1. Introduction
Ankle arthroplasty is increasingly being used for the treatment of ankle osteoarthritis [
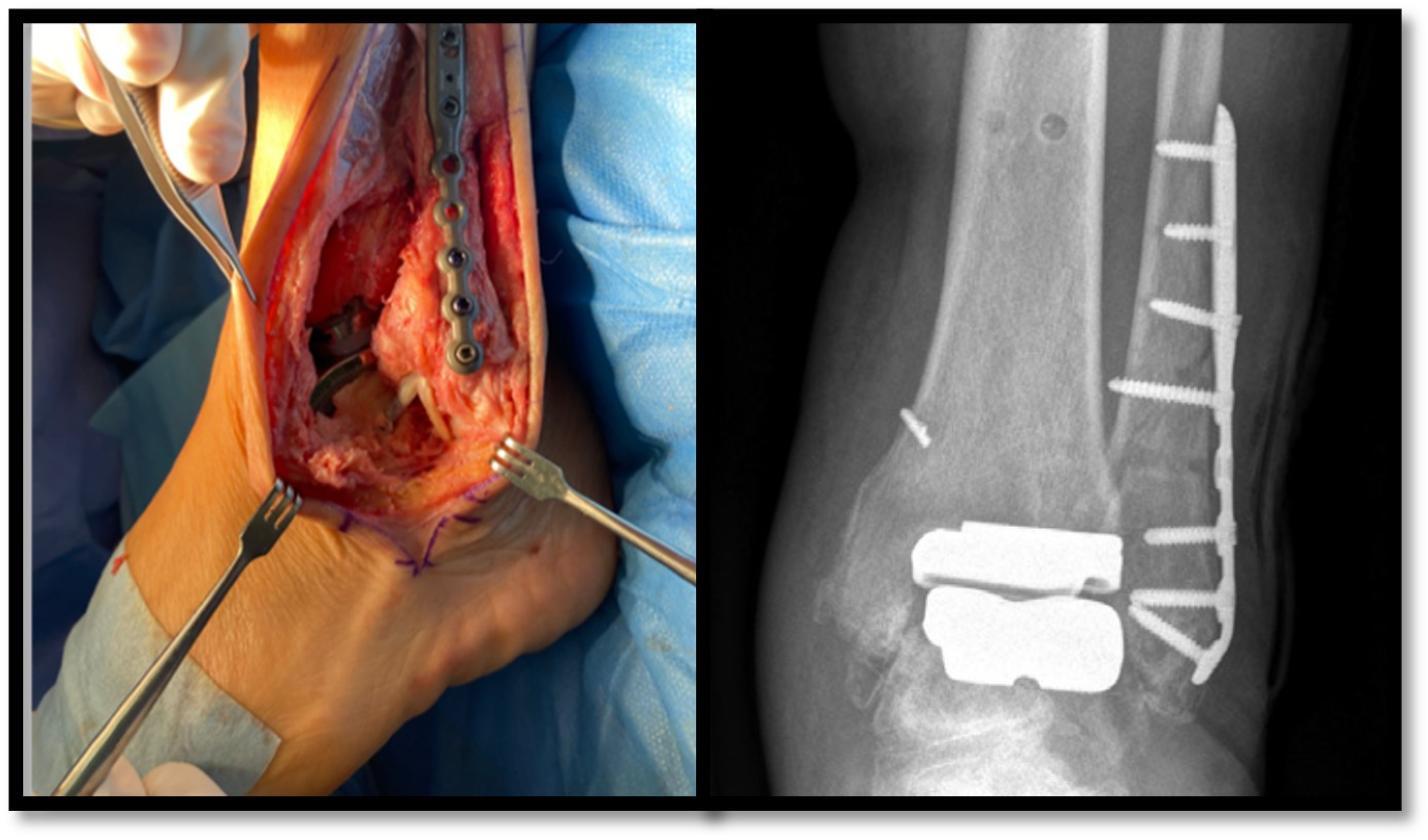
1]. In recent years, the transfibular approach has been shown to have functional advantages over the classic anterior incision, despite being more technically demanding and requiring the implantation of osteosynthesis material in the fibula (
Figure 1) [
2].
Prosthetic joint infection (PJI) after ankle arthroplasty is less common compared to hip or knee PJI, but the incidence is higher (2–10%) [
3,
4]. The indications for the treatment of ankle PJI are the same as for knee and hip PJI [
5]. However, there is limited experience in the appropriate management of ankle PJI in implants inserted using the transfibular approach, especially when debridement with implant retention is indicated. Re-opening the fibular route is controversial in this scenario because of the subsequent risk of instability and the difficulties of fibular bone resynthesis, which would jeopardise the mechanical goals of the prosthesis. However, this can lead to suboptimal debridement of the prosthetic components and thus a theoretically higher probability of failure. By contrast, in ankle prostheses placed through the anterior approach, an iterative incision leads directly to the prosthetic compartment, allowing for the proper debridement of the prosthetic components.
In our centre, starting in 2014, a progressive switch from the anterior approach to the lateral prosthesis was made, based on the best-published results of this new technique [
2,
6,
7]. The aim of this study was to analyse our experience in the management of prosthetic ankle infections (PAI) by focusing on the peculiarities of transfibular approach with special attention to cases managed with irrigation and debridement limited to the fibular plate. We analysed and compared the results with a cohort of infected fibular plates without ankle prostheses with the aim of delimiting the involvement of the prosthetic component in the process of infection.
2. Results
Among a total of 291 episodes of prosthetic joint infection (PJI) identified in our centre, 10 happened in ankle arthroplasties (3.4%) (7 transfibular and 3 anterior ankle prostheses). The total number of ankle prostheses implanted during the described period was 84 in the transfibular approach, resulting in an overall infection incidence of 8.3% (7/84). Among the seven cases of transfibular PAI, arthroplasty was indicated in four patients (57%) with post-traumatic injury, two (29%) with arthrosis, and one (14%) with an inflammatory chronic disease. Two patients (29%) had undergone a prior lateral approach for their original orthopaedic disease. The median age was 64 years (range 54–74), and five (71%) were women. Diabetes was the most common baseline condition [three cases (43%)]. There was only one case of rheumatoid arthritis with corticosteroid and immunosuppressant therapy, with no cases of cancer or other immunosuppressants. Three patients (43%) were active smokers. Other patients’ baseline conditions are resumed in
Table 1.
During the period 2018–2022, there were eleven cases of infection related to osteosynthesis material in fibular fractures with a median age was 56 years (range 39–86), six (55%) being women. Comorbidities were distributed in 27% (three) diabetes and 18% (two) rheumatoid arthritis and chronic pneumopathy each. Eight patients (73%) had an early infection and four (27%) had a delayed infection [
8]. Only three patients were smokers (27%). The main aspects of these cases are summarised in
Table 2.
Infection was classified as early post-surgical in all the cases of PJI. Median time from initial surgery to the diagnosis of infection was 40 days (range 11–82). All the patients presented with wound dehiscence, three of them with local inflammatory signs and pain (43%), and fever in two cases (28.6%). Radiological loosening and sinus tract were absent in all cases. Aetiology was polymicrobial in three cases (43%). The main bacteria involved were Staphylococcus aureus (four cases, 57%, all of them methicillin-susceptible), followed by Enterobacter cloacae (two cases, 29%) and S. epidermidis (one case, 14%). X-rays of the ankle showed prostheses stability in all cases. No CTs or MRIs were performed prior to the surgical treatment.
The main aspects of therapeutic management are summarised in
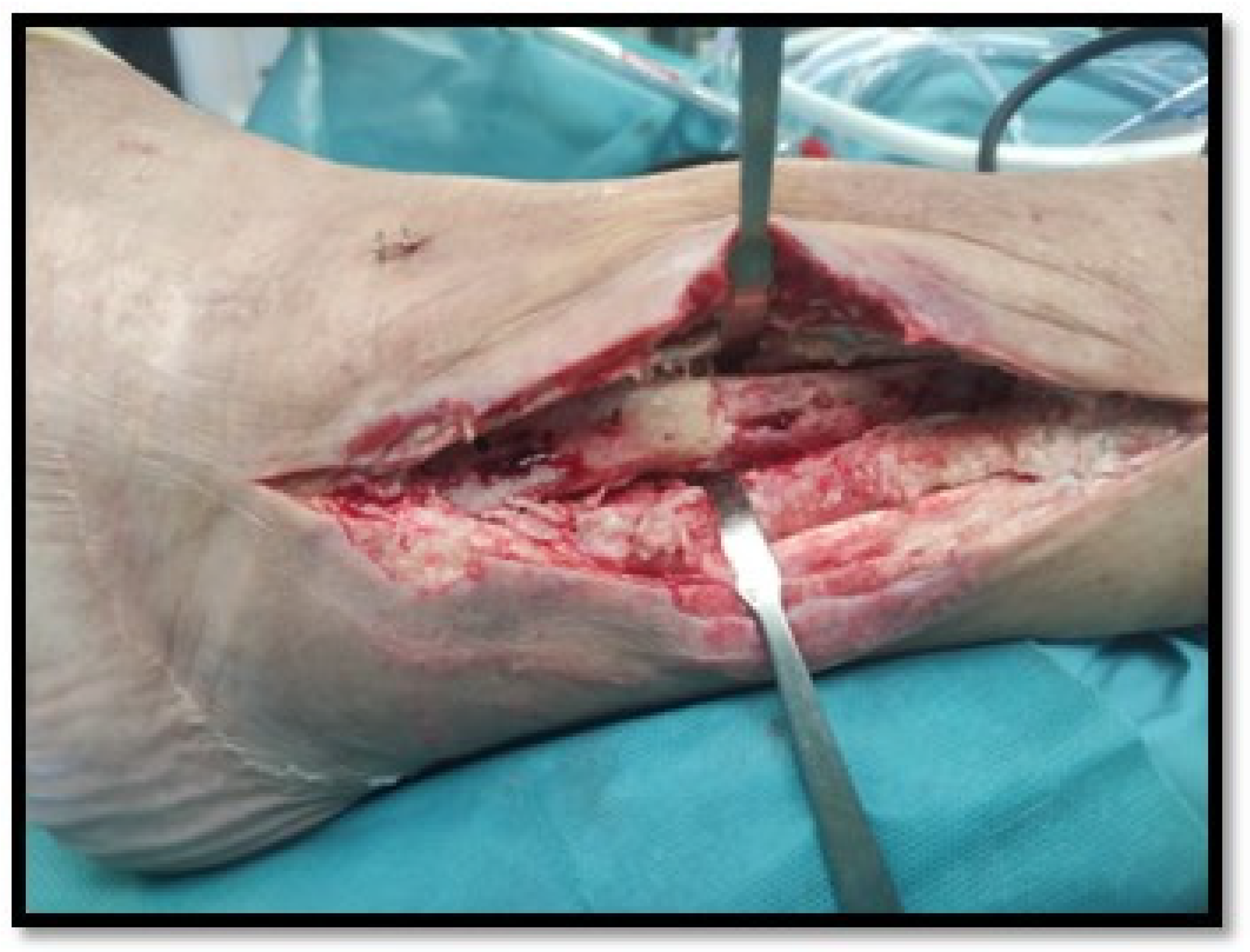
Table 3. All seven patients were managed with irrigation and debridement (I&D) of the fibular plate by an iterative surgical transfibular approach, with two cases requiring an anterior compartment dissection conducted through the same surgical approach. The osteosynthesis fibular plate could be removed in three cases (43%) (
Figure 2). To prevent joint instability, in none of these seven cases was the joint space approached or the prosthetic components debrided, nor the polyethylene liner exchanged. Four cases required flap wound coverage and two cases needed vacuum therapy. In four cases, wound recovery was unfavourable requiring further wound closure therapies.
The empirical treatment protocol encompassed the parenteral administration of β-lactam antibiotics exhibiting antipseudomonal activity, along with either vancomycin or daptomycin. Subsequent to a 3–7-day interval, therapeutic adjustments were made based on microbiologic findings, with a transition to oral administration over a total duration of 8 weeks.
Three patients (43%) were considered cured and unassisted walking was achieved in all three. The median follow-up time was 0.9, 7 and 7.3 years, respectively. The other four patients (57%) presented with failure after a median of 220 days, in the range of 60–582. Salvage therapy consisted of a second I&D of the fibular plate with arthroscopy associated in one case (25%), a removal of the fibular osteosynthesis material in one case (25%), and two-stage revision in two cases (50%), involving the placement of a cement spacer loaded with vancomycin and vancomycin plus clindamycin in one case each. All four cases were again approached via transfibular. Median follow-up in this group was of 3.5 years (range 2.6–6.4). As per functional outcome, at last follow-up two patients were able to walk without support (
Table 2).
A comparison between patients with a favourable and unfavourable outcome revealed no differences in demographic or clinical characteristics except for a non-significant higher frequency of diabetes mellitus in failed cases (3/4 versus 0/3; p = 0.14). Polymicrobial infection was also more frequent in failed cases (3/4 vs. 0/3, p = 0.14). A trend towards a longer delay from the onset of symptoms to surgery was observed among failed (median 59 days, range 56–363) versus cured cases (median 21.5 days, range 5–38) (p = 0.06). This delay may have contributed to the evolution of acute infections into chronic infections in some cases. We did not observe a higher rate of failure among patients with a need for soft tissue coverage (p = 0.714).
The eleven patients with a fibular plate infection (no prosthetic) had similar basic characteristics to patients with PJI. The main microbiological aetiology was methicillin-susceptible S. aureus (73%). Seven cases were treated with the removal of osteosynthesis material (64%) and four cases with I&D (36%). Infection recurred in only two cases (18%, one I&D and one two-stage revision), both of which were salvaged by orthopaedic hardware removal. A non-statistically significant higher rate of infection recurrence was observed among patients with transfibular ankle PJI than in cases of fibular plate-associated infection (57% vs. 18%, p = 0.087).
3. Discussion
Ankle PJI is an emerging disease that has increased in recent years with advances in orthopaedic techniques but remains infrequent. Despite being a less frequent arthroplasty than knee or hip prostheses, the incidence of PAI is higher, probably due to its anatomical nature. To our knowledge, this is the first study focusing on the management of infected transfibular ankle prostheses. While previous studies have reported the incidence of infection in anterior devices, as well as the differences between that technique and the transfibular approach regarding functional outcomes, none of these has addressed the technical problems of the surgical management of PAI, especially for transfibular arthroplasties [
3,
4,
9].
The treatment of PAI is often based on guidelines from other PJI locations. While I&D has proven to be as effective as in the hip or knee joints for the classic anterior approach, our results show a considerable failure rate for infected transfibular ankle arthroplasties (57%). This rate is higher than that reported in other series of infection in prostheses implanted by an anterior approach; Mazzoti et al. reported a rate of 48%, while Lachman et al. reported a rate of 54%, which may have been influenced by the incidence of methicillin-resistant
S. aureus [
5,
10,
11]. The patients in our series had similar baseline conditions and microbiological aetiologies to those reported in other series [
11,
12]. We hypothesise that suboptimal debridement of the joint compartment in patients with a transfibular ankle prosthesis may contribute to the poor outcomes observed in some cases. This hypothesis is supported by the comparison between transfibular ankle prostheses and infected peroneal plates, which suggests that the presence of intra-articular orthopaedic material could be a key factor responsible for the higher failure rate observed in the former group.
Indeed, a meticulous joint debridement and cleaning of orthopaedic material is at the heart of the surgical treatment. It is essential to remove as much necrotic material, debris, and purulence as possible, as well as bacterial biofilm [
13]. This, however, may be problematic for a transfibular ankle prosthesis: on the one hand, reopening the transfibular route (refracturing and re-repairing the fibula) can lead to joint instability; on the other, not accessing the joint compartment and restricting lavage to the peroneal plate may lead to suboptimal debridement and ultimately failure.
Given the difficulties of reusing the transfibular approach, the question of how to perform debridement in these patients remains open. While a new anterior incision of the ankle could lead to problems of skin ischaemia [
9], the possibility of an arthroscopic lavage could perhaps be explored. In other prosthetic joints, arthroscopy carries poorer results in I&D compared with open debridement, among other reasons, because the exchange of removable components is not possible [
14,
15]. However, some authors report successful outcomes with arthroscopic debridement of knee prostheses using a supplementary posterior port [
16,
17]. Analogically, in such a specific and complex scenario as transfibular PAI, the combination of a peroneal debridement plus an arthroscopic lavage could be an acceptable approach to preserve joint stability and ameliorate the thoroughness of the debridement. Alternatively, a less invasive re-synthesis of the fibula (e.g., revision of the fibula osteotomy with K-wires or screws intramedullary to avoid hardware over the fibula) could also be explored to provide the necessary capacity for deep joint surgical exploration while preserving the stability of the joint.
Our study has some limitations. Firstly, it was conducted at a single centre, and the number of cases was too small to obtain conclusive results. Secondly, the joint cavity was not explored, which could cast doubt on the involvement of the joint in the infection, except when it recurred. Lastly, the orthopaedic function assessment scale employed during follow-up was a basic tool that did not discriminate fine degrees of functionality or specific ranges of joint mobility. Nevertheless, this clinical problem has not previously been specifically addressed, and its strengths must also be noted. It is a long, prospective study conducted over 10 years at a renowned orthopaedic centre with extensive experience in osteoarticular infections.
In conclusion, the optimal surgical management of acute infections of transfibular ankle prostheses is challenging due to the risk of loss of joint stability. Our results show a high rate of failure of treatment when debridement is limited to the fibular component, suggesting that complementary surgical manoeuvres are needed. Larger multicentre studies are needed to confirm these results and consolidate collective experience with this technique.
4. Materials and Methods
This is an observational, prospective, single-centre study including all cases of transfibular PAI between 2014 and 2022 treated in a tertiary hospital. The centre is a 1300-bed acute-care teaching hospital with an Orthopaedic Unit that serves as a referral centre for a patient population of 550,000 inhabitants. Ethical approval for this study was obtained from the Hospital Research Ethics Committee (ref. 19/145).
Prostheses implanted via transfibular were TM AnkleR (ZimmerBiomet, Warsaw, IN, USA). The demographic characteristics of the identified cases were collected, including sex, age, comorbidities, immunosuppression status, type of prosthesis (primary or revision), infection characteristics (clinical, radiological, microbiological), and type of treatment administered (surgical and antibiotic therapy).
Cases of infection were defined according to the European Bone Joint Infection Society (EBJIS) [
18]. All microbiological results were obtained exclusively from intraoperative tissue samples or joint fluid aspirations. The type of infection was classified according to the modified Tsukayama classification, in which early post-surgical infection was defined as infection presenting with symptoms in the first 90 days after implantation [
19,
20].
The treatment followed individualised and multidisciplinary recommendations of the Spanish national guidelines for PJI (SEIMC), with irrigation and debridement being indicated for acute infections (i.e., early post-surgical and haematogenous) when appropriate, or removal of the prosthesis for chronic infections, with one- or two-stage revision or arthrodesis at the discretion of the responsible orthopaedic surgeon [
21]. All previous surgical incisions were used whenever possible, and in cases with significant soft-tissue loss, coverage techniques were employed (e.g., flaps or vacuum therapy). Functional rehabilitation of transfibular prostheses during the postoperative period took longer compared to the anterior approach, with mobility and partial carrying beginning 3 and 6 weeks after the surgery, respectively. Patients were followed up during hospitalisation and later in the outpatient clinic.
The primary endpoint was the failure of the first surgical and medical treatment used, defined as infection-related death, persistence or recurrence of infection, and/or the need for salvage therapy during follow-up, including suppressive antimicrobial therapy.
Since I&D of these cases was limited to the fibular plate, we compared the results with a cohort of patients with peroneal fracture-related infection, also involving the placement of a peroneal plate but without a joint prosthesis.
Data analysis was performed using the STATA 15.1 statistical software package. The Wilcoxon rank-sum test was used to compare continuous variables with non-normal distributions, while Fisher’s exact test was used for categorical variables. Confidence intervals were set at 95%, with a significance level of
p < 0.05 for all tests. This study adhered to the STROBE guidelines for cohort studies [
22]. The reporting checklist may be found in
Table S1.
Author Contributions
Conceptualization, P.H.-J., M.M.-L., M.Á.M.-C., M.Á.M.-R., P.B., C.L.-B., J.E.V.y.R. and J.L.-T.; methodology, P.H.-J., M.M.-L., M.Á.M.-C., M.Á.M.-R., P.B., C.L.-B., J.E.V.y.R. and J.L.-T.; software, P.H.-J., M.M.-L. and J.L.-T.; validation, P.H.-J., M.M.-L. and J.L.-T.; formal analysis, P.H.-J., M.M.-L. and J.L.-T.; investigation, P.H.-J., M.M.-L., M.Á.M.-C., M.Á.M.-R., P.B., C.L.-B., J.E.V.y.R. and J.L.-T.; resources, P.H.-J., M.M.-L., M.Á.M.-C., M.Á.M.-R., P.B., C.L.-B., J.E.V.y.R. and J.L.-T.; data curation, P.H.-J., M.M.-L. and J.L.-T.; writing—original draft preparation, P.H.-J. and J.L.-T.; writing—review and editing, P.H.-J., M.M.-L., M.Á.M.-C., M.Á.M.-R., P.B., C.L.-B., J.E.V.y.R. and J.L.-T.; visualisation, P.H.-J., M.M.-L., M.Á.M.-C., M.Á.M.-R., P.B., C.L.-B., J.E.V.y.R. and J.L.-T.; supervision, P.H.-J. and J.L.-T.; project administration, P.H.-J. and J.L.-T.; funding acquisition, P.H.-J. and J.L.-T. All authors have read and agreed to the published version of the manuscript.
Funding
PH was supported by a research contract ‘Río Hortega’ (Instituto de Salud Carlos III, Expte CM23/00259).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Hospital Research Ethics Committee (ref. 19/145).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are unavailable due to privacy and ethical restrictions.
Acknowledgments
We are grateful to Janet Dawson for reviewing the English version of the manuscript. We also express our gratitude for the collaboration of Juan Martin Romero Sabariego and Ana Pilar García-Almonacid from the ‘Coding’ and ‘Sterilization and Logistics of Implantable Material’ teams at the “12 de Octubre” University Hospital, Madrid, Spain.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | Linear dichroism |
References
- Novoa-Parra, C.D.; Gil-Monzó, E.; Díaz-Fernández, R.; Lizaur-Utrilla, A. Tendencia en España en el uso de artroplastia total de tobillo frente a artrodesis en el periodo 1997–2017. Rev. Esp. Cir. Ortopédica. Traumatol. 2021, 65, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Abarquero-Diezhandino, A.; Vacas Sánchez, E.; Diaz Fernandez, R.; Rico, J.V.Y. Results of Transfibular Total Ankle Arthroplasty. A Series of 50 Implants. J. Foot Ankle Surg. 2023, 62, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Alhaddab, Y.A.; Mittal, R.; Symes, M.J.; Wines, A.P. Rate of Infection and Causative Organisms in a Lateral Approach Total Ankle Replacement. Foot Ankle Spec. 2023, 19386400231184960, Advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Kessler, B.; Sendi, P.; Graber, P.; Knupp, M.; Zwicky, L.; Hintermann, B.; Zimmerli, W. Risk factors for periprosthetic ankle joint infection: A case-control study. J. Bone Jt. Surg. Am. 2012, 94, 1871–1876. [Google Scholar] [CrossRef]
- Kessler, B.; Knupp, M.; Graber, P.; Zwicky, L.; Hintermann, B.; Zimmerli, W.; Sendi, P. The treatment and outcome of peri-prosthetic infection of the ankle: A single cohort-centre experience of 34 cases. Bone Jt. J. 2014, 96-B, 772–777. [Google Scholar] [CrossRef]
- Clugston, E.; Ektas, N.; Scholes, C.; Symes, M.; Wilton, A.; Wines, A.; Mittal, R. Early Clinical Outcomes and Complications of Transfibular Total Ankle Arthroplasty: The Australian Experience. Foot Ankle Int. 2023, 44, 40–47. [Google Scholar] [CrossRef]
- Fletcher, A.N.; Day, J.; Motsay, M.; Manchester, M.; Zhang, Z.; Schon, L.C. Transfibular Total Ankle Arthroplasty: Clinical, Functional, and Radiographic Outcomes and Complications at a Minimum of 5-Year Follow-up. Foot Ankle Int. 2025, 46, 1–8. [Google Scholar] [CrossRef]
- Metsemakers, W.J.; Kuehl, R.; Moriarty, T.F.; Richards, R.; Verhofstad, M.; Borens, O.; Kates, S.; Morgenstern, M. Infection after fracture fixation: Current surgical and microbiological concepts. Injury 2018, 49, 511–522. [Google Scholar] [CrossRef]
- Usuelli, F.G.; Indino, C.; Maccario, C.; Manzi, L.; Liuni, F.M.; Vulcano, E. Infections in primary total ankle replacement: Anterior approach versus lateral transfibular approach. Foot Ankle Surg. 2019, 25, 19–23. [Google Scholar] [CrossRef]
- Marculescu, C.E.; Berbari, E.F.; Hanssen, A.D.; Steckelberg, J.M.; Harmsen, S.W.; Mandrekar, J.N.; Osmon, D.R. Outcome of prosthetic joint infections treated with debridement and retention of components. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2006, 42, 471–478. [Google Scholar] [CrossRef]
- Mazzotti, A.; Geraci, G.; Panciera, A.; Perna, F.; Stefanini, N.; Pilla, F.; Ruffilli, A.; Faldini, C. Trends in surgical management of the infected total ankle arthroplasty. Eur. Rev. Med. Pharmacol. Sci. 2019, 23 (Suppl. S2), 159–172. [Google Scholar] [CrossRef] [PubMed]
- Lachman, J.R.; Ramos, J.A.; DeOrio, J.K.; Easley, M.E.; Nunley, J.A.; Adams, S.B. Outcomes of Acute Hematogenous Periprosthetic Joint Infection in Total Ankle Arthroplasty Treated With Irrigation, Debridement, and Polyethylene Exchange. Foot Ankle Int. 2018, 39, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Tande, A.J.; Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef] [PubMed]
- Byren, I.; Bejon, P.; Atkins, B.L.; Angus, B.; Masters, S.; McLardy-Smith, P.; Gundle, R.; Berendt, A. One hundred and twelve infected arthroplasties treated with “DAIR” (debridement, antibiotics and implant retention): Antibiotic duration and outcome. J. Antimicrob. Chemother. 2009, 63, 1264–1271. [Google Scholar] [CrossRef]
- Waldman, B.J.; Hostin, E.; Mont, M.A.; Hungerford, D.S. Infected total knee arthroplasty treated by arthroscopic irrigation and débridement. J. Arthroplast. 2000, 15, 430–436. [Google Scholar] [CrossRef]
- Chung, J.Y.; Ha, C.W.; Park, Y.B.; Song, Y.J.; Yu, K.S. Arthroscopic debridement for acutely infected prosthetic knee: Any role for infection control and prosthesis salvage? Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 2014, 30, 599–606. [Google Scholar] [CrossRef]
- Dixon, P.; Parish, E.N.; Cross, M.J. Arthroscopic debridement in the treatment of the infected total knee replacement. J. Bone Jt. Surg. Br. 2004, 86, 39–42. [Google Scholar] [CrossRef]
- McNally, M.; Sousa, R.; Wouthuyzen-Bakker, M.; Chen, A.F.; Soriano, A.; Vogely, H.C.; Clauss, M.; Higuera, C.A.; Trebše, R. The EBJIS definition of periprosthetic joint infection: A practical guide for clinicians. Bone Jt. J. 2021, 103-B, 18–25. [Google Scholar] [CrossRef]
- Tsukayama, D.T.; Estrada, R.; Gustilo, R.B. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J. Bone Jt. Surg. Am. 1996, 78, 512–523. [Google Scholar] [CrossRef]
- Zimmerli, W.; Ochsner, P.E. Management of Infection Associated with Prosthetic Joints. Infection 2003, 31, 99–108. [Google Scholar] [CrossRef]
- Ariza, J.; Cobo, J.; Baraia-Etxaburu, J.; de Benito, N.; Bori, G.; Cabo, J.; Corona, P.; Esteban, J.; Horcajada, J.P.; Lora-Tamayo, J.; et al. Executive summary of management of prosthetic joint infections. Clinical practice guidelines by the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC). Enferm. Infecc. Microbiol. Clin. 2017, 35, 189–195. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).