Prevalence and Variability of Helicobacter pylori Clarithromycin Resistance Mutations in Pediatric Patients in Poland: A Genotypic Analysis Using the Bosphore Genotyping Kit
Abstract
1. Introduction
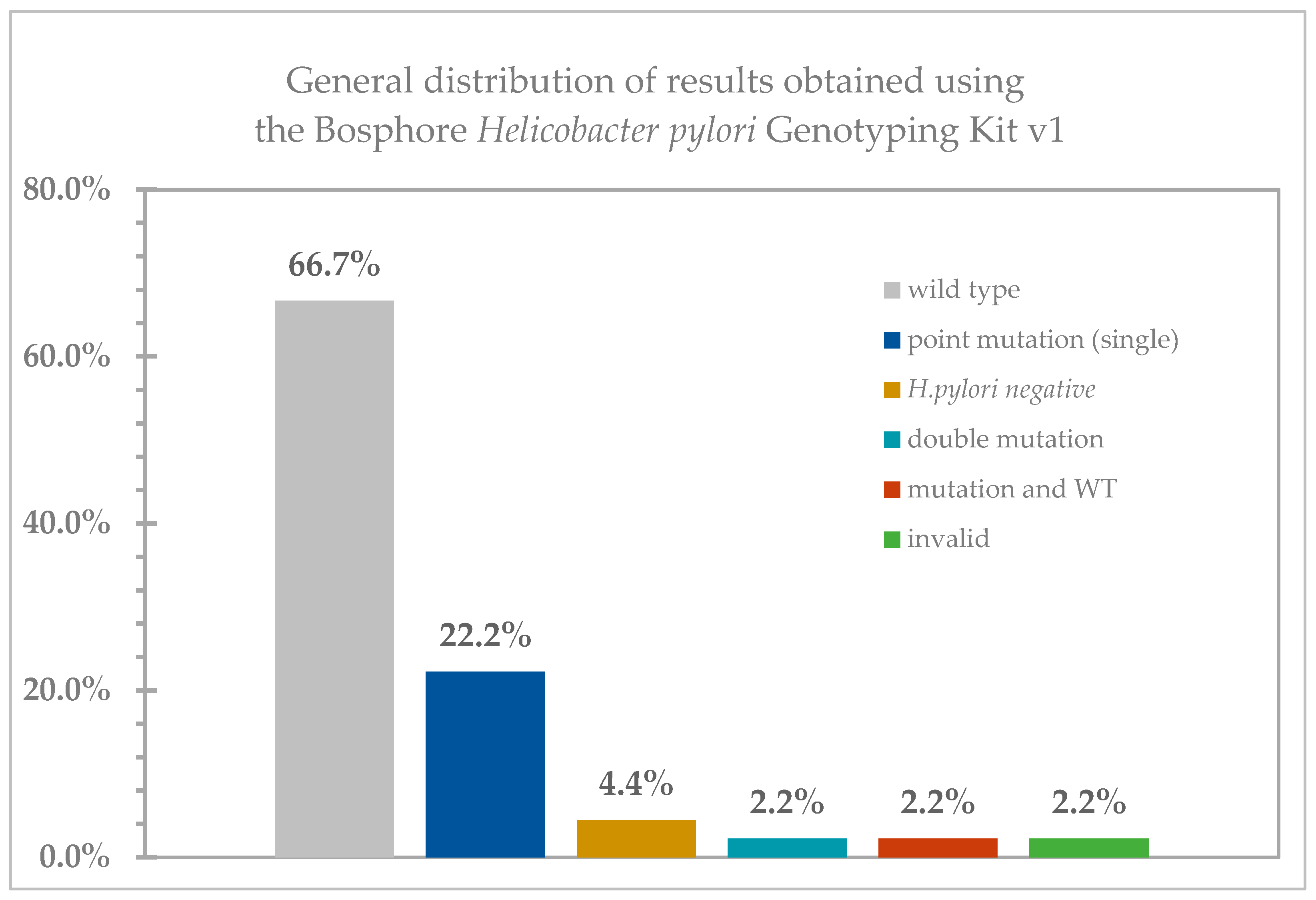
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. DNA Extraction
4.3. Bosphore Helicobacter pylori Genotyping Kit v1
4.4. Data Interpretation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A2142G | adenine to guanine point mutations at position 2142 |
| A2143G | adenine to guanine point mutations at position 2143 |
| Ct | cycle treshold |
| MDR | multidrug resistance |
| PBPs | penicillin-binding proteins |
| WT | wild-type |
References
- Roszczenko-Jasińska, P.; Wojtyś, M.I.; Jagusztyn-Krynicka, E.K. Helicobacter pylori Treatment in the Post-Antibiotics Era—Searching for New Drug Targets. Appl. Microbiol. Biotechnol. 2020, 104, 9891–9905. [Google Scholar] [CrossRef]
- De Brito, B.B.; Da Silva, F.A.F.; Soares, A.S.; Pereira, V.A.; Cordeiro Santos, M.L.; Sampaio, M.M.; Moreira Neves, P.H.; De Melo, F.F. Pathogenesis and Clinical Management of Helicobacter pylori Gastric Infection. World J. Gastroenterol. 2019, 25, 5578–5589. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter pylori Infection. Nat. Rev. Dis. Prim. 2023, 9, 19. [Google Scholar] [CrossRef]
- Tshibangu-Kabamba, E.; Yamaoka, Y. Helicobacter pylori Infection and Antibiotic Resistance—From Biology to Clinical Implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 613–629. [Google Scholar] [CrossRef]
- Wang, D.; Guo, Q.; Yuan, Y.; Gong, Y. The Antibiotic Resistance of Helicobacter pylori to Five Antibiotics and Influencing Factors in an Area of China with a High Risk of Gastric Cancer. BMC Microbiol. 2019, 19, 152. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, Y.; Lu, N.H. Novel and Effective Therapeutic Regimens for Helicobacter pylori in an Era of Increasing Antibiotic Resistance. Front. Cell Infect. Microbiol. 2017, 7, 168. [Google Scholar] [CrossRef]
- Puah, S.M.; Goh, K.L.; Ng, H.K.; Chua, K.H. Current Status of Helicobacter pylori Resistance to Clarithromycin and Levofloxacin in Malaysia-Findings from a Molecular Based Study. PeerJ 2021, 9, e11518. [Google Scholar] [CrossRef]
- Ziver-Sarp, T.; Yuksel-Mayda, P.; Saribas, S.; Demiryas, S.; Gareayaghi, N.; Ergin, S.; Tasci, I.; Ozbey, D.; Bal, K.; Erzin, Y.; et al. Point Mutations at GyrA and GyrB Genes of Levofloxacin Resistant Helicobacter pylori Strains and Dual Resistance with Clarithromycin. Clin. Lab. 2021, 67, 2369–2377. [Google Scholar] [CrossRef]
- Li, Y.; Lv, T.; He, C.; Wang, H.; Cram, D.S.; Zhou, L.; Zhang, J.; Jiang, W. Evaluation of Multiplex ARMS-PCR for Detection of Helicobacter pylori Mutations Conferring Resistance to Clarithromycin and Levofloxacin. Gut Pathog. 2020, 12, 35. [Google Scholar] [CrossRef]
- Abdollahi, H.; Savari, M.; Zahedi, M.J.; Moghadam, S.D.; Abasi, M.H. Detection of A2142C, A2142G, and A2143G Mutations in 23s RRNA Gene Conferring Resistance to Clarithromycin among Helicobacter pylori Isolates in Kerman, Iran. Iran. J. Med. Sci. 2011, 36, 104–110. [Google Scholar]
- Marques, B.; Donato, M.M.; Cardoso, O.; Luxo, C.; Martinho, A.; Almeida, N. Study of RdxA and FrxA Genes Mutations in Metronidazole-Resistant and -Susceptible Helicobacter pylori Clinical Isolates from the Central Region of Portugal. J. Glob. Antimicrob. Resist. 2019, 17, 300–304. [Google Scholar] [CrossRef]
- Qureshi, N.N.; Gallaher, B.; Schiller, N.L. Evolution of Amoxicillin Resistance of Helicobacter pylori In Vitro: Characterization of Resistance Mechanisms. Microb. Drug Resist. 2014, 20, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Haumaier, F.; Schneider-Fuchs, A.; Backert, S.; Vieth, M.; Sterlacci, W.; Wöhrl, B.M. Rapid Detection of Quinolone Resistance Mutations in GyrA of Helicobacter pylori by Real-Time PCR. Pathogens 2022, 11, 59. [Google Scholar] [CrossRef]
- Rimbara, E.; Noguchi, N.; Kawai, T.; Sasatsu, M. Fluoroquinolone Resistance in Helicobacter pylori: Role of Mutations at Position 87 and 91 of GyrA on the Level of Resistance and Identification of a Resistance Conferring Mutation in GyrB. Helicobacter 2012, 17, 36–42. [Google Scholar] [CrossRef]
- Miftahussurur, M.; Shrestha, P.K.; Subsomwong, P.; Sharma, R.P.; Yamaoka, Y. Emerging Helicobacter pylori Levofloxacin Resistance and Novel Genetic Mutation in Nepal. BMC Microbiol. 2016, 16, 256. [Google Scholar] [CrossRef]
- Argueta, E.A.; Ho, J.J.C.; Elfanagely, Y.; D’Agata, E.; Moss, S.F. Clinical Implication of Drug Resistance for H. pylori Management. Antibiotics 2022, 11, 1684. [Google Scholar] [CrossRef]
- Srisuphanunt, M.; Wilairatana, P.; Kooltheat, N.; Duangchan, T.; Katzenmeier, G.; Rose, J.B. Molecular Mechanisms of Antibiotic Resistance and Novel Treatment Strategies for Helicobacter pylori Infections. Trop. Med. Infect. Dis. 2023, 8, 163. [Google Scholar] [CrossRef]
- Seriki, A.T.; Smith, S.I.; Adeleye, A.I.; Fowora, M.A. Molecular Analysis of Low-Level Tetracycline Resistance in Clinical Isolates of Helicobacter pylori among Dyspeptic Patients in South West Nigeria. J. Glob. Antimicrob. Resist. 2018, 13, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Gerrits, M.M.; Berning, M.; Van Vliet, A.H.M.; Kuipers, E.J.; Kusters, J.G. Effects of 16S RRNA Gene Mutations on Tetracycline Resistance in Helicobacter pylori. Antimicrob. Agents Chemother. 2003, 47, 2984–2986. [Google Scholar] [CrossRef]
- Noh, J.H.; Ahn, J.Y.; Choi, J.; Park, Y.S.; Na, H.K.; Lee, J.H.; Jung, K.W.; Kim, D.H.; Choi, K.D.; Song, H.J.; et al. Real-Time Polymerase Chain Reaction for the Detection of Helicobacter pylori and Clarithromycin Resistance. Gut Liver 2023, 17, 375–381. [Google Scholar] [CrossRef]
- Eder, P. The Problem of Helicobacter pylori Antibiotic Resistance—Overview and Recent Insights. Lek. POZ 2016, 2, 171–174. [Google Scholar]
- Alarcón-Millán, J.; Bonilla-Delgado, J.; Fernández-Tilapa, G.; Nieto-Velázquez, N.G.; Sierra-Martínez, M.; Alvarado-Castro, V.M.; Cortés-Malagón, E.M. Helicobacter pylori Virulence Factors and Clarithromycin Resistance-Associated Mutations in Mexican Patients. Pathogens 2023, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Thung, I.; Aramin, H.; Vavinskaya, V.; Gupta, S.; Park, J.Y.; Crowe, S.E.; Valasek, M.A. Review Article: The Global Emergence of Helicobacter pylori Antibiotic Resistance. Aliment. Pharmacol. Ther. 2016, 43, 514–533. [Google Scholar] [CrossRef]
- Ribeiro, M.L.; Vitiello, L.; Miranda, M.C.; Benvengo, Y.H.; Godoy, A.P.; Mendonca, S.; Pedrazzoli, J. Mutations in the 23S RRNA Gene Are Associated with Clarithromycin Resistance in Helicobacter pylori Isolates in Brazil. Ann. Clin. Microbiol. Antimicrob. 2003, 2, 11. [Google Scholar] [CrossRef]
- Bińkowska, A.; Biernat, M.M.; Łaczmański, Ł.; Gościniak, G. Molecular Patterns of Resistance Among Helicobacter pylori Strains in South-Western Poland. Front. Microbiol. 2018, 9, 3154. [Google Scholar] [CrossRef]
- Gareayaghi, N.; Kocazeybek, B. Detection of A2143G, A2142C, and A2142G Point Mutations with Real-Time PCR in Stool Specimens from Children Infected with Helicobacter pylori. Diagnostics 2022, 12, 2119. [Google Scholar] [CrossRef]
- Albasha, A.M.; Elnosh, M.M.; Osman, E.H.; Zeinalabdin, D.M.; Fadl, A.A.M.; Ali, M.A.; Altayb, H.N. Helicobacter pylori 23S RRNA Gene A2142G, A2143G, T2182C, and C2195T Mutations Associated with Clarithromycin Resistance Detected in Sudanese Patients. BMC Microbiol. 2021, 21, 38. [Google Scholar] [CrossRef]
- Ghaith, D.; Elzahry, M.; Mostafa, G.; Mostafa, S.; Elsherif, R.; Ramzy, I. Mutations Affecting Domain V of the 23S RRNA Gene in Helicobacter pylori from Cairo, Egypt. J. Chemother. 2016, 28, 367–370. [Google Scholar] [CrossRef]
- Mégraud, F. H. pylori Antibiotic Resistance: Prevalence, Importance, and Advances in Testing. Gut 2004, 53, 1374–1384. [Google Scholar] [CrossRef]
- Kuo, Y.T.; Liou, J.M.; El-Omar, E.M.; Wu, J.Y.; Leow, A.H.R.; Goh, K.L.; Das, R.; Lu, H.; Lin, J.T.; Tu, Y.K.; et al. Primary Antibiotic Resistance in Helicobacter pylori in the Asia-Pacific Region: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 707–715. [Google Scholar] [CrossRef]
- Megraud, F. Epidemiology and Mechanism of Antibiotic Resistance in Helicobacter pylori. Gastroenterology 1998, 115, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Megraud, F.; Bruyndonckx, R.; Coenen, S.; Wittkop, L.; Huang, T.D.; Hoebeke, M.; Bénéjat, L.; Lehours, P.; Goossens, H.; Glupczynski, Y.; et al. Helicobacter pylori Resistance to Antibiotics in Europe in 2018 and Its Relationship to Antibiotic Consumption in the Community. Gut 2021, 70, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.M.; Chang, C.Y.; Chen, M.J.; Chen, C.C.; Fang, Y.J.; Lee, J.Y.; Wu, J.Y.; Luo, J.C.; Liou, T.C.; Chang, W.H.; et al. The Primary Resistance of Helicobacter pylori in Taiwan after the National Policy to Restrict Antibiotic Consumption and Its Relation to Virulence Factors-A Nationwide Study. PLoS ONE 2015, 10, e0124199. [Google Scholar] [CrossRef]
- Leeuwendaal, N.K.; Stanton, C.; O’toole, P.W.; Beresford, T.P. Fermented Foods, Health and the Gut Microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef]
- Seo, S.I.; Do, B.J.; Kang, J.G.; Kim, H.S.; Jang, M.K.; Kim, H.Y.; Shin, W.G. Helicobacter pylori Eradication According to Sequencing-Based 23S Ribosomal RNA Point Mutation Associated with Clarithromycin Resistance. J. Clin. Med. 2019, 9, 54. [Google Scholar] [CrossRef]
- Seo, J.W.; Park, J.Y.; Shin, T.S.; Kim, J.G. The Analysis of Virulence Factors and Antibiotic Resistance between Helicobacter pylori Strains Isolated from Gastric Antrum and Body. BMC Gastroenterol. 2019, 19, 140. [Google Scholar] [CrossRef]
- Krashias, G.; Bashiardes, S.; Potamitou, A.; Potamitis, G.S.; Christodoulou, C. Prevalence of Helicobacter pylori CagA and VacA Genes in Cypriot Patients. J. Infect. Dev. Ctries. 2013, 7, 642–650. [Google Scholar] [CrossRef]
- Tran, V.H.; Ha, T.M.T.; Le, P.T.Q.; Nguyen, V.N.; Phan, T.N.; Paglietti, B. Helicobacter pylori 23S RRNA Gene Mutations Associated with Clarithromycin Resistance in Chronic Gastritis in Vietnam. J. Infect. Dev. Ctries. 2018, 12, 526–532. [Google Scholar] [CrossRef]
- Raymond, J.; Lamarque, D.; Kalach, N.; Chaussade, S.; Burucoa, C. High Level of Antimicrobial Resistance in French Helicobacter pylori Isolates. Helicobacter 2010, 15, 21–27. [Google Scholar] [CrossRef]
- Oleastro, M.; Ménard, A.; Santos, A.; Lamouliatte, H.; Monteiro, L.; Barthélémy, P.; Mégraud, F. Real-Time PCR Assay for Rapid and Accurate Detection of Point Mutations Conferring Resistance to Clarithromycin in Helicobacter pylori. J. Clin. Microbiol. 2003, 41, 397–402. [Google Scholar] [CrossRef]
- Ma, Q.; Li, H.; Liao, J.; Cai, Z.; Zhang, B. Tailored Therapy for Helicobacter pylori Eradication: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 13, 908202. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, B.W.; Kim, J.I.; Chung, W.C.; Jung, S.W.; Bang, C.S.; Kim, G.H.; Jeon, S.W.; Joo, M.K.; Lee, S.H.; et al. Empirical Therapy Versus Tailored Therapy of Helicobacter pylori in Korea: Results of the K-CREATE Study. Helicobacter 2024, 29, e13126. [Google Scholar] [CrossRef] [PubMed]
- Rokkas, T.; Ekmektzoglou, K.; Graham, D.Y. Current Role of Tailored Therapy in Treating Helicobacter pylori Infections. A Systematic Review, Meta-Analysis and Critical Analysis. Helicobacter 2023, 28, e12936. [Google Scholar] [CrossRef] [PubMed]
- Helicobacter pylori—Anatolia Geneworks. Available online: https://www.anatoliageneworks.com/kits/microbiology/helicobacter-pylori-kits/ (accessed on 13 January 2025).

| Step | Temperature | Time | Repeats |
|---|---|---|---|
| Initial denaturation | 95°C | 14:30 min. | N/A |
| Denaturation | 97°C | 00:30 min. | 50 cycles |
| Annealing and synthesis | 63°C | 00:45 min. | |
| Hold | 22°C | 05:00 min. | N/A |
| Channel/Detection Mix No. | FAM | HEX (Internal Control) | Cy5 | Interpretation |
|---|---|---|---|---|
| 1 | + | − | − | Clarithromycin resistance mutation 2142 A->G |
| 1 | − | − | + | Wild type |
| 1 | − | − | − | Test must be repeated |
| 2 | + | − | − | Clarithromycin resistance mutation 2143 A->G |
| 2 | − | − | + | Double mutation (2142 A->G & 2143 A->G) |
| 2 | − | − | − | Test must be repeated |
| 3 | + | − | − | Double mutation (2142 A->C & 2143 A->G) |
| 3 | − | − | + | Clarithromycin resistance mutation 2142 A->C |
| 3 | − | − | − | Test must be repeated |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogiel, T.; Szaflarska-Popławska, A.; Krawczyk, A. Prevalence and Variability of Helicobacter pylori Clarithromycin Resistance Mutations in Pediatric Patients in Poland: A Genotypic Analysis Using the Bosphore Genotyping Kit. Antibiotics 2025, 14, 352. https://doi.org/10.3390/antibiotics14040352
Bogiel T, Szaflarska-Popławska A, Krawczyk A. Prevalence and Variability of Helicobacter pylori Clarithromycin Resistance Mutations in Pediatric Patients in Poland: A Genotypic Analysis Using the Bosphore Genotyping Kit. Antibiotics. 2025; 14(4):352. https://doi.org/10.3390/antibiotics14040352
Chicago/Turabian StyleBogiel, Tomasz, Anna Szaflarska-Popławska, and Agnieszka Krawczyk. 2025. "Prevalence and Variability of Helicobacter pylori Clarithromycin Resistance Mutations in Pediatric Patients in Poland: A Genotypic Analysis Using the Bosphore Genotyping Kit" Antibiotics 14, no. 4: 352. https://doi.org/10.3390/antibiotics14040352
APA StyleBogiel, T., Szaflarska-Popławska, A., & Krawczyk, A. (2025). Prevalence and Variability of Helicobacter pylori Clarithromycin Resistance Mutations in Pediatric Patients in Poland: A Genotypic Analysis Using the Bosphore Genotyping Kit. Antibiotics, 14(4), 352. https://doi.org/10.3390/antibiotics14040352






