Multidrug Resistance, Biofilm-Forming Ability, and Molecular Characterization of Vibrio Species Isolated from Foods in Thailand
Abstract
1. Introduction
2. Results
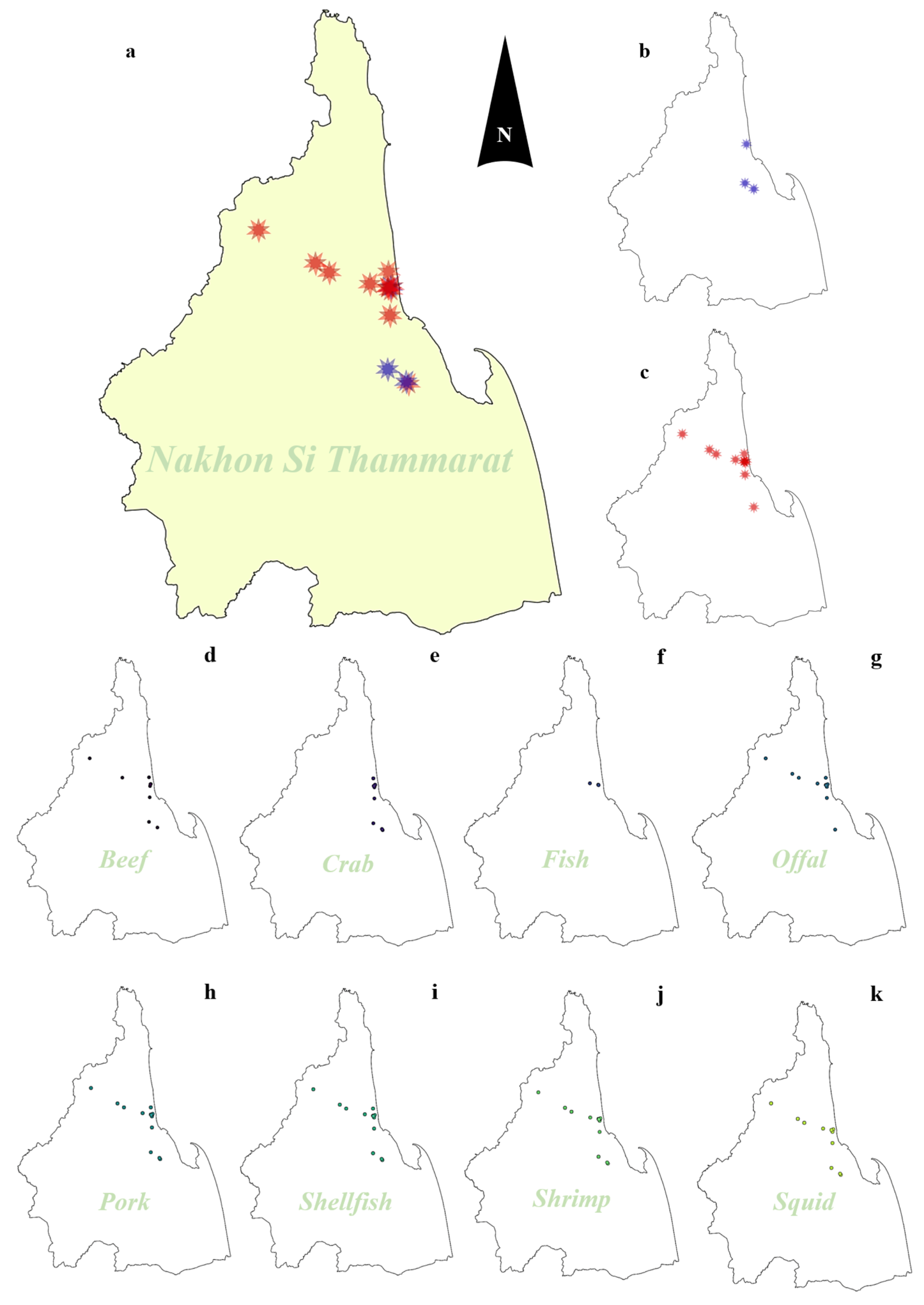
2.1. Prevalence of Vibrio Species Isolated from Food Samples
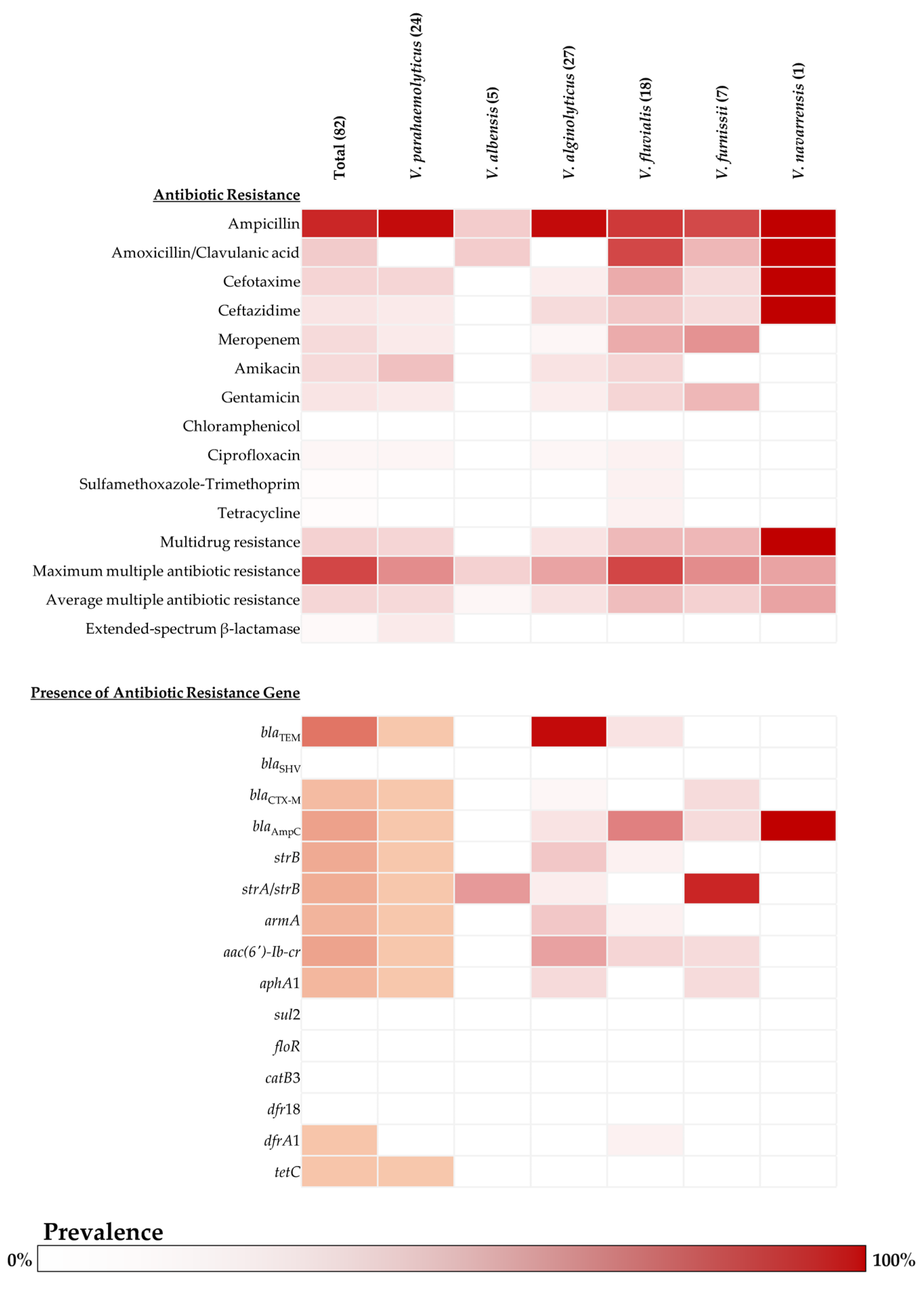
2.2. Antibiotic Susceptibility and Antibiotic Resistance Genes of Vibrio Isolates
2.3. Determination of ESBL Producer and ESBL-Related Genes in Vibrio Isolates
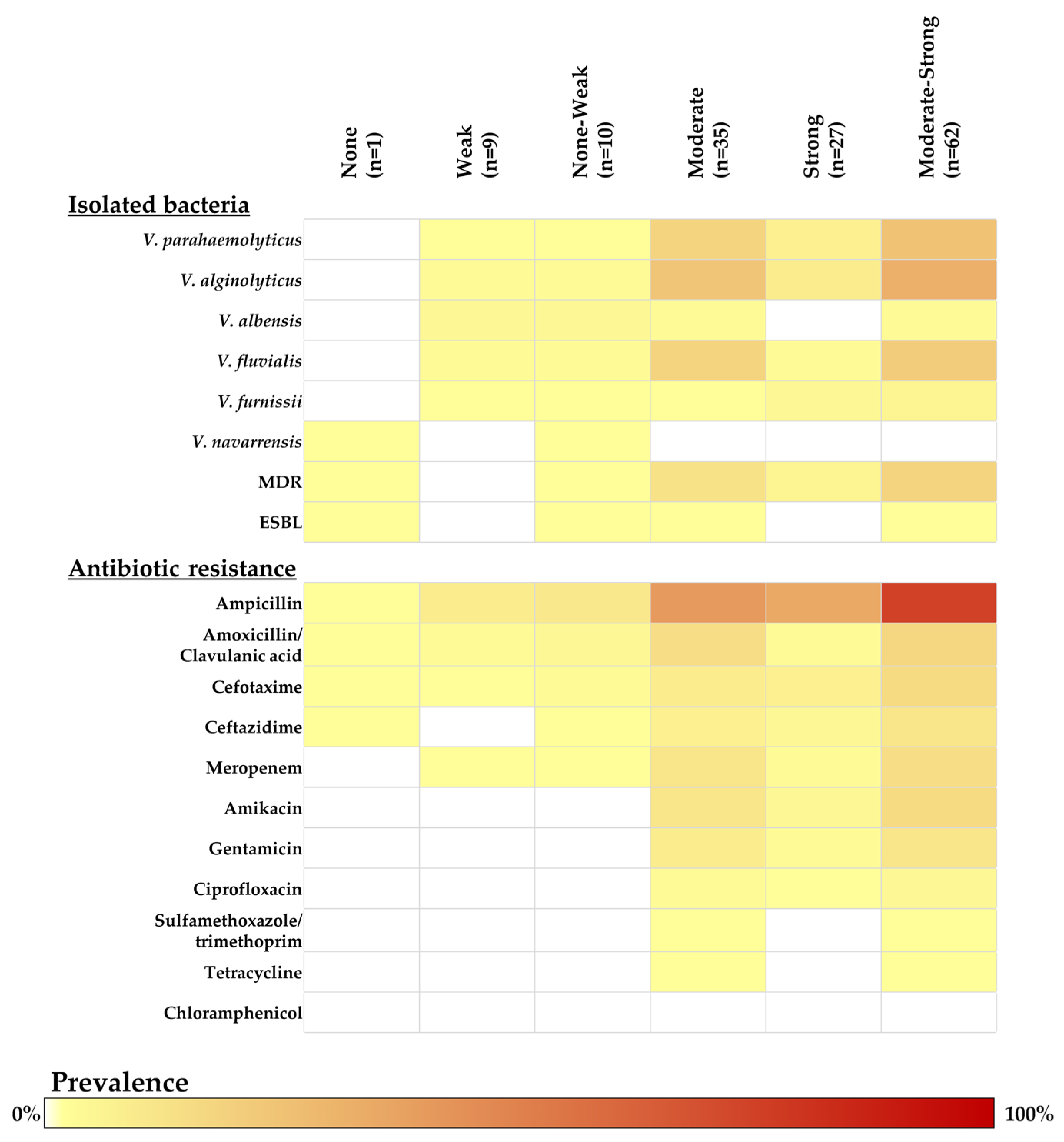
2.4. Biofilm-Forming Ability Determination in Vibrio Isolates
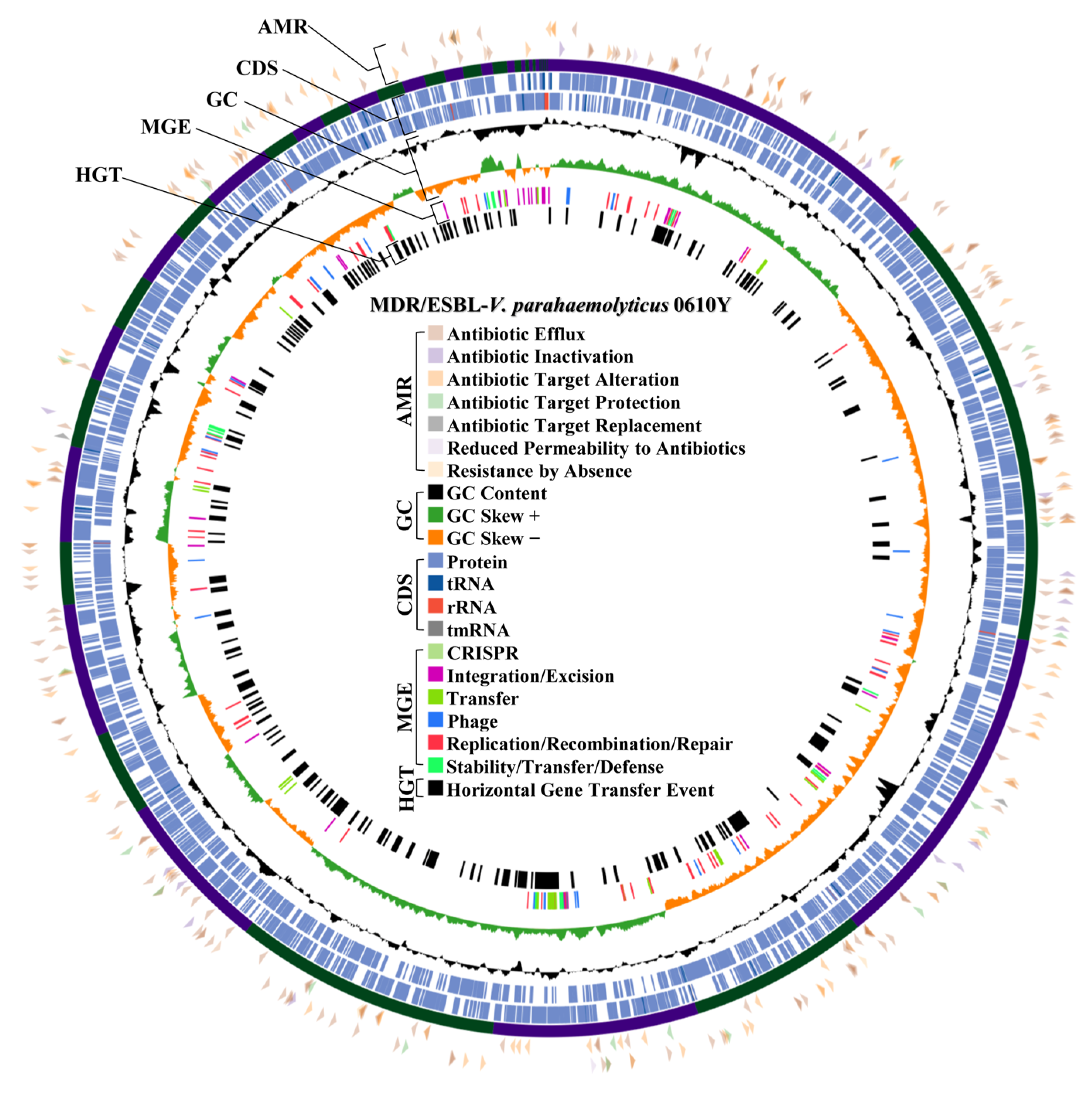
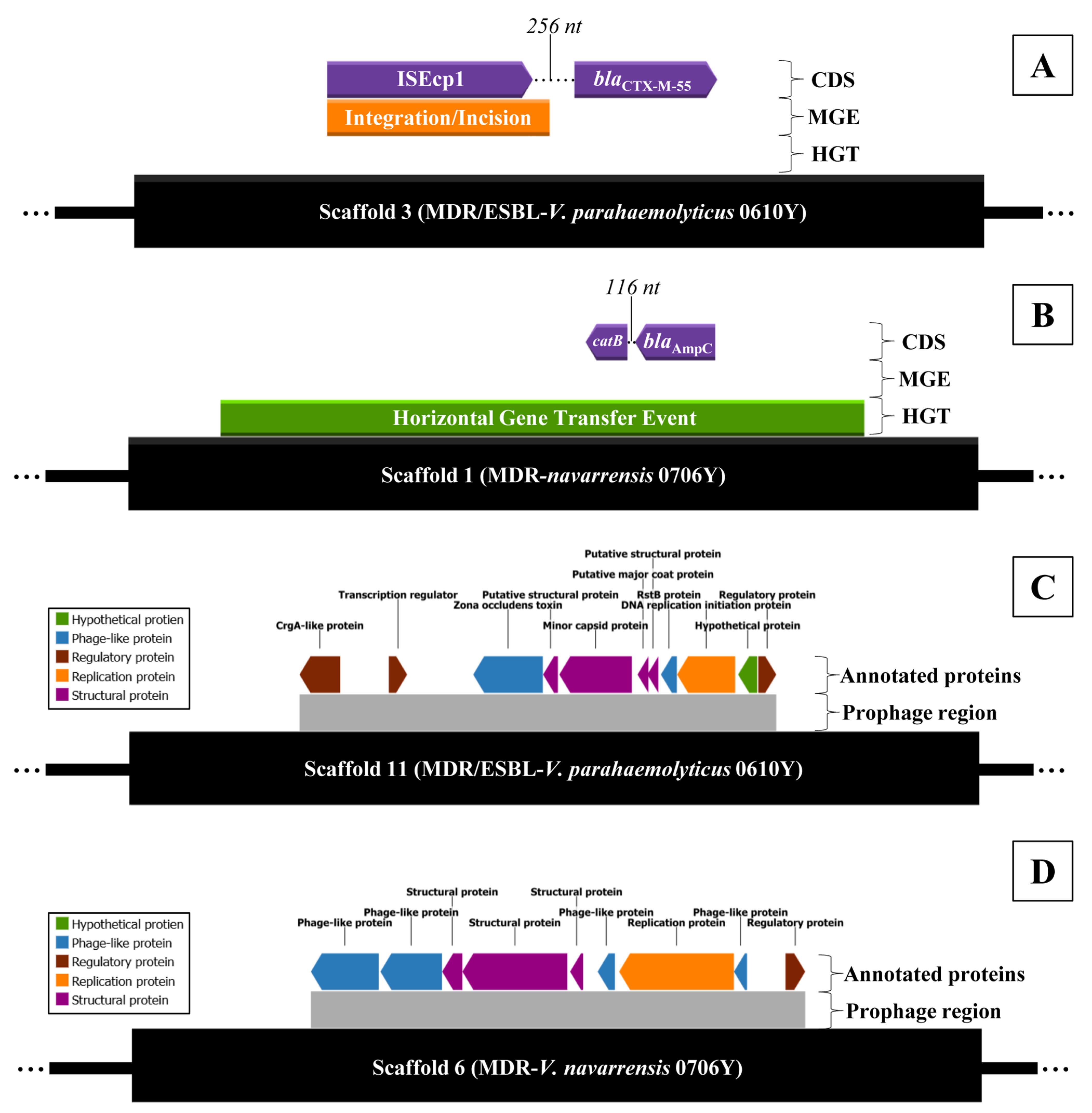
2.5. Antibiotic Resistance Mechanism and Prophage Region Analysis from WGS of MDR/ESBL-Producing Vibrio parahaemolyticus 0610Y and MDR-Vibrio navarrensis 0706Y
3. Discussion
4. Materials and Methods
4.1. Sample Size and Sampling Techniques
4.2. Sample Collection
4.3. Vibrio Isolation and Confirmation
4.4. Antibiotic Susceptibility Testing
4.5. Determination of ESBL by Phenotypic Confirmatory Disc Diffusion Test (PCDDT)
4.6. Biofilm-Forming Ability Determination
4.7. Molecular Detection of Antibiotic Resistance Gene
4.8. Whole Genome Sequencing (WGS)
4.9. Bioinformatic Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Souza Valente, C.; Wan, A.H.L. Vibrio and Major Commercially Important Vibriosis Diseases in Decapod Crustaceans. J. Invertebr. Pathol. 2021, 181, 107527. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Contreras, A.K.; Quiñones-Ramírez, E.I.; Vázquez-Salinas, C. Prevalence, Detection of Virulence Genes and Antimicrobial Susceptibility of Pathogen Vibrio Species Isolated from Different Types of Seafood Samples at “La Nueva Viga” Market in Mexico City. Antonie Van Leeuwenhoek 2021, 114, 1417–1429. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.-Y.; Zhu, X.-K.; Hu, R.-G.; Qi, Z.-Z.; Sun, W.-C.; Hao, Z.-P.; Cong, W.; Kang, Y.-H. A Systematic Review, Meta-Analysis and Meta-Regression of the Global Prevalence of Foodborne Vibrio spp. Infection in Fishes: A Persistent Public Health Concern. Mar. Pollut. Bull. 2023, 187, 114521. [Google Scholar] [CrossRef] [PubMed]
- Canellas, A.L.B.; Lopes, I.R.; Mello, M.P.; Paranhos, R.; de Oliveira, B.F.R.; Laport, M.S. Vibrio Species in an Urban Tropical Estuary: Antimicrobial Susceptibility, Interaction with Environmental Parameters, and Possible Public Health Outcomes. Microorganisms 2021, 9, 1007. [Google Scholar] [CrossRef]
- Sheng, L.; Wang, L. The Microbial Safety of Fish and Fish Products: Recent Advances in Understanding Its Significance, Contamination Sources, and Control Strategies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 738–786. [Google Scholar] [CrossRef]
- Dutta, D.; Kaushik, A.; Kumar, D.; Bag, S. Foodborne Pathogenic Vibrios: Antimicrobial Resistance. Front. Microbiol. 2021, 12, 638331. [Google Scholar] [CrossRef]
- Jeong, H.-W.; Kim, J.-A.; Jeon, S.-J.; Choi, S.-S.; Kim, M.-K.; Yi, H.-J.; Cho, S.-J.; Kim, I.-Y.; Chon, J.-W.; Kim, D.-H.; et al. Prevalence, Antibiotic-Resistance, and Virulence Characteristics of Vibrio parahaemolyticus in Restaurant Fish Tanks in Seoul, South Korea. Foodborne Pathog. Dis. 2020, 17, 209–214. [Google Scholar] [CrossRef]
- Xie, T.; Yu, Q.; Tang, X.; Zhao, J.; He, X. Prevalence, Antibiotic Susceptibility and Characterization of Vibrio parahaemolyticus Isolates in China. FEMS Microbiol. Lett. 2020, 367, fnaa136. [Google Scholar] [CrossRef]
- Nakayama, T.; Yamaguchi, T.; Jinnai, M.; Kumeda, Y.; Hase, A. ESBL-Producing Vibrio vulnificus and V. alginolyticus Harbour a Plasmid Encoding ISEc9 Upstream of bla(CTX-M-55) and qnrS2 Isolated from Imported Seafood. Arch. Microbiol. 2023, 205, 241. [Google Scholar] [CrossRef]
- Dahanayake, P.S.; Hossain, S.; Wickramanayake, M.V.K.S.; Heo, G.-J. Prevalence of Virulence and Extended-Spectrum β-Lactamase (ESBL) Genes Harbouring Vibrio spp. Isolated from Cockles (Tegillarca granosa) Marketed in Korea. Lett. Appl. Microbiol. 2020, 71, 61–69. [Google Scholar] [CrossRef]
- Wang, D.; Fletcher, G.C.; On, S.L.W.; Palmer, J.S.; Gagic, D.; Flint, S.H. Biofilm Formation, Sodium Hypochlorite Susceptibility and Genetic Diversity of Vibrio parahaemolyticus. Int. J. Food Microbiol. 2023, 385, 110011. [Google Scholar] [CrossRef] [PubMed]
- Shree, P.; Singh, C.K.; Sodhi, K.K.; Surya, J.N.; Singh, D.K. Biofilms: Understanding the Structure and Contribution towards Bacterial Resistance in Antibiotics. Med. Microecol. 2023, 16, 100084. [Google Scholar] [CrossRef]
- Arunkumar, M.; LewisOscar, F.; Thajuddin, N.; Pugazhendhi, A.; Nithya, C. In Vitro and In Vivo Biofilm Forming Vibrio spp: A Significant Threat in Aquaculture. Process Biochem. 2020, 94, 213–223. [Google Scholar] [CrossRef]
- Lopez, A.L.; Dutta, S.; Qadri, F.; Sovann, L.; Pandey, B.D.; Bin Hamzah, W.M.; Memon, I.; Iamsirithaworn, S.; Dang, D.A.; Chowdhury, F.; et al. Cholera in Selected Countries in Asia. Vaccine 2020, 38, A18–A24. [Google Scholar] [CrossRef]
- Bureau of Epidemiology, Department of Disease Control, Ministry of Public Health. National Disease Surveillance. Available online: http://doe.moph.go.th/surdata/ (accessed on 29 December 2024).
- Woodring, J.; Srijan, A.; Puripunyakom, P.; Oransathid, W.; Wongstitwilairoong, B.; Mason, C. Prevalence and Antimicrobial Susceptibilities of Vibrio, Salmonella, and Aeromonas Isolates from Various Uncooked Seafoods in Thailand. J. Food Prot. 2012, 75, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Beshiru, A.; Igbinosa, E.O. Surveillance of Vibrio parahaemolyticus Pathogens Recovered from Ready-to-Eat Foods. Sci. Rep. 2023, 13, 4186. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Hiyoshi, H.; Tandhavanant, S.; Kodama, T. Advances on Vibrio parahaemolyticus Research in the Postgenomic Era. Microbiol. Immunol. 2020, 64, 167–181. [Google Scholar] [CrossRef]
- Yu, Y.; Li, H.; Wang, Y.; Zhang, Z.; Liao, M.; Rong, X.; Li, B.; Wang, C.; Ge, J.; Zhang, X. Antibiotic Resistance, Virulence and Genetic Characteristics of Vibrio alginolyticus Isolates from Aquatic Environment in Costal Mariculture Areas in China. Mar. Pollut. Bull. 2022, 185, 114219. [Google Scholar] [CrossRef]
- Jacobs Slifka, K.M.; Newton, A.E.; Mahon, B.E. Vibrio alginolyticus Infections in the USA, 1988-2012. Epidemiol. Infect. 2017, 145, 1491–1499. [Google Scholar] [CrossRef]
- Neetoo, H.; Reega, K.; Manoga, Z.S.; Nazurally, N.; Bhoyroo, V.; Allam, M.; Jaufeerally-Fakim, Y.; Ghoorah, A.W.; Jaumdally, W.; Hossen, A.M.; et al. Prevalence, Genomic Characterization, and Risk Assessment of Human Pathogenic Vibrio Species in Seafood. J. Food Prot. 2022, 85, 1553–1565. [Google Scholar] [CrossRef]
- Zhou, K.; Tian, K.-Y.; Liu, X.-Q.; Liu, W.; Zhang, X.-Y.; Liu, J.-Y.; Sun, F. Characteristic and Otopathogenic Analysis of a Vibrio alginolyticus Strain Responsible for Chronic Otitis Externa in China. Front. Microbiol. 2021, 12, 750642. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Huang, Y.; Liu, P.; Yan, L.; Zhou, Y.; Yang, C.; Wu, Y.; Qin, J.; Guo, Y.; Pei, X.; et al. Population Genomics of the Food-Borne Pathogen Vibrio fluvialis Reveals Lineage Associated Pathogenicity-Related Genetic Elements. Microb. Genom. 2022, 8, 769. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Ibne Momen, A.M.; Rahman, A.; Biswas, J.; Yasmin, M.; Nessa, J.; Ahsan, C.R. Draft-Genome Analysis Provides Insights into the Virulence Properties and Genome Plasticity of Vibrio fluvialis Organisms Isolated from Shrimp Farms and Turag River in Bangladesh. Arch. Microbiol. 2022, 204, 527. [Google Scholar] [CrossRef]
- Kothary, M.H.; Lowman, H.; McCardell, B.A.; Tall, B.D. Purification and Characterization of Enterotoxigenic El Tor-Like Hemolysin Produced by Vibrio fluvialis. Infect. Immun. 2003, 71, 3213–3220. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.O.E.; Ali, G.A.; Hassen, S.S.; Goravey, W. Vibrio albensis Bacteremia: A Case Report and Systematic Review. IDCases 2022, 29, e01551. [Google Scholar] [CrossRef]
- Araj, G.F.; Taleb, R.; El Beayni, N.K.; Goksu, E. Vibrio albensis: An Unusual Urinary Tract Infection in a Healthy Male. J. Infect. Public Health 2019, 12, 712–713. [Google Scholar] [CrossRef]
- Ballal, M.; Shetty, V.; Bangera, S.R.; Prabhu, M.; Umakanth, S. Vibrio furnissii, an Emerging Pathogen Causing Acute Gastroenteritis: A Case Report. JMM Case Rep. 2017, 4, e005111. [Google Scholar] [CrossRef]
- Gladney, L.M.; Tarr, C.L. Molecular and Phenotypic Characterization of Vibrio navarrensis Isolates Associated with Human Illness. J. Clin. Microbiol. 2014, 52, 4070–4074. [Google Scholar] [CrossRef]
- Magdalena, L.; Kinga, W.; Jacek, O. Antimicrobial Resistance, Virulence Factors, and Genetic Profiles of Vibrio parahaemolyticus from Seafood. Appl. Environ. Microbiol. 2018, 84, e00537-18. [Google Scholar] [CrossRef]
- Ahmed, H.A.; El Bayomi, R.M.; Hussein, M.A.; Khedr, M.H.E.; Abo Remela, E.M.; El-Ashram, A.M.M. Molecular Characterization, Antibiotic Resistance Pattern and Biofilm Formation of Vibrio parahaemolyticus and V. cholerae Isolated from Crustaceans and Humans. Int. J. Food Microbiol. 2018, 274, 31–37. [Google Scholar] [CrossRef]
- García-Zamora, J.L.; Alonso-Arenas, J.; Rebollar-Pérez, G.; Pacheco-Aguirre, F.M.; García-Diaz, E.; Torres, E. Detection of Ampicillin Based on the Fluorescence of a Biocatalytic Oxidation Product. Front. Environ. Sci. 2022, 10, 1–12. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Committee on Food Additives. Ampicillin. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/dde71439-7a1c-49fe-816a-fbc0dabf1d30/content (accessed on 16 February 2025).
- Fu, S.; Wang, Q.; Wang, R.; Zhang, Y.; Lan, R.; He, F.; Yang, Q. Horizontal Transfer of Antibiotic Resistance Genes within the Bacterial Communities in Aquacultural Environment. Sci. Total Environ. 2022, 820, 153286. [Google Scholar] [CrossRef] [PubMed]
- Marques, P.H.; Prado, L.C.d.S.; Felice, A.G.; Rodrigues, T.C.V.; Pereira, U.D.P.; Jaiswal, A.K.; Azevedo, V.; Oliveira, C.J.F.; Soares, S. Insights into the Vibrio Genus: A One Health Perspective from Host Adaptability and Antibiotic Resistance to In Silico Identification of Drug Targets. Antibiotics 2022, 11, 1399. [Google Scholar] [CrossRef] [PubMed]
- Sudan, P.; Tyagi, A.; Dar, R.A.; Sharma, C.; Singh, P.; Kumar B.T., N.; Chandra, M.; Arora, A.K. Prevalence and Antimicrobial Resistance of Food Safety Related Vibrio Species in Inland Saline Water Shrimp Culture Farms. Int. Microbiol. 2023, 26, 591–600. [Google Scholar] [CrossRef]
- Abdel-Raheem, S.M.; Al-Sultan, S.I.; El-Tarabili, R.M. First Detection of Vibrio parahaemolyticus in Migratory Birds in Egypt: Antibiogram, Virulence, and Resistance Gene Profiles Indicating Zoonotic and Public Health Risks. Curr. Microbiol. 2024, 82, 15. [Google Scholar] [CrossRef]
- Abioye, O.E.; Nontongana, N.; Osunla, C.A.; Okoh, A.I. Antibiotic Resistance and Virulence Genes Profiling of Vibrio cholerae and Vibrio mimicus Isolates from Some Seafood Collected at the Aquatic Environment and Wet Markets in Eastern Cape Province, South Africa. PLoS ONE 2023, 18, e0290356. [Google Scholar] [CrossRef]
- Zheng, Z.; Ye, L.; Li, R.; Chen, S. Whole-Genome Sequencing of Strains of Vibrio spp. from China Reveals Different Genetic Contexts of blaCTX-M-14 among Diverse Lineages. J. Antimicrob. Chemother. 2021, 76, 950–956. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, R.; Ye, L.; Wai-chi Chan, E.; Xia, X.; Chen, S. Genetic Characterization of blaCTX–M–55-Bearing Plasmids Harbored by Food-Borne Cephalosporin-Resistant Vibrio parahaemolyticus Strains in China. Front. Microbiol. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Dahanayake, P.S.; Hossain, S.; Wickramanayake, M.V.K.S.; Wimalasena, S.H.M.P.; Heo, G.-J. Manila Clam (Ruditapes philippinarum) Marketed in Korea as a Source of Vibrios Harbouring Virulence and β-Lactam Resistance Genes. Lett. Appl. Microbiol. 2020, 71, 46–53. [Google Scholar] [CrossRef]
- Lan, N.P.H.; Hien, N.H.; Le Thi Phuong, T.; Thanh, D.P.; Thieu, N.T.V.; Ngoc, D.T.T.; Tuyen, H.T.; Vinh, P.V.; Ellington, M.J.; Thwaites, G.E.; et al. Phenotypic and Genotypic Characteristics of ESBL and AmpC Producing Organisms Associated with Bacteraemia in Ho Chi Minh City, Vietnam. Antimicrob. Resist. Infect. Control 2017, 6, 105. [Google Scholar] [CrossRef]
- Adesiyan, I.M.; Bisi-Johnson, M.A.; Okoh, A.I. Incidence of Antibiotic Resistance Genotypes of Vibrio Species Recovered from Selected Freshwaters in Southwest Nigeria. Sci. Rep. 2022, 12, 18912. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, A.; Delerue, T.; Morel, H.; Le Monnier, A.; Carbonnelle, E.; Pilmis, B.; Zahar, J.R. Infections Caused by Naturally AmpC-Producing Enterobacteriaceae: Can We Use Third-Generation Cephalosporins? A Narrative Review. Int. J. Antimicrob. Agents 2020, 55, 105834. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, G.H.; Koh, Y.J.; Jung, J.S. Identification of strA-strB Genes in Streptomycin-Resistant Pseudomonas syringae pv. actinidiae Biovar 2 Strains Isolated in Korea. Plant Pathol. J. 2021, 37, 489–493. [Google Scholar] [CrossRef]
- Fihman, V.; Lartigue, M.F.; Jacquier, H.; Meunier, F.; Schnepf, N.; Raskine, L.; Riahi, J.; Sanson-le Pors, M.-J.; Berçot, B. Appearance of aac(6’)-Ib-Cr Gene among Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae in a French Hospital . J. Infect. 2008, 56, 454–459. [Google Scholar] [CrossRef]
- Kim, C.; Cha, J.Y.; Yan, H.; Vakulenko, S.B.; Mobashery, S. Hydrolysis of ATP by Aminoglycoside 3’-Phosphotransferases: An Unexpected Cost to Bacteria for Harboring an Antibiotic Resistance Enzyme. J. Biol. Chem. 2006, 281, 6964–6969. [Google Scholar] [CrossRef]
- Stogios, P.J.; Spanogiannopoulos, P.; Evdokimova, E.; Egorova, O.; Shakya, T.; Todorovic, N.; Capretta, A.; Wright, G.D.; Savchenko, A. Structure-Guided Optimization of Protein Kinase Inhibitors Reverses Aminoglycoside Antibiotic Resistance. Biochem. J. 2013, 454, 191–200. [Google Scholar] [CrossRef]
- Galimand, M.; Sabtcheva, S.; Courvalin, P.; Lambert, T. Worldwide Disseminated armA Aminoglycoside Resistance Methylase Gene Is Borne by Composite Transposon Tn1548. Antimicrob. Agents Chemother. 2005, 49, 2949–2953. [Google Scholar] [CrossRef]
- Falco, A.; Villaquirán-Muriel, M.Á.; Gallo Pérez, J.D.; Mondragón-Quiguanas, A.; Aranaga, C.; Correa, A. Identification of Vibrio metschnikovii and Vibrio injensis Isolated from Leachate Ponds: Characterization of Their Antibiotic Resistance and Virulence-Associated Genes. Antibiotics 2023, 12, 1571. [Google Scholar] [CrossRef]
- De Silva, L.A.D.S.; Heo, G.-J. Biofilm Formation of Pathogenic Bacteria Isolated from Aquatic Animals. Arch. Microbiol. 2022, 205, 36. [Google Scholar] [CrossRef]
- Nguyen, K.C.T.; Truong, P.H.; Thi, H.T.; Ho, X.T.; Nguyen, P. Van Prevalence, Multidrug Resistance, and Biofilm Formation of Vibrio parahaemolyticus Isolated from Fish Mariculture Environments in Cat Ba Island, Vietnam. Osong Public Health Res. Perspect. 2024, 15, 56–67. [Google Scholar] [CrossRef]
- Ashrafudoulla, M.; Mizan, M.F.R.; Park, H.; Byun, K.-H.; Lee, N.; Park, S.H.; Ha, S.-D. Genetic Relationship, Virulence Factors, Drug Resistance Profile and Biofilm Formation Ability of Vibrio parahaemolyticus Isolated From Mussel. Front. Microbiol. 2019, 10, 513. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Yuan, M.; Tao, Q.; Xu, T.; Liu, J.; Huang, Z.; Wu, Q.; Pan, Y.; Zhao, Y.; Zhang, Z. Discovery of Novel Resistance Mechanisms of Vibrio parahaemolyticus Biofilm against Aminoglycoside Antibiotics. Antibiotics 2023, 12, 638. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Simner, P.J.; Bradford, P.A. Extended-Spectrum β-Lactamases: An Update on Their Characteristics, Epidemiology and Detection. JAC-Antimicrob. Resist. 2021, 3, dlab092. [Google Scholar] [CrossRef] [PubMed]
- Stephen, J.; Lekshmi, M.; Ammini, P.; Kumar, S.H.; Varela, M.F. Membrane Efflux Pumps of Pathogenic Vibrio Species: Role in Antimicrobial Resistance and Virulence. Microorganisms 2022, 10, 382. [Google Scholar] [CrossRef]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. V Molecular Mechanisms of Antibiotic Resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Liu, C.-W.; Liu, P.-Y. Extended-Spectrum β-Lactamases (ESBL) Producing Bacteria in Animals. Antibiotics 2023, 12, 661. [Google Scholar] [CrossRef]
- Bush, K.; Fisher, J.F. Epidemiological Expansion, Structural Studies, and Clinical Challenges of New β-Lactamases from Gram-Negative Bacteria. Annu. Rev. Microbiol. 2011, 65, 455–478. [Google Scholar] [CrossRef]
- Yang, J.-T.; Zhang, L.-J.; Lu, Y.; Zhang, R.-M.; Jiang, H.-X. Genomic Insights into Global bla(CTX-M-55)-Positive Escherichia coli Epidemiology and Transmission Characteristics. Microbiol. Spectr. 2023, 11, e0108923. [Google Scholar] [CrossRef]
- Wang, Y.; Song, C.; Duan, G.; Zhu, J.; Yang, H.; Xi, Y.; Fan, Q. Transposition of ISEcp1 Modulates blaCTX-M-55-Mediated Shigella flexneri Resistance to Cefalothin. Int. J. Antimicrob. Agents 2013, 42, 507–512. [Google Scholar] [CrossRef]
- Zeng, S.; Luo, J.; Li, X.; Zhuo, C.; Wu, A.; Chen, X.; Huang, L. Molecular Epidemiology and Characteristics of CTX-M-55 Extended-Spectrum β-Lactamase-Producing Escherichia coli From Guangzhou, China. Front. Microbiol. 2021, 12, 730012. [Google Scholar] [CrossRef]
- Zhao, W.-H.; Hu, Z.-Q. Epidemiology and Genetics of CTX-M Extended-Spectrum β-Lactamases in Gram-Negative Bacteria. Crit. Rev. Microbiol. 2013, 39, 79–101. [Google Scholar] [CrossRef] [PubMed]
- Gxalo, O.; Digban, T.O.; Igere, B.E.; Olapade, O.A.; Okoh, A.I.; Nwodo, U.U. Virulence and Antibiotic Resistance Characteristics of Vibrio Isolates from Rustic Environmental Freshwaters. Front. Cell. Infect. Microbiol. 2021, 11, 732001. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lin, D.; Chen, K.; Wong, M.H.Y.; Chen, S. First Detection of AmpC β-Lactamase bla(CMY-2) on a Conjugative IncA/C Plasmid in a Vibrio parahaemolyticus Isolate of Food Origin. Antimicrob. Agents Chemother. 2015, 59, 4106–4111. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, P.; Adriaenssens, E.M.; Consortium, I.R. ICTV Virus Taxonomy Profile: Inoviridae. J. Gen. Virol. 2021, 102, 1–2. [Google Scholar] [CrossRef]
- Tyagi, A.; Dubey, S.; Sharma, C.; Sudan, P.; Rai, S.; Kumar, B.T.N.; Chandra, M.; Arora, A.K. Complete Genome Sequencing and Characterization of Single-Stranded DNA Vibrio parahaemolyticus Phage from Inland Saline Aquaculture Environment. Virus Genes 2022, 58, 483–487. [Google Scholar] [CrossRef]
- Foxall, R.L.; Means, J.; Marcinkiewicz, A.L.; Schillaci, C.; DeRosia-Banick, K.; Xu, F.; Hall, J.A.; Jones, S.H.; Cooper, V.S.; Whistler, C.A. Inoviridae Prophage and Bacterial Host Dynamics during Diversification, Succession, and Atlantic Invasion of Pacific-Native Vibrio parahaemolyticus. MBio 2024, 15, e0285123. [Google Scholar] [CrossRef]
- Sweere, J.M.; Van Belleghem, J.D.; Ishak, H.; Bach, M.S.; Popescu, M.; Sunkari, V.; Kaber, G.; Manasherob, R.; Suh, G.A.; Cao, X.; et al. Bacteriophage Trigger Antiviral Immunity and Prevent Clearance of Bacterial Infection. Science 2019, 363, eaat9691. [Google Scholar] [CrossRef]
- Roux, S.; Krupovic, M.; Daly, R.A.; Borges, A.L.; Nayfach, S.; Schulz, F.; Sharrar, A.; Matheus Carnevali, P.B.; Cheng, J.-F.; Ivanova, N.N.; et al. Cryptic Inoviruses Revealed as Pervasive in Bacteria and Archaea across Earth’s Biomes. Nat. Microbiol. 2019, 4, 1895–1906. [Google Scholar] [CrossRef]
- Michniewski, S. Phages Infecting Marine Vibrios: Prevalence, Diversity and Role in the Dissemination of Antibiotic Resistance Genes. Ph.D. Thesis, University of Warwick, Coventry, UK, 2020. [Google Scholar]
- Clinical & Laboratory Standards Institute. M45: Methods for Antimicrobial Dilution and Disk Susceptibility Testing on Infrequently Isolated or Fastidious Bacteria, 3rd ed.; Clinical and Laboratory Standards Institute: Harrisburg, PA, USA, 2016; pp. 56–59. [Google Scholar]
- Mitsuwan, W.; Intongead, S.; Saengsawang, P.; Romyasamit, C.; Narinthorn, R.; Nissapatorn, V.; de Lourdes Pereira, M.; Paul, A.K.; Wongtawan, T.; Boripun, R. Occurrence of Multidrug Resistance Associated with Extended-Spectrum β-lactamase and the Biofilm Forming Ability of Escherichia coli in Environmental Swine Husbandry. Comp. Immunol. Microbiol. Infect. Dis. 2023, 103, 102093. [Google Scholar] [CrossRef]
- Mitsuwan, W.; Saengsawang, P.; Thaikoed, S.; Tanthanathipchai, N.; Saedan, P.; Chaisiri, K.; Boonmar, S.; Morita, Y. Rattus spp. as Reservoirs of Multidrug Resistance- and Biofilm-Forming Escherichia coli in Urban Community from Southern Thailand. Foodborne Pathog. Dis. 2024. [Google Scholar] [CrossRef]
- Kulnanan, P.; Chuprom, J.; Thomrongsuwannakij, T.; Romyasamit, C.; Sangkanu, S.; Manin, N.; Nissapatorn, V.; de Lourdes Pereira, M.; Wilairatana, P.; Kitpipit, W.; et al. Antibacterial, Antibiofilm, and Anti-Adhesion Activities of Piper Betle Leaf Extract against Avian Pathogenic Escherichia coli. Arch. Microbiol. 2021, 204, 49. [Google Scholar] [CrossRef] [PubMed]
- Falbo, V.; Carattoli, A.; Tosini, F.; Pezzella, C.; Dionisi, A.M.; Luzzi, I. Antibiotic Resistance Conferred by a Conjugative Plasmid and a Class I Integron in Vibrio cholerae O1 El Tor Strains Isolated in Albania and Italy. Antimicrob. Agents Chemother. 1999, 43, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, M.; Toma, C.; Miyazato, T.; Insisiengmay, S.; Nakasone, N.; Ehara, M. Antibiotic Resistance Conferred by a Class I Integron and SXT Constin in Vibrio cholerae O1 Strains Isolated in Laos. Antimicrob. Agents Chemother. 2004, 48, 2364–2369. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kwon, T.-H.; Jung, S.-M.; Cho, S.-H.; Jin, S.Y.; Park, N.-H.; Kim, C.-G.; Kim, J.-S. Antibiotic Resistance of Bacteria Isolated from the Internal Organs of Edible Snow Crabs. PLoS ONE 2013, 8, e70887. [Google Scholar] [CrossRef]
- Dalsgaard, A.; Forslund, A.; Tam, N.V.; Vinh, D.X.; Cam, P.D. Cholera in Vietnam: Changes in Genotypes and Emergence of Class I Integrons Containing AminoglycosideResistance Gene Cassettes in Vibrio cholerae O1 Strains Isolated from 1979 to 1996. J. Clin. Microbiol. 1999, 37, 734–741. [Google Scholar] [CrossRef]
- Hossain, S.; Wickramanayake, M.V.K.S.; Dahanayake, P.S.; Heo, G.-J. Occurrence of Virulence and Extended-Spectrum β-Lactamase Determinants in Vibrio spp. Isolated from Marketed Hard-Shelled Mussel (Mytilus coruscus). Microb. Drug Resist. 2020, 26, 391–401. [Google Scholar] [CrossRef]
- Liao, H.; Liu, C.; Zhou, S.; Liu, C.; Eldridge, D.J.; Ai, C.; Wilhelm, S.W.; Singh, B.K.; Liang, X.; Radosevich, M.; et al. Prophage-Encoded Antibiotic Resistance Genes Are Enriched in Human-Impacted Environments. Nat. Commun. 2024, 15, 8315. [Google Scholar] [CrossRef]


| Feature | MDR/ESBL-Vp0610Y | MDR-Vn0706Y |
|---|---|---|
| 1. Draft genome characteristics | ||
| Submitted GenBank assembly | GCA040530925 | GCA040530935 |
| Genome size | 5.21 Mb | 4.62 Mb |
| GC content | 45.5% | 48.0% |
| Number of genes | 4794 | 4224 |
| 4620 | 4030 |
| 1499 | 1356 |
Gene annotation
| 338 | 270 |
| 2. Antibiotic resistance mechanism | ||
| Antibiotic efflux | 55.65% | 55.40% |
| Antibiotic target alteration | 30.14% | 30.58% |
| Antibiotic target protection | 6.09% | 6.83% |
| Antibiotic inactivation | 4.35% | 3.96% |
| Resistance by absence | 1.74% | 1.44% |
| Reduced permeability to antibiotics | 1.16% | 1.08% |
| Antibiotic target replacement | 0.87% | 0.72% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitsuwan, W.; Boripun, R.; Saengsawang, P.; Intongead, S.; Boonplu, S.; Chanpakdee, R.; Morita, Y.; Boonmar, S.; Rojanakun, N.; Suksriroj, N.; et al. Multidrug Resistance, Biofilm-Forming Ability, and Molecular Characterization of Vibrio Species Isolated from Foods in Thailand. Antibiotics 2025, 14, 235. https://doi.org/10.3390/antibiotics14030235
Mitsuwan W, Boripun R, Saengsawang P, Intongead S, Boonplu S, Chanpakdee R, Morita Y, Boonmar S, Rojanakun N, Suksriroj N, et al. Multidrug Resistance, Biofilm-Forming Ability, and Molecular Characterization of Vibrio Species Isolated from Foods in Thailand. Antibiotics. 2025; 14(3):235. https://doi.org/10.3390/antibiotics14030235
Chicago/Turabian StyleMitsuwan, Watcharapong, Ratchadaporn Boripun, Phirabhat Saengsawang, Sutsiree Intongead, Sumaree Boonplu, Rawiwan Chanpakdee, Yukio Morita, Sumalee Boonmar, Napapat Rojanakun, Natnicha Suksriroj, and et al. 2025. "Multidrug Resistance, Biofilm-Forming Ability, and Molecular Characterization of Vibrio Species Isolated from Foods in Thailand" Antibiotics 14, no. 3: 235. https://doi.org/10.3390/antibiotics14030235
APA StyleMitsuwan, W., Boripun, R., Saengsawang, P., Intongead, S., Boonplu, S., Chanpakdee, R., Morita, Y., Boonmar, S., Rojanakun, N., Suksriroj, N., Ruekaewma, C., & Tenitsara, T. (2025). Multidrug Resistance, Biofilm-Forming Ability, and Molecular Characterization of Vibrio Species Isolated from Foods in Thailand. Antibiotics, 14(3), 235. https://doi.org/10.3390/antibiotics14030235









