Antibacterial Effects of Synthetic Plantaricins Against Staphylococcus aureus
Abstract
1. Introduction
2. Results
2.1. Antimicrobial Activities of Synthetic Plantaricins Against S. aureus ATCC 12692
2.2. Antibacterial Activity Effect of Synthetic Plantaricins on S. aureus ATCC 12692 Using Ra Man Spectroscopy
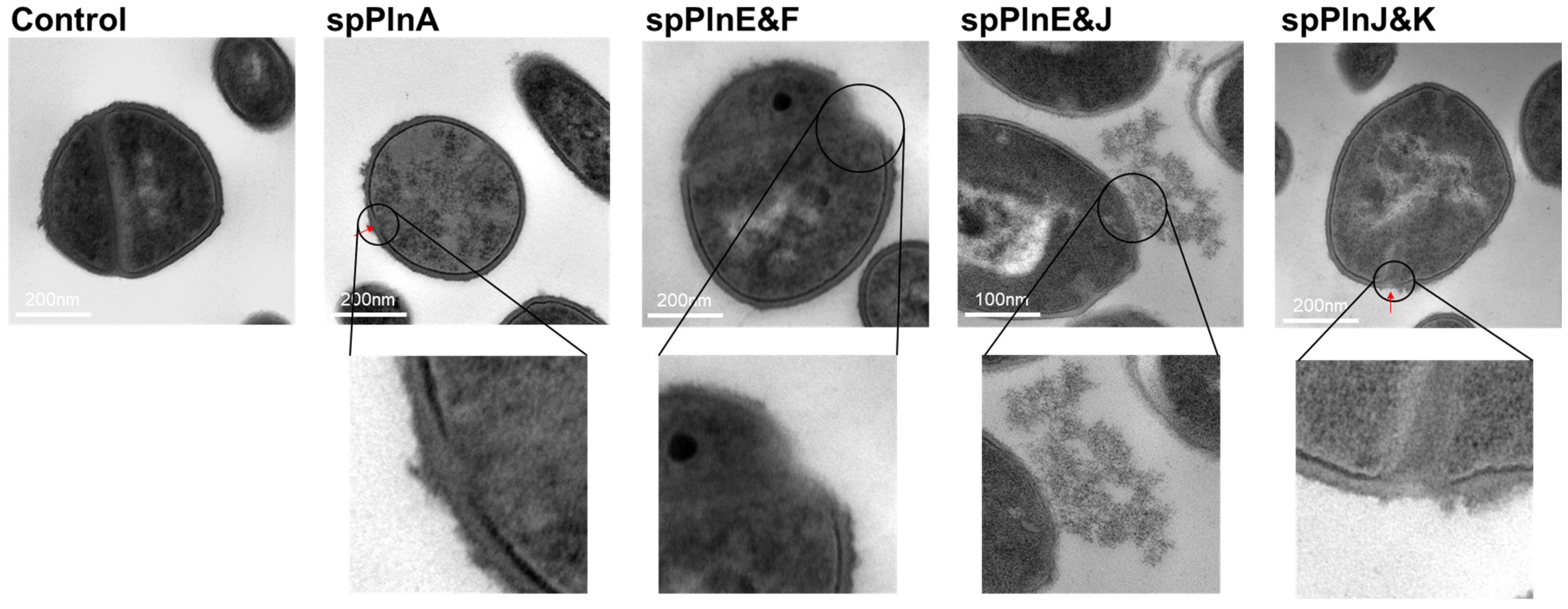
2.3. Synthetic Plantaricins Cause the Cell Lysis of S. aureus ATCC 12692
2.4. Effects of Heat, pH, and Enzymes on Antibacterial Activity
3. Discussion
4. Materials and Methods
4.1. Synthetic Plantaricins
4.2. Bacterial Minimum Inhibitory Concentration
4.3. Raman Spectrum
4.4. TEM Analysis
4.5. Effects of Temperature, pH, and Enzyme
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hennekinne, J.A.; De Buyser, M.L.; Dragacci, S. Staphylococcus aureus and its food poisoning toxins: Characterization and outbreak investigation. FEMS Microbiol. Rev. 2012, 36, 815–836. [Google Scholar] [CrossRef] [PubMed]
- Ogston, A. Classics in infectious diseases. “On abscesses”. Rev. Infect. Dis. 1984, 6, 122–128. [Google Scholar] [CrossRef]
- Gutierrez, D.; Delgado, S.; Vazquez-Sanchez, D.; Martinez, B.; Cabo, M.L.; Rodriguez, A.; Herrera, J.J.; Garcia, P. Incidence of Staphylococcus aureus and analysis of associated bacterial communities on food industry surfaces. Appl. Environ. Microbiol. 2012, 78, 8547–8554. [Google Scholar] [CrossRef] [PubMed]
- Kadariya, J.; Smith, T.C.; Thapaliya, D. Staphylococcus aureus and staphylococcal food-borne disease: An ongoing challenge in public health. BioMed Res. Int. 2014, 2014, 827965. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.S.; Da, F.; Liu, R.; Li, Y.; Wang, Z.; Zhang, H.; Zhou, H.; Chen, W. Contribution of Staphylococcal Enterotoxin B to Staphylococcus aureus Systemic Infection. J. Infect. Dis. 2021, 223, 1766–1775. [Google Scholar] [CrossRef]
- Regenthal, P.; Hansen, J.S.; Andre, I.; Smith, L.; Johnson, M.; Brown, R.; Taylor, D. Thermal stability and structural changes in bacterial toxins responsible for food poisoning. PLoS ONE 2017, 12, e0172445. [Google Scholar]
- Fisher, E.L.; Otto, M.; Cheung, G.Y.C. Basis of Virulence in Enterotoxin-Mediated Staphylococcal Food Poisoning. Front. Microbiol. 2018, 9, 436. [Google Scholar] [CrossRef]
- Galie, S.; Garcia-Gutierrez, C.; Miguelez, E.M.; Vazquez, F.; Perez, B.; Martinez, A.; Lopez, R. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Miao, J.; Lin, S.; Soteyome, T.; Peters, B.M.; Li, Y.; Chen, H.; Su, J.; Li, L.; Li, B.; Xu, Z.; et al. Biofilm Formation of Staphylococcus aureus under Food Heat Processing Conditions: First Report on CML Production within Biofilm. Sci. Rep. 2019, 9, 1312. [Google Scholar] [CrossRef]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- DeLeo, F.R.; Diep, B.A.; Otto, M. Host defense and pathogenesis in Staphylococcus aureus infections. Infect. Dis. Clin. N. Am. 2009, 23, 17–34. [Google Scholar] [CrossRef]
- Zecconi, A.; Scali, F. Staphylococcus aureus virulence factors in evasion from innate immune defenses in human and animal diseases. Immunol. Lett. 2013, 150, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.W.; Cho, H.; Lee, H.; Li, C.; Garza, J.; Fried, M.; Bae, T. Identification of the P3 promoter and distinct roles of the two promoters of the SaeRS two-component system in Staphylococcus aureus. J. Bacteriol. 2011, 193, 4672–4684. [Google Scholar] [CrossRef]
- Jeong, D.W.; Cho, H.; Jones, M.B.; Shatzkes, K.; Liu, Q.; Peterson, S.N.; Bae, T. The auxiliary protein complex SaePQ activates the phosphatase activity of sensor kinase SaeS in the SaeRS two-component system of Staphylococcus aureus. Mol. Microbiol. 2012, 86, 331–348. [Google Scholar] [CrossRef]
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef]
- Piewngam, P.; Otto, M. Staphylococcus aureus colonisation and strategies for decolonisation. Lancet Microbe 2024, 5, e606–e618. [Google Scholar] [CrossRef] [PubMed]
- Howden, B.P.; Giulieri, S.G.; Wong Fok Lung, T.; Baines, S.L.; Sharkey, L.K.; Lee, J.Y.H.; Hachani, A.; Monk, I.R.; Stinear, T.P. Staphylococcus aureus host interactions and adaptation. Nat. Rev. Microbiol. 2023, 21, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef]
- Kumariya, R.; Garsa, A.K.; Rajput, Y.S.; Sood, S.K.; Akhtar, N.; Patel, S. Bacteriocins: Classification, synthesis, mechanism of action and resistance development in food spoilage causing bacteria. Microb. Pathog. 2019, 128, 171–177. [Google Scholar] [CrossRef]
- Umu, O.C.; Bauerl, C.; Oostindjer, M.; Pope, P.B.; Hernández, P.E.; Pérez-Martínez, G.; Diep, D.B. The Potential of Class II Bacteriocins to Modify Gut Microbiota to Improve Host Health. PLoS ONE 2016, 11, e0164036. [Google Scholar] [CrossRef]
- Gradisteanu Pircalabioru, G.; Popa, L.I.; Marutescu, L.; Gheorghe, I.; Popa, M.; Czobor Barbu, I.; Cristescu, R.; Chifiriuc, M.-C. Bacteriocins in the era of antibiotic resistance: Rising to the challenge. Pharmaceutics 2021, 13, 196. [Google Scholar] [CrossRef]
- Mills, S.; Serrano, L.M.; Griffin, C.; O’Connor, P.M.; Schaad, G.; Bruining, C.; Hill, C.; Ross, R.P.; Meijer, W.C. Inhibitory activity of Lactobacillus plantarum LMG P-26358 against Listeria innocua when used as an adjunct starter in the manufacture of cheese. Microb. Cell Factories 2011, 10 (Suppl. 1), S7. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef]
- Settanni, L.; Corsetti, A. Application of bacteriocins in vegetable food biopreservation. Int. J. Food Microbiol. 2008, 121, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to Qualify Microorganisms as “Probiotic” in Foods and Dietary Supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef]
- Ammor, M.S.; Mayo, B. Selection criteria for lactic acid bacteria to be used as functional starter cultures in dry sausage production: An update. Meat Sci. 2007, 76, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Zendo, T.; Sugimoto, S.; Iwase, T.; Tajima, A.; Yamada, S.; Sonomoto, K.; Mizunoe, Y. Effects of bacteriocins on methicillin-resistant Staphylococcus aureus biofilm. Antimicrob. Agents Chemother. 2013, 57, 5572–5579. [Google Scholar] [CrossRef]
- Kranjec, C.; Ovchinnikov, K.V.; Gronseth TEbineshan, K.; Srikantam, A.; Diep, D.B. A bacteriocin-based antimicrobial formulation to effectively disrupt the cell viability of methicillin-resistant Staphylococcus aureus (MRSA) biofilms. Npj Biofilms Microbiomes 2020, 6, 58. [Google Scholar] [CrossRef]
- Foulquie Moreno, M.R.; Sarantinopoulos, P.; Tsakalidou, E.; De Vuyst, L. The role and application of enterococci in food and health. Int. J. Food Microbiol. 2006, 106, 1–24. [Google Scholar] [CrossRef]
- Heo, S.; Kim, J.-H.; Kwak, M.-S.; Jeong, D.-W.; Sung, M.-H. Complete genome sequence of Lactiplantibacillus plantarum KM2 from low-temperature aging beef. Korean J. Microbiol. 2021, 57, 303–306. [Google Scholar]
- Oh, S.-E.; Heo, S.; Lee, G.; Kim, J.; Park, S.; Jeong, D.-W. Synthetic plantaricins show significantly enhanced antibacterial activity against Flavobacterium sp. Food Biosci. 2024, 62, 105285. [Google Scholar] [CrossRef]
- Ho, C.S.; Jean, N.; Hogan, C.A.; Blackmon, L.; Jeffrey, S.S.; Holodniy, M.; Banaei, N.; Saleh, A.A.E.; Ermon, S.; Dionne, J. Rapid identification of pathogenic bacteria using Raman spectroscopy and deep learning. Nat. Commun. 2019, 10, 4927. [Google Scholar] [CrossRef]
- Liu, F.; Wu, T.; Tian, A.; He, C.; Bi, X.; Ye, J. Intracellular metabolic profiling of drug resistant cells by surface enhanced Raman scattering. Anal. Chim. Acta 2023, 1279, 341809. [Google Scholar] [CrossRef]
- Lin, L.; Qu, F.; Nie, P.; Zhang, H.; Chu, B.; He, Y. Rapid and Quantitative Determination of Sildenafil in Cocktail Based on Surface Enhanced Raman Spectroscopy. Molecules 2019, 24, 1790. [Google Scholar] [CrossRef]
- El Khalfi, A.I.; Ech-chamikh, E.M.; Ijdiyaou, Y.; Azizan, M.; Essafti, A.; Nkhaili, L.; Outzourhit, A. Infrared and Raman study of amorphous silicon carbide thin films deposited by radiofrequency cosputtering. Spectrosc. Lett. 2014, 47, 392–396. [Google Scholar] [CrossRef]
- Notingher, I. Raman spectroscopy cell-based biosensors. Sensors 2007, 7, 1343–1358. [Google Scholar] [CrossRef]
- Nergui, N.; Chen, M.J.; Wang, J.K.; Wang, Y.L.; Hsing, C.R.; Wei, C.M.; Takahashi, K. Dependence of adenine raman spectrum on excitation laser wavelength: Comparison between experiment and theoretical simulations. J. Phys. Chem. A 2016, 120, 8114–8122. [Google Scholar] [CrossRef] [PubMed]
- Schuster, K.C.; Urlaub, E.; Gapes, J.R. Single-cell analysis of bacteria by Raman microscopy: Spectral information on the chemical composition of cells and on the heterogeneity in a culture. J. Microbiol. Methods 2000, 42, 29–38. [Google Scholar] [CrossRef]
- Beraza-Millor, M.; Rodríguez-Castejón, J.; Miranda, J.; del Pozo-Rodríguez, A.; Rodríguez-Gascón, A.; Solinís, M.Á. Novel golden lipid nanoparticles with small interference ribonucleic acid for substrate reduction therapy in Fabry disease. Pharmaceutics 2023, 15, 1936. [Google Scholar] [CrossRef]
- Singh, R.K.; Bhriguvansh, P.; Asthana, B.P.; Verma, A.L. Raman study of vibrational dephasing in hydrogen-bonded binary and ternary complexes of C6H5Cl and methanol. Chem. Phys. Lett. 1998, 296, 611–618. [Google Scholar] [CrossRef]
- Luo, H.; Liu, J.; He, X.; Li, J. Low-Temperature Polymorphic Transformation of β-Lactam Antibiotics. Crystals 2019, 9, 460. [Google Scholar] [CrossRef]
- Kalp, M.; Carey, P.R. Carbapenems and SHV-1 β-lactamase form different acyl-enzyme populations in crystals and solution. Biochemistry 2008, 47, 11830–11837. [Google Scholar] [CrossRef]
- de Siqueira e Oliveira, F.S.; da Silva, A.M.; Pacheco, M.T.T.; Giana, H.E.; Silveira, L. Biochemical characterization of pathogenic bacterial species using Raman spectroscopy and discrimination model based on selected spectral features. Lasers Med. Sci. 2021, 36, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Westerhuis, J.A.; Hoefsloot, H.C.; Smit, S.; Vis, D.J.; Smilde, A.K.; van Velzen, E.J.; van Duijnhoven, J.P.M.; van Dorsten, F.A. Assessment of PLSDA cross validation. Metabolomics 2008, 4, 81–89. [Google Scholar] [CrossRef]
- Chambers, H.F.; Deleo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic Pollution in the Environment: From Microbial Ecology to Public Policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef]
- Foster, T.J. Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol. Rev. 2017, 41, 430–449. [Google Scholar] [CrossRef]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef]
- Galvez, A.; Abriouel, H.; Lopez, R.L.; Ben Omar, N. Bacteriocin-based strategies for food biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70. [Google Scholar] [CrossRef]
- Rea, M.C.; Dobson, A.; O’Sullivan, O.; Crispie, F.; Fouhy, F.; Cotter, P.D.; Shanahan, F.; Kiely, B.; Hill, C.; Ross, R.P. Effect of broad- and narrow-spectrum antimicrobials on Clostridium difficile and microbial diversity in a model of the distal colon. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4639–4644. [Google Scholar] [CrossRef]
- Hassan, M.; Kjos, M.; Nes, I.F.; Diep, D.B.; Lotfipour, F. Natural antimicrobial peptides from bacteria: Characteristics and potential applications to fight against antibiotic resistance. J. Appl. Microbiol. 2012, 113, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.H.; Zendo, T.; Sonomoto, K. Novel bacteriocins from lactic acid bacteria (LAB): Various structures and applications. Microb. Cell Factories 2014, 13 (Suppl. 1), S3. [Google Scholar] [CrossRef] [PubMed]
- Deegan, L.H.; Cotter, P.D.; Hill, C.; Ross, P. Bacteriocins: Biological tools for bio-preservation and shelf-life extension. Int. Dairy J. 2006, 16, 1058–1071. [Google Scholar] [CrossRef]
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 2001, 71, 1–20. [Google Scholar] [CrossRef]
- Yadav, M.K.; Baldia, A.; Tiwari, S.K. Plantaricin LD1 inhibits the growth and biofilm formation of Staphylococcus aureus in Milk. J. Explor. Res. Pharmacol. 2024, 9, 1–7. [Google Scholar] [CrossRef]
- Musa, A.; Wiman, E.; Selegård, R.; Aili, D.; Bengtsson, T.; Khalaf, H. Plantaricin NC8 αβ prevents Staphylococcus aureus-mediated cytotoxicity and inflammatory responses of human keratinocytes. Sci. Rep. 2021, 11, 12514. [Google Scholar] [CrossRef]
- Bengtsson, T.; Selegård, R.; Musa, A.; Hultenby, K.; Utterström, J.; Sivlér, P.; Skog, M.; Nayeri, F.; Hellmark, B.; Söderquist, B.; et al. Plantaricin NC8 αβ exerts potent antimicrobial activity against Staphylococcus spp. and enhances the effects of antibiotics. Sci. Rep. 2020, 10, 3580. [Google Scholar]
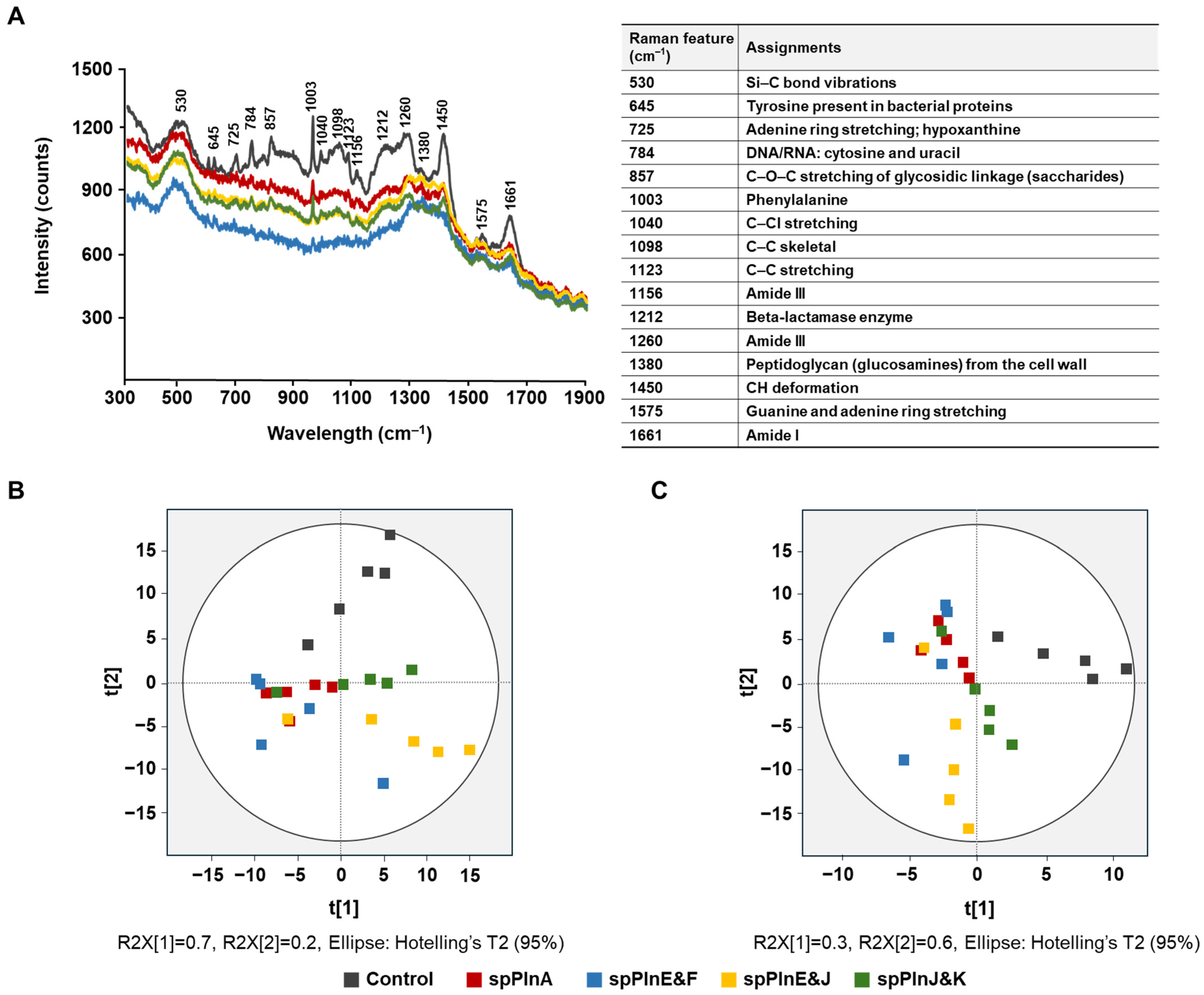
| spPlnA | spPlnE&F | spPlnE&J | spPlnJ&K | |
|---|---|---|---|---|
| Effects of temperature | ||||
| 20 °C | 11.3 ± 1.2 a | 11.0 ± 0.0 a | 17.0 ± 1.7 b | 11.3 ± 0.6 a |
| 30 °C | 11.0 ± 1.0 a | 11.7 ± 0.6 a | 13.7 ± 1.2 b | 9.0 ± 1.0 c |
| 40 °C | 8.7 ± 0.6 a | 11.0 ± 0.0 b | 10.3 ± 0.6 b | 7.3 ± 0.6 c |
| pH stability | ||||
| pH4 | 7.0 ± 1.7 a | 10.0 ± 0.0 b | 10.7 ± 0.6 b | 7.7 ± 0.6 a |
| pH5 | 7.0 ± 0.0 a | 10.0 ± 0.0 b | 13.0 ± 0.0 c | 9.0 ± 0.0 b |
| pH6 | 8.7 ± 0.6 a | 12.0 ± 1.0 b | 14.3 ± 0.6 c | 11.3 ± 1.5 b |
| pH7 | 10.7 ± 0.6 a | 14.0 ± 0.0 a | 18.7 ± 1.2 a | 14.7 ± 0.6 b |
| Effects of enzyme | ||||
| α–Amylase | 11.3 ± 1.5 a | 12.7 ± 1.2 a | 11.0 ± 0.0 a | 12.7 ± 0.6 a |
| Proteinase K | − | − | − | − |
| Lysozyme | 7.3 ± 1.2 a | 9.3 ± 0.6 a | 13.0 ± 1.7 b | 1.7 ± 0.6 c |
| Heat w/o enzyme | 11.4 ± 0.5 a | 12.0 ± 0.5 a | 11.0 ± 0.0 a | 11.7 ± 1.0 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.-E.; Heo, S.; Lee, G.; Kim, J.; Kwak, M.-S.; Jeong, D.-W. Antibacterial Effects of Synthetic Plantaricins Against Staphylococcus aureus. Antibiotics 2025, 14, 311. https://doi.org/10.3390/antibiotics14030311
Oh S-E, Heo S, Lee G, Kim J, Kwak M-S, Jeong D-W. Antibacterial Effects of Synthetic Plantaricins Against Staphylococcus aureus. Antibiotics. 2025; 14(3):311. https://doi.org/10.3390/antibiotics14030311
Chicago/Turabian StyleOh, Seung-Eun, Sojeong Heo, Gawon Lee, Jina Kim, Mi-Sun Kwak, and Do-Won Jeong. 2025. "Antibacterial Effects of Synthetic Plantaricins Against Staphylococcus aureus" Antibiotics 14, no. 3: 311. https://doi.org/10.3390/antibiotics14030311
APA StyleOh, S.-E., Heo, S., Lee, G., Kim, J., Kwak, M.-S., & Jeong, D.-W. (2025). Antibacterial Effects of Synthetic Plantaricins Against Staphylococcus aureus. Antibiotics, 14(3), 311. https://doi.org/10.3390/antibiotics14030311







