.; et al. Diagnosis and Management of Diabetic Foot Infections. Compendia 2020, 2020, 1–24. [Google Scholar] [CrossRef]
- Ciofu, O.; Tolker-Nielsen, T. Tolerance and Resistance of Pseudomonas aeruginosa Biofilms to Antimicrobial Agents-How P. aeruginosa Can Escape Antibiotics. Front. Microbiol. 2019, 10, 913. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A. Diabetic Foot Infections: Current Treatment and Delaying the ‘Post-antibiotic Era’. Diabetes Metab. Res. 2016, 32, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Berendt, A.R.; Cornia, P.B.; Pile, J.C.; Peters, E.J.G.; Armstrong, D.G.; Deery, H.G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W.; et al. 2012 Infectious Diseases Society of America Clinical Practice Guideline for the Diagnosis and Treatment of Diabetic Foot Infectionsa. Clin. Infect. Dis. 2012, 54, e132–e173. [Google Scholar] [CrossRef] [PubMed]
- Serrano, I.; Alhinho, B.; Cunha, E.; Tavares, L.; Trindade, A.; Oliveira, M. Bacteriostatic and Antibiofilm Efficacy of a Nisin Z Solution against Co-Cultures of Staphylococcus aureus and Pseudomonas aeruginosa from Diabetic Foot Infections. Life 2023, 13, 504. [Google Scholar] [CrossRef]
- Shin, J.M.; Gwak, J.W.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Biomedical Applications of Nisin. J. Appl. Microbiol. 2016, 120, 1449–1465. [Google Scholar] [CrossRef]
- Field, D.; O’ Connor, R.; Cotter, P.D.; Ross, R.P.; Hill, C. In Vitro Activities of Nisin and Nisin Derivatives Alone and In Combination with Antibiotics against Staphylococcus Biofilms. Front. Microbiol. 2016, 7, 508. [Google Scholar] [CrossRef]
- Pinto, S.N.; Mil-Homens, D.; Pires, R.F.; Alves, M.M.; Serafim, G.; Martinho, N.; Melo, M.; Fialho, A.M.; Bonifácio, V.D.B. Core–Shell Polycationic Polyurea Pharmadendrimers: New-Generation of Sustainable Broad-Spectrum Antibiotics and Antifungals. Biomater. Sci. 2022, 10, 5197–5207. [Google Scholar] [CrossRef]
- Boyd, N.K.; Lee, G.C.; Teng, C.; Frei, C.R. In Vitro Activity of Non-Antibiotic Drugs against Staphylococcus aureus Clinical Strains. J. Glob. Antimicrob. Resist. 2021, 27, 167–171. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Wang, D.; Sun, S.; Lu, C. In Vitro Interactions of Ambroxol Hydrochloride or Amlodipine in Combination with Antibacterial Agents against Carbapenem-Resistant Acinetobacter baumannii. Lett. Appl. Microbiol. 2020, 70, 189–195. [Google Scholar] [CrossRef]
- Mazumdar, K.; Asok Kumar, K.; Dutta, N.K. Potential Role of the Cardiovascular Non-Antibiotic (Helper Compound) Amlodipine in the Treatment of Microbial Infections: Scope and Hope for the Future. Int. J. Antimicrob. Agents 2010, 36, 295–302. [Google Scholar] [CrossRef]
- Verma, D.; Sharma, S.K. Recent Advances in Guar Gum Based Drug Delivery Systems and Their Administrative Routes. Int. J. Biol. Macromol. 2021, 181, 653–671. [Google Scholar] [CrossRef]
- Santos, R.; Gomes, D.; Macedo, H.; Barros, D.; Tibério, C.; Veiga, A.S.; Tavares, L.; Castanho, M.; Oliveira, M. Guar Gum as a New Antimicrobial Peptide Delivery System against Diabetic Foot Ulcers Staphylococcus aureus Isolates. J. Med. Microbiol. 2016, 65, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.; Santos, R.; Soares, S.R.; Reis, S.; Carvalho, S.; Rego, P.; Peleteiro, C.M.; Tavares, L.; Oliveira, M. Pexiganan in Combination with Nisin to Control Polymicrobial Diabetic Foot Infections. Antibiotics 2020, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.S.; Santos, R.; Cunha, E.; Tavares, L.; Trindade, A.; Oliveira, M. Influence of Storage on the Antimicrobial and Cytotoxic Activities of a Nisin-Biogel with Potential to Be Applied to Diabetic Foot Infections Treatment. Antibiotics 2020, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Delvesbroughton, J. The Use of EDTA to Enhance the Efficacy of Nisin towards Gram-Negative Bacteria. Int. Biodeterior. Biodegrad. 1993, 32, 87–97. [Google Scholar] [CrossRef]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A Viable Alternative to Antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef]
- Santos, S.; Oliveira, M.; Lemsaddek, T. Bacteriocins. In Frontiers in Antimicrobial Agents—The Challenges of Antibiotic Resistance in the Development of New Therapeutics; Bentham Science Publishers: Sharjah, United Arab Emirates, 2015; Volume 1, pp. 178–207. [Google Scholar]
- Simons, A.; Alhanout, K.; Duval, R.E. Bacteriocins, Antimicrobial Peptides from Bacterial Origin: Overview of Their Biology and Their Impact against Multidrug-Resistant Bacteria. Microorganisms 2020, 8, 639. [Google Scholar] [CrossRef]
- Suárez, A.M.; Rodríguez, J.M.; Hernández, P.E.; Azcona-Olivera, J.I. Generation of Polyclonal Antibodies against Nisin: Immunization Strategies and Immunoassay Development. Appl. Environ. Microbiol. 1996, 62, 2117–2121. [Google Scholar] [CrossRef]
- Zainal Baharin, N.H.; Khairil Mokhtar, N.F.; Mohd Desa, M.N.; Gopalsamy, B.; Mohd Zaki, N.N.; Yuswan, M.H.; Muthanna, A.; Dzaraly, N.D.; Abbasiliasi, S.; Mohd Hashim, A.; et al. The Characteristics and Roles of Antimicrobial Peptides as Potential Treatment for Antibiotic-Resistant Pathogens: A Review. PeerJ 2021, 9, e12193. [Google Scholar] [CrossRef]
- Lam, S.J.; O’Brien-Simpson, N.M.; Pantarat, N.; Sulistio, A.; Wong, E.H.H.; Chen, Y.-Y.; Lenzo, J.C.; Holden, J.A.; Blencowe, A.; Reynolds, E.C.; et al. Combating Multidrug-Resistant Gram-Negative Bacteria with Structurally Nanoengineered Antimicrobial Peptide Polymers. Nat. Microbiol. 2016, 1, 16162. [Google Scholar] [CrossRef]
- Ram, C.V.S. Therapeutic Usefulness of a Novel Calcium Channel Blocker Azelnidipine in the Treatment of Hypertension: A Narrative Review. Cardiol. Ther. 2022, 11, 473–489. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.M.; Adenwalla, N.; Wiles, S.; Proft, T. Galleria mellonella Larvae as an Infection Model for Group A Streptococcus. Virulence 2013, 4, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Verdial, C.; Serrano, I.; Tavares, L.; Gil, S.; Oliveira, M. Mechanisms of Antibiotic and Biocide Resistance That Contribute to Pseudomonas aeruginosa Persistence in the Hospital Environment. Biomedicines 2023, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Huang, X.; Wang, Q.; Yao, D.; Lu, W. Virulence Factors of Pseudomonas aeruginosa and Antivirulence Strategies to Combat Its Drug Resistance. Front. Cell Infect. Microbiol. 2022, 12, 926758. [Google Scholar] [CrossRef]
- Desbois, A.P.; Coote, P.J. Utility of Greater Wax Moth Larva (Galleria mellonella) for Evaluating the Toxicity and Efficacy of New Antimicrobial Agents. Adv. Appl. Microbiol. 2012, 78, 25–53. [Google Scholar]
- Desbois, A.P.; Coote, P.J. Wax Moth Larva (Galleria mellonella): An In Vivo Model for Assessing the Efficacy of Antistaphylococcal Agents. J. Antimicrob. Chemother. 2011, 66, 1785–1790. [Google Scholar] [CrossRef]
- Gibreel, T.M.; Upton, M. Synthetic Epidermicin NI01 Can Protect Galleria mellonella Larvae from Infection with Staphylococcus aureus. J. Antimicrob. Chemother. 2013, 68, 2269–2273. [Google Scholar] [CrossRef]
- Mouritzen, M.V.; Andrea, A.; Qvist, K.; Poulsen, S.S.; Jenssen, H. Immunomodulatory Potential of Nisin A with Application in Wound Healing. Wound Repair. Regen. 2019, 27, 650–660. [Google Scholar] [CrossRef]
- Silva, F.P.D.; Fernandes, K.M.; Freitas, L.L.; Cascardo, R.S.; Bernardes, R.C.; Oliveira, L.L.; Martins, G.F.; Vanetti, M.C.D. Effects of Sub-Lethal Doses of Nisin on the Virulence of Salmonella enterica in Galleria mellonella Larvae. Res. Microbiol. 2021, 172, 103836. [Google Scholar] [CrossRef]
- Pereira, M.F.; Rossi, C.C.; da Silva, G.C.; Rosa, J.N.; Bazzolli, D.M.S. Galleria mellonella as an Infection Model: An in-Depth Look at Why It Works and Practical Considerations for Successful Application. Pathog. Dis. 2020, 78, ftaa056. [Google Scholar] [CrossRef]
- Tsai, C.J.; Loh, J.M.; Proft, T. Galleria mellonella Infection Models for the Study of Bacterial Diseases and for Antimicrobial Drug Testing. Virulence 2016, 7, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Serrano, I.; Verdial, C.; Tavares, L.; Oliveira, M. The Virtuous Galleria mellonella Model for Scientific Experimentation. Antibiotics 2023, 12, 505. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, J.C. Galleria mellonella as a Model Host for Human Pathogens: Recent Studies and New Perspectives. Virulence 2012, 3, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.M.; McArthur, J.D. Developing Galleria mellonella as a Model Host for Human Pathogens. Virulence 2013, 4, 350–353. [Google Scholar] [CrossRef]
- Jander, G.; Rahme, L.G.; Ausubel, F.M. Positive Correlation between Virulence of Pseudomonas aeruginosa Mutants in Mice and Insects. J. Bacteriol. 2000, 182, 3843–3845. [Google Scholar] [CrossRef]
- Tharmalingam, N.; Jayamani, E.; Rajamuthiah, R.; Castillo, D.; Fuchs, B.B.; Kelso, M.J.; Mylonakis, E. Activity of a Novel Protonophore against Methicillin-Resistant Staphylococcus aureus. Future Med. Chem. 2017, 9, 1401–1411. [Google Scholar] [CrossRef]
- Leive, L. Release of Lipopolysaccharide by EDTA Treatment of E. coli. Biochem. Biophys. Res. Commun. 1965, 21, 290–296. [Google Scholar] [CrossRef]
- Foster, T.J. Immune Evasion by Staphylococci. Nat. Rev. Microbiol. 2005, 3, 948–958. [Google Scholar] [CrossRef]
- Hauser, A.R. The Type III Secretion System of Pseudomonas aeruginosa: Infection by Injection. Nat. Rev. Microbiol. 2009, 7, 654–665. [Google Scholar] [CrossRef]
- Browne, N.; Heelan, M.; Kavanagh, K. An Analysis of the Structural and Functional Similarities of Insect Hemocytes and Mammalian Phagocytes. Virulence 2013, 4, 597–603. [Google Scholar] [CrossRef]
- Mendes, J.J.; Marques-Costa, A.; Vilela, C.; Neves, J.; Candeias, N.; Cavaco-Silva, P.; Melo-Cristino, J. Clinical and Bacteriological Survey of Diabetic Foot Infections in Lisbon. Diabetes Res. Clin. Pract. 2012, 95, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Mottola, C.; Semedo-Lemsaddek, T.; Mendes, J.J.; Melo-Cristino, J.; Tavares, L.; Cavaco-Silva, P.; Oliveira, M. Molecular Typing, Virulence Traits and Antimicrobial Resistance of Diabetic Foot Staphylococci. J. Biomed. Sci. 2016, 23, 33. [Google Scholar] [CrossRef] [PubMed]
- Mottola, C.; Mendes, J.J.; Cristino, J.M.; Cavaco-Silva, P.; Tavares, L.; Oliveira, M. Polymicrobial Biofilms by Diabetic Foot Clinical Isolates. Folia Microbiol. 2016, 61, 35–43. [Google Scholar] [CrossRef]
- Mottola, C.; Matias, C.S.; Mendes, J.J.; Melo-Cristino, J.; Tavares, L.; Cavaco-Silva, P.; Oliveira, M. Susceptibility Patterns of Staphylococcus aureus Biofilms in Diabetic Foot Infections. BMC Microbiol. 2016, 16, 119. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Mil-Homens, D.; Martins, M.; Barbosa, J.; Serafim, G.; Sarmento, M.J.; Pires, R.F.; Rodrigues, V.; Bonifácio, V.D.B.; Pinto, S.N. Carbapenem-Resistant Klebsiella pneumoniae Clinical Isolates: In Vivo Virulence Assessment in Galleria mellonella and Potential Therapeutics by Polycationic Oligoethyleneimine. Antibiotics 2021, 10, 56. [Google Scholar] [CrossRef]
- Mil-Homens, D.; Barahona, S.; Moreira, R.N.; Silva, I.J.; Pinto, S.N.; Fialho, A.M.; Arraiano, C.M. Stress Response Protein BolA Influences Fitness and Promotes Salmonella enterica Serovar Typhimurium Virulence. Appl. Environ. Microbiol. 2018, 84, e02850-17. [Google Scholar] [CrossRef]

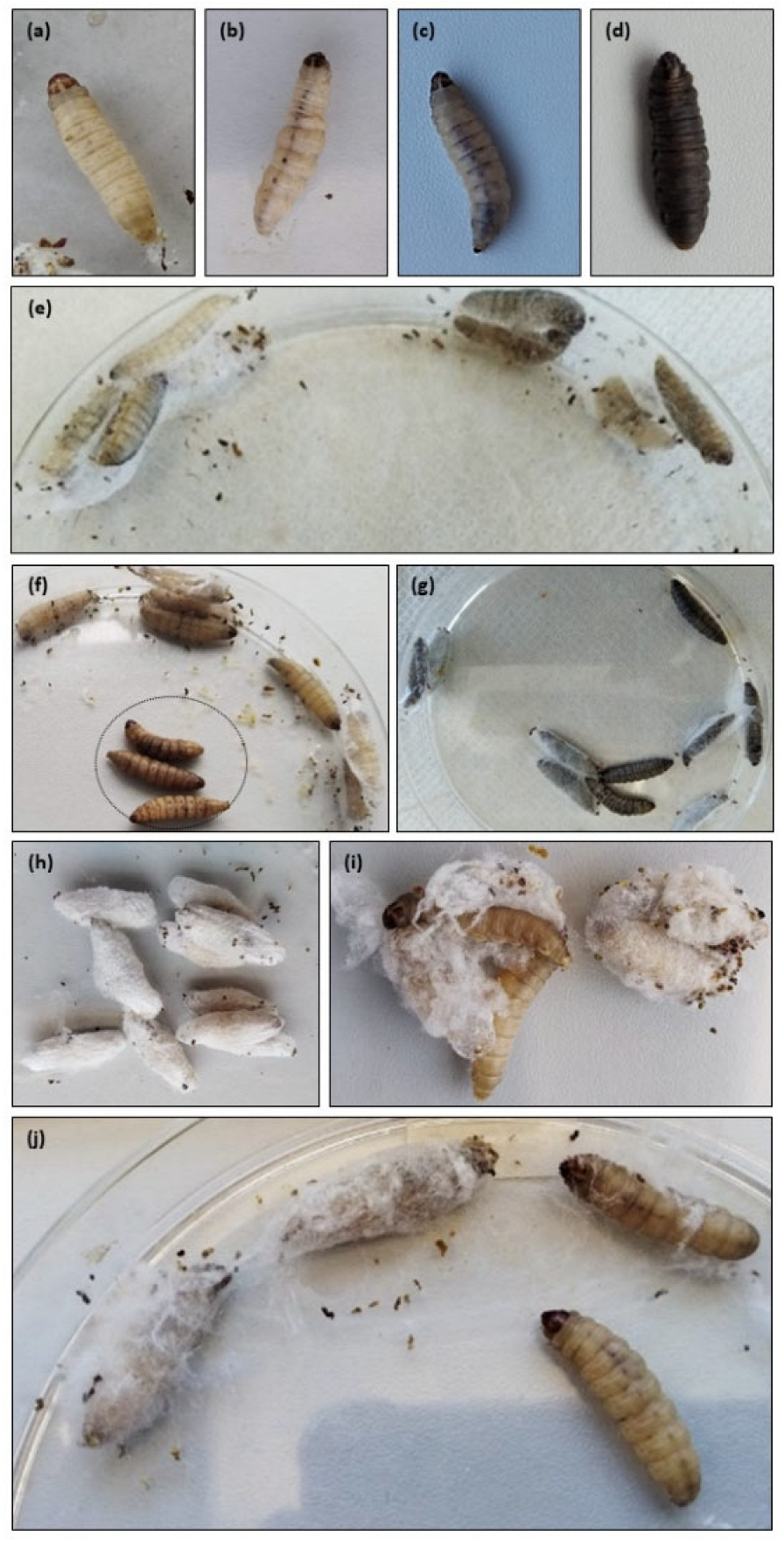
| Strains | MIC mg/mL (µM 1) | ||
|---|---|---|---|
| Nisin Z | PUREG4OEI48 | Amlodipine | |
| S. aureus Z25.2 | 0.01 (3) | 2.07 (40.5) | 0.04 (97.8) |
| P. aeruginosa Z25.1 | >0.4 2 (>120) | 0.52 (10.2) | 0.16 (391.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, I.; Mil-Homens, D.; Pires, R.F.; Bonifácio, V.D.B.; Guerreiro, J.F.; Cunha, E.; Costa, S.S.; Tavares, L.; Oliveira, M. In Vivo Antimicrobial Activity of Nisin Z Against S. aureus and Polyurea Pharmadendrimer PUREG4OEI48 Against P. aeruginosa from Diabetic Foot Infections. Antibiotics 2025, 14, 444. https://doi.org/10.3390/antibiotics14050444
Serrano I, Mil-Homens D, Pires RF, Bonifácio VDB, Guerreiro JF, Cunha E, Costa SS, Tavares L, Oliveira M. In Vivo Antimicrobial Activity of Nisin Z Against S. aureus and Polyurea Pharmadendrimer PUREG4OEI48 Against P. aeruginosa from Diabetic Foot Infections. Antibiotics. 2025; 14(5):444. https://doi.org/10.3390/antibiotics14050444
Chicago/Turabian StyleSerrano, Isa, Dalila Mil-Homens, Rita F. Pires, Vasco D. B. Bonifácio, Joana F. Guerreiro, Eva Cunha, Sofia S. Costa, Luís Tavares, and Manuela Oliveira. 2025. "In Vivo Antimicrobial Activity of Nisin Z Against S. aureus and Polyurea Pharmadendrimer PUREG4OEI48 Against P. aeruginosa from Diabetic Foot Infections" Antibiotics 14, no. 5: 444. https://doi.org/10.3390/antibiotics14050444
APA StyleSerrano, I., Mil-Homens, D., Pires, R. F., Bonifácio, V. D. B., Guerreiro, J. F., Cunha, E., Costa, S. S., Tavares, L., & Oliveira, M. (2025). In Vivo Antimicrobial Activity of Nisin Z Against S. aureus and Polyurea Pharmadendrimer PUREG4OEI48 Against P. aeruginosa from Diabetic Foot Infections. Antibiotics, 14(5), 444. https://doi.org/10.3390/antibiotics14050444









