Biological Activity and Chemical Composition of Essential Oil from Leaves and Fruits of Zanthoxylum mantaro (J.F.Macbr.) J.F.Macbr
Abstract
1. Introduction
2. Results
2.1. Essential Oil Isolation
2.2. Chemical Composition of Essential Oil
2.3. Antimicrobial Activity of the Essential Oils of Z. mantaro
2.4. Scavenging Radical Capacity of Essential Oil
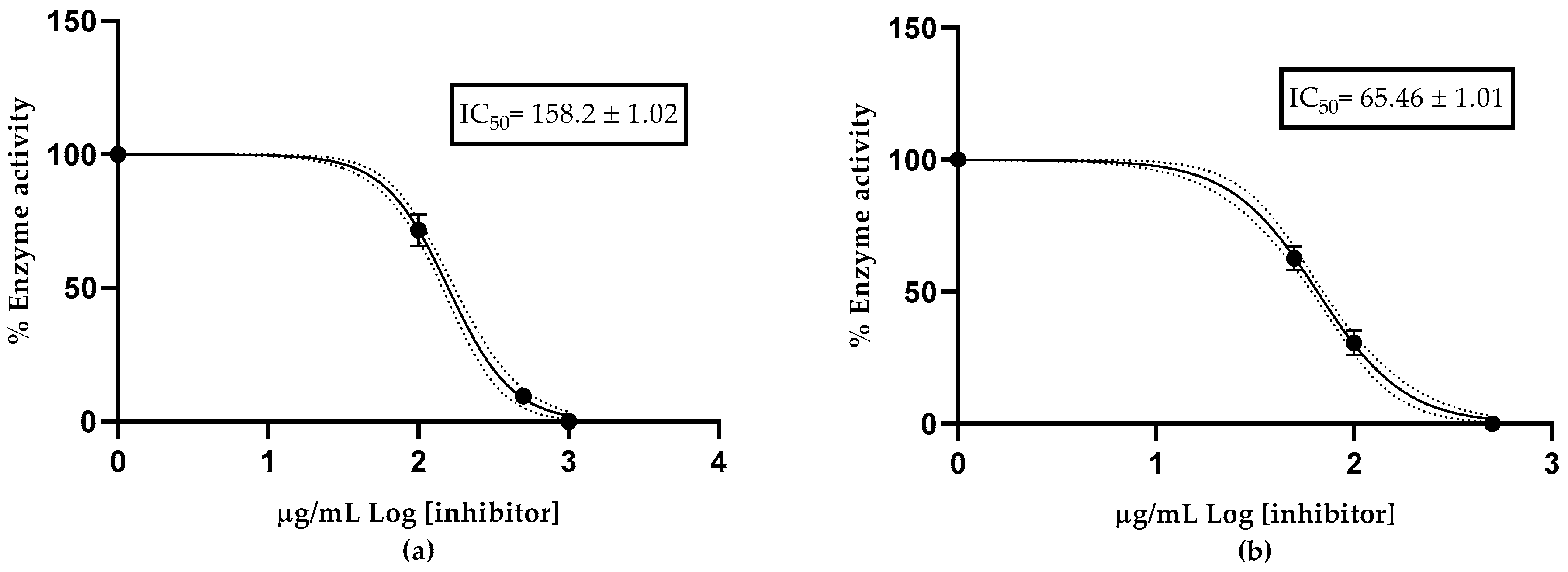
2.5. Anticholinesterase Activity
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. Plant Material
4.3. Extraction of Essential Oil
4.4. Identification and Quantification of Essential Oil
4.4.1. Sample Preparation
4.4.2. Qualitative Analysis (GC-MC) of the EOs
4.4.3. Quantitative Analysis (GC-FID) of the EOs
4.4.4. Identification and Quantification of Compounds
4.5. Antimicrobial Activity
4.6. Radical Scavenging Capacity
4.7. Cholinesterase Assay
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sierra Sarmiento, M.A.; Barros Algarra, R.; Gómez Paternina, D.; Mejía Terán, A.; Suarez Rivero, D. Productos Naturales: Metabolitos Secundarios y Aceites Esenciales; Bogotá, D.C., Ed.; Fundación Universitaria Agraria de Colombia-UNIAGRARIA: Cundinamarca, Colombia, 2018; ISBN 978-958-56645-4-8 (impreso), ISBN 978-958-56645-5-5 (e-book). [Google Scholar]
- Waterman, P.G. Rutaceae. In Phytochemical Dictionary: A Handbook of Bioactive Compounds from Plants; Harborne, J.B., Baxter, H., Eds.; Taylor & Francis: London, UK, 1993; pp. 604–628. [Google Scholar]
- Gupta, S.; Sharma, R.; Sharma, P.; Sharma, N. Phytochemical and Pharmacological Attributes of Rutaceae: A Review. Pharmacogn. Rev. 2020, 14, 1–10. [Google Scholar]
- Marzouk, M.M.; Mohamed, T.K.; Hussein, S.R. Flavonoids and Biological Activities of Ruta Species (Rutaceae): An Updated Review. J. Med. Plants Res. 2017, 11, 701–717. [Google Scholar]
- Wink, M. Medicinal Plants of the World. Chem. Biol. Med. 2015, 5, 12–25. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, X.; Wei, Y.; Yang, Z. Essential Oil Composition and Antimicrobial Activity of Zanthoxylum bungeanum Seeds. Molecules 2019, 24, 291. [Google Scholar]
- Kiem, P.V.; Quang, T.H.; Minh, C.V.; Tai, B.H. Alkaloids from the Genus Zanthoxylum and Their Biological Activities. Nat. Prod. Commun. 2012, 7, 921–931. [Google Scholar]
- Shah, S.S.; Ahmed, S.; Zhou, B.; Shi, L. A review on pharmacological activities and phytochemical constituents of Zanthoxylum armatum DC. Nat. Prod. Res. 2024, 38, 1–20. [Google Scholar] [CrossRef]
- Duke, J.A. Handbook of Medicinal Herbs; CRC Press: Boca Raton, FL, USA, 2002; pp. 601–605. [Google Scholar]
- Okagu, I.U.; Ndefo, J.C.; Aham, E.C.; Udenigwe, C.C. Zanthoxylum Species: A Comprehensive Review of Traditional Uses, Phytochemistry, Pharmacological and Nutraceutical Applications. Molecules 2021, 26, 4023. [Google Scholar] [CrossRef]
- Rios, J.L.; Recio, M.C. Medicinal Plants and Antimicrobial Activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.; Kim, H. Insecticidal and Repellent Activities of Essential Oils from Zanthoxylum piperitum Against Mosquitoes. Parasitol. Res. 2014, 113, 1057–1065. [Google Scholar]
- Chen, G.; Yuan, Y.; Wu, H.; He, F. Biological Activities and Phytochemical Profile of Zanthoxylum armatum. Ind. Crops Prod. 2020, 145, 112137. [Google Scholar]
- Morocho, V.; Aguilar, Y.; Cruz, C.; Cumbicus, N.; Andrade, J.M.; Montalvan, M. Chemical Composition, Enantiomeric Distribution, and Physical Properties of the Fruit Essential Oil from Zanthoxylum lepidopteriphilum (Reynel) Rutaceae from Ecuador. Plants 2024, 13, 2834. [Google Scholar] [CrossRef] [PubMed]
- Tatsadjieu, L.N.; Essia Ngang, J.J.; Ngassoum, M.B.; Etoa, F.X. Antibacterial and Antifungal Activity of Xylopia aethiopica, Monodora myristica, Zanthoxylum xanthoxyloïdes, and Zanthoxylum leprieurii from Cameroon. Fitoterapia 2003, 74, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Phuyal, N.; Jha, P.K.; Raturi, P.P.; Gurung, S.; Rajbhandary, S. Essential oil composition of Zanthoxylum armatum leaves as a function of growing conditions. Int. J. Food Prop. 2019, 22, 1873–1885. [Google Scholar] [CrossRef]
- Joshi, R.K. Chemical composition, biological activities and medicinal properties of genus Zanthoxylum: An updated review. Am. J. Essent. Oils Nat. Prod. 2024, 12, 23–27. [Google Scholar] [CrossRef]
- Silva, F.G.; Cazal, C.M. Characterization of Zanthoxylum rhoifolium Essential Oil Nanospheres and Insecticidal Effects to Bemisia tabaci. Plants 2022, 11, 1135. [Google Scholar]
- Liu, Z.L.; Du, S.S.; Wang, Y.Y.; Deng, Z.W.; Zhou, L. Components and Insecticidal Activity against the Maize Weevils of Zanthoxylum schinifolium Fruits and Leaves. Molecules 2011, 16, 3077–3088. [Google Scholar] [CrossRef]
- Bhandari, H.R.; Bhanu, A.N.; Srivastava, K.; Singh, M.N.; Shreya, H.A. Assessment of Genetic Diversity in Crop Plants—An Overview. Adv. Plants Agric. Res. 2017, 7, 279–286. [Google Scholar]
- Quian, Q.; Zhuo, Z.; Peng, Y.; Xu, D. Chemical Composition Variation in Essential Oil and Their Correlation with Climate Factors in Chinese Prickly Ash Peels (Zanthoxylum armatum DC.) from Different Habitats. Molecules 2024, 29, 1343. [Google Scholar] [CrossRef]
- Yang, J.; Song, X.; Hu, H.; Zhong, W.; Cao, R.; Xu, Y.; Li, R. Chemical Composition and Antifungal, Anti-inflammatory, Antiviral, and Larvicidal Activities of the Essential Oils of Zanthoxylum acanthopodium DC. from China and Myanmar. Molecules 2022, 27, 5243. [Google Scholar] [CrossRef]
- Diep, T.T.; Dung, L.V.; Trung, P.V.; Hoai, N.T.; Thao, D.T.; Uyen, N.T.T.; Linh, T.T.H.; Ha, T.H.N.; Truc, H.T. Chemical Composition, Antimicrobial, Nitric Oxide Inhibition, and Cytotoxic Activity of Essential Oils from Zanthoxylum acanthopodium DC. Leaves and Stems from Vietnam. Chem. Biodivers. 2023, 20, e202300649. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Hu, W.; Cheng, W.; Zhang, S.; Zou, R. Zanthoxylum bungeanum Essential Oil: Extraction and Component Analysis for α-Glucosidase Inhibitory Activity and the Underlying Mechanism Based on Molecular Docking. Appl. Sci. 2023, 13, 2627. [Google Scholar] [CrossRef]
- Hudaib, M.; Speroni, E.; Di Pietra, A.M.; Cavrini, V. GC/MS Evaluation of Thyme (Thymus vulgaris L.) Oil Composition and Variations during the Vegetative Cycle. J. Pharm. Biomed. Anal. 2002, 29, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.I.; Chang, K.M.; Lee, Y.S.; Kim, G.H. Antibacterial Activity of Essential Oils from Zanthoxylum piperitum A.P. DC. and Zanthoxylum schinifolium. Food Sci. Biotechnol. 2008, 17, 195–198. [Google Scholar]
- Bhanu Prakash, P.; Singh, P.; Kumar Mishra, P.; Dubey, N.K. Safety Assessment of Zanthoxylum alatum Roxb. Essential Oil, Its Antifungal, Antiaflatoxin, Antioxidant Activity and Efficacy as Antimicrobial in Preservation of Piper nigrum L. Fruits. Int. J. Food Microbiol. 2012, 153, 183–191. [Google Scholar] [CrossRef]
- Van Vuuren, S.; Holl, D. Antimicrobial Natural Product Research: A Review from a South African Perspective for the Years 2009–2016. J. Ethnopharmacol. 2017, 208, 236–252. [Google Scholar] [CrossRef]
- Teker, T.; Sefer, Ö.; Gazdağlı, A.; Yörük, E.; Varol, G.İ.; Albayrak, G. α-Thujone Exhibits an Antifungal Activity Against F. graminearum by Inducing Oxidative Stress, Apoptosis, Epigenetics Alterations and Reduced Toxin Synthesis. Eur. J. Plant Pathol. 2021, 160, 611–622. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, R.; Yang, Q.; Zhang, J.; Zhao, Y.; Zheng, Y.; Yang, J. Chapter One-Recent Advances in the Biosynthesis of Isoprenoids in Engineered Saccharomyces cerevisiae. In Advances in Industrial Biotechnology; Gadd, G.M., Sariaslani, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 114, pp. 1–35. [Google Scholar]
- Mata, A.T.; Proença, C.; Ferreira, A.R.; Serralheiro, M.L.M.; Nogueira, J.M.F.; Araújo, M.E.M. Antioxidant and Anti-acetylcholinesterase Activities of Five Plants Used as Portuguese Food Spices. Food Chem. 2007, 103, 778–786. [Google Scholar] [CrossRef]
- Andrade, M.A.; Das Graças Cardoso, M.; de Andrade, J.; Silva, L.F.; Teixeira, M.L.; Valério Resende, J.M.; da Silva Figueiredo, A.C.; Barroso, J.G. Chemical Composition and Antioxidant Activity of Essential Oils from Cinnamodendron dinisii Schwacke and Siparuna guianensis Aublet. Antioxidants 2013, 2, 384–397. [Google Scholar] [CrossRef]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The Versatility of Antioxidant Assays in Food Science and Safety-Chemistry, Applications, Strengths, and Limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miguel, M.G. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wen, J.; Xiang, Q.; Guo, J.; Zhang, J.; Yang, N.; Huang, Y.; Chen, Y.; Hu, T.; Rao, C. Pharmacological activities of Zanthoxylum L. Plants and its exploitation and utilization. Heliyon 2024, 10, e33207. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhou, L.; Mou, Y.; Mao, Z. Extraction optimization of polysaccharide from Zanthoxylum bungeanum using RSM and its antioxidant activity. Int. J. Biol. Macromol. 2015, 72, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ren, T.; Yang, M.; Lu, G.; Tan, S. Zanthoxylum alkylamides activate the Shh/Ptch1/Smo signaling pathway to ameliorate cognitive impairment in Alzheimer’s disease mice. J. Funct. Foods 2024, 120, 106376. [Google Scholar] [CrossRef]
- Chaudhary, N.; Kaur, S.; Kumar, N.; Mir, P.A.; Priyanka, S.A.; Sethi, N. Essential Oils as A Potential Neuroprotective Remedy for Alzheimer’s Disease. J. Pharm. Res. Rep. 2023, 4, 1–12. [Google Scholar]
- Negi, J.S.; Bisht, V.K.; Bhandari, A.K.; Singh, P.; Sundriyal, R.C. Chemical constituents and biological activities of the genus Zanthoxylum: A review. Afr. J. Pure Appl. Chem. 2011, 5, 412–416. [Google Scholar]
- Liu, S.L.; Wei, L.X.; Wang, D.; Gao, C.Y. Studies on the chemical constituents from the peel of Zanthoxylum schinifolium Sieb et Zucc. Yao Xue Xue Bao = Acta Pharm. Sin. 1991, 26, 836–840. [Google Scholar]
- Yang, Z.; Zhang, D.; Ren, J.; Yang, M. Skimmianine, a furoquinoline alkaloid from Zanthoxylum nitidum as a potential acetylcholinesterase inhibitor. Med. Chem. Res. 2011, 21, 722–725. [Google Scholar] [CrossRef]
- Hieu, T.T.; Kim, S.-I.; Ahn, Y.-J. Toxicity of Zanthoxylum piperitum and Zanthoxylum armatum oil constituents and related compounds to Stomoxys calcitrans (Diptera: Muscidae). J. Med. Entomol. 2014, 49, 1084–1091. [Google Scholar] [CrossRef]
- Min, S.L.S.; Liew, S.Y.; Chear, N.J.Y.; Goh, B.H.; Tan, W.-N.; Khaw, K.Y. Plant Terpenoids as the Promising Source of Cholinesterase Inhibitors for Anti-AD Therapy. Biology 2022, 11, 307. [Google Scholar] [CrossRef]
- Kang, J.S.; Kim, E.; Lee, S.H.; Park, I.-K. Inhibition of acetylcholinesterases of the pinewood nematode, Bursaphelenchus xylophilus, by phytochemicals from plant essential oils. Pestic. Biochem. Physiol. 2013, 105, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D.; Baranowska-Wójcik, E. Terpenes and Phenylpropanoids as Acetyl- and Butyrylcholinesterase Inhibitors: A Comparative Study. Curr. Alzheimer Res. 2019, 16, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Menichini, F.; Tundis, R.; Loizzo, M.R.; Bonesi, M.; Marrelli, M.; Statti, G.A.; Menichini, F.; Conforti, F. Acetylcholinesterase and butyrylcholinesterase inhibition of ethanolic extract and monoterpenes from Pimpinella anisoides V Brig. (Apiaceae). Fitoterapia 2009, 80, 297–300. [Google Scholar] [CrossRef]
- Politeo, O.; Cajic, I.; Simic, A.; Ruscic, M.; Bektaevi, M. Comparative Study of Chemical Composition and Cholinesterase Inhibition Potential of Essential Oils Isolated from Artemisia Plants from Croatia. Separations 2023, 10, 546. [Google Scholar] [CrossRef]
- Adams, R. Identification of Essential Oil Components by Gas Cromatography/Mass Spectrometry, 4th ed.; Allured Publ.: Carol Stream, IL, USA, 2017; Volume 1. [Google Scholar]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Cartuche, L.; Calva, J.; Valarezo, E.; Chuchuca, N.; Morocho, V. Chemical and Biological Activity Profiling of Hedyosmum strigosum Todzia Essential Oil, an Aromatic Native Shrub from Southern Ecuador. Plants 2022, 11, 2832. [Google Scholar] [CrossRef]
- Ellman, G.; Courtney, D.; Andres, V.; Featherstone, R. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Andrade, J.M.; Pachar, P.; Trujillo, L.; Cartuche, L. Suillin: A Mixed-Type Acetylcholinesterase Inhibitor from Suillus luteus Which Is Used by Saraguros Indigenous, Southern Ecuador. PLoS ONE 2022, 17, 1–13. [Google Scholar] [CrossRef]

| N° | Compound a | CLRI | LLRI | Leaves | Fruits | CF | ||
|---|---|---|---|---|---|---|---|---|
| % | SD | % | SD | |||||
| 1 | α-Thujene | 926 | 924 | 0.45 | 0.022 | 0.32 | 0.004 | C10H16 |
| 2 | α-Pinene | 933 | 932 | - | - | 0.64 | 0.007 | C10H16 |
| 3 | Sabinene | 975 | 969 | - | - | 10.71 | 0.112 | C10H16 |
| 4 | β-Pinene | 979 | 974 | 0.15 | 0.06 | 0.60 | 0.007 | C10H16 |
| 5 | Myrcene | 993 | 988 | - | - | 1.86 | 0.020 | C10H16 |
| 6 | α-Phellandrene | 1009 | 1002 | - | - | 0.93 | 0.010 | C10H16 |
| 7 | α-Terpinene | 1020 | 1014 | - | - | 0.51 | 0.006 | C10H16 |
| 8 | ο-Cymene | 1030 | 1022 | - | - | 1.24 | 0.010 | C10H14 |
| 9 | Limonene | 1033 | 1024 | - | - | 0.97 | 0.010 | C10H16 |
| 10 | β-Phellandrene | 1034 | 1025 | - | - | 0.62 | 0.006 | C10H16 |
| 11 | 1,8-Cineole | 1037 | 1026 | 0.48 | 0.02 | 0.32 | 0.005 | C10H18O |
| 12 | trans-Ocimene | 1052 | 1044 | - | - | 0.98 | 0.010 | C10H16 |
| 13 | γ-Terpinene | 1063 | 1054 | - | - | 0.99 | 0.010 | C10H16 |
| 14 | trans-4-Thujanol | 1079 | 1065 | - | - | 0.64 | 0.006 | C10H18O |
| 15 | p-Mentha-2,4(8)-diene | 1089 | 1085 | - | - | 0.31 | 0.003 | C10H18O |
| 16 | trans-Pinene hydrate | 1111 | 1119 | - | - | 0.41 | 0.012 | C10H18O |
| 17 | α-thujone | 1117 | 1101 | 1.04 | 0.05 | 39.85 | 0.363 | C10H16O |
| 18 | β-thujone | 1128 | 1112 | 0.74 | 0.04 | 25.04 | 0.302 | C10H16O |
| 19 | trans-p-Menth-2-en-1-ol | 1134 | 1136 | - | - | 0.27 | 0.004 | C10H18O |
| 20 | trans-Pinocamphone | 1187 | 1169 | - | - | 0.72 | 0.012 | C10H18O |
| 21 | Terpinen-4-ol | 1190 | 1175 | 0.48 | 0.02 | 4.38 | 0.040 | C10H18O |
| 22 | Isothujyl acetate | 1277 | 1266 | - | - | 0.76 | 0.007 | C12H20O2 |
| 23 | trans-Sabinyl acetate | 1297 | 1297 | - | - | 0.53 | 0.007 | C12H20O2 |
| 24 | Isoledene | 1381 | 1374 | 0.60 | 0.019 | - | - | C15H24 |
| 25 | β-Copaene | 1437 | 1430 | 0.56 | 0.011 | - | - | C15H24 |
| 26 | α-neo-Clovene | 1456 | 1452 | 0.44 | 0.008 | - | - | C15H24 |
| 27 | Geranyl acetone | 1459 | 1453 | 0.40 | 0.010 | - | - | C13H22O |
| 28 | γ-Muurolene | 1483 | 1478 | 0.24 | 0.004 | - | - | C15H24 |
| 29 | Germacrene D | 1489 | 1480 | 21.75 | 0.279 | - | - | C15H24 |
| 30 | n-Pentadecane | 1500 | 1500 | 1.53 | 0.013 | - | - | C15H32 |
| 31 | Isolepidozene | 1504 | 1480 | 2.00 | 0.043 | - | - | C15H24 |
| 32 | δ-Amorphene | 1510 | 1511 | 0.27 | 0.005 | - | - | C15H24 |
| 33 | γ-Cadinene | 1522 | 1513 | 0.31 | 0.210 | - | - | C15H24 |
| 34 | δ-Cadinene | 1526 | 1522 | 2.69 | 0.032 | - | - | C15H24 |
| 35 | Zonarene | 1532 | 1528 | 0.56 | 0.068 | - | - | C15H24 |
| 36 | α-Cadinene | 1546 | 1537 | 0.14 | 0.122 | - | - | C15H24 |
| 37 | (E)-Nerolidol | 1570 | 1561 | 12.39 | 0.083 | - | - | C15H26O |
| 38 | (Z)-dihydro-Apofarnesol | 1578 | 1571 | 2.63 | 0.110 | - | - | C15H28O |
| 39 | Dodecanoic acid | 1581 | 1580 | 1.96 | 0.009 | - | - | C12H24O2 |
| 40 | (Z)-3-Hexen-1-ol, benzoate | 1588 | 1580 | 0.53 | 0.004 | - | - | C13H16O2 |
| 41 | Isoaromadendrene epoxide | 1597 | 1594 | 0.75 | 0.006 | - | - | C15H24O2 |
| 42 | Tetradecanal | 1622 | 1615 | 0.52 | 0.007 | - | - | C14H28O |
| 43 | Junenol | 1638 | 1618 | 0.39 | 0.214 | - | - | C15H26O |
| 44 | (2Z,6Z)-Farnesal | 1671 | 1684 | 2.91 | 0.021 | - | - | C15H26O |
| 45 | 9,12,15-Octadecatrienal | 1676 | 1676 | 2.10 | 0.034 | - | - | C18H30O |
| 46 | n-Tetradecanol | 1679 | 1671 | 1.49 | 0.045 | - | - | C14H30O |
| 47 | 13-Methyltetradecanal | 1688 | 1680.3 | 1.24 | 0.062 | - | - | C15H30O |
| 48 | n-Heptadecane | 1700 | 1700 | 0.25 | 0.020 | - | - | C17H36 |
| 49 | Pentadecanal- | 1726 | 1717 | 7.14 | 0.047 | - | - | C15H30O |
| 50 | Isobicyclogermacrenal | 1758 | 1733 | 1.23 | 0.005 | - | - | C15H22O |
| 51 | Tetradecanoic acid | 1778 | 1768 | 1.21 | 0.007 | - | - | C14H28O |
| 52 | 2-Pentadecanone, 6,10,14-trimethyl- | 1848 | 1846.7 | 0.71 | 0.002 | - | - | C18H36O |
| 53 | n-Nonadecane | 1899 | 1900 | 0.42 | 0.220 | - | - | C19H40 |
| 54 | (5E,9E)-Farnesyl acetone | 1921 | 1913 | 0.53 | 0.004 | - | - | C18H28O |
| 55 | n-Eicosane | 1999 | 2000 | 0.33 | 0.008 | - | - | C20H42 |
| 56 | (E,E)-Geranyl linalool | 2031 | 2026 | 2.11 | 0.024 | - | - | C20H34O |
| 57 | n-Octadecanol | 2095 | 2077 | 0.62 | 0.041 | - | - | C18H38O |
| 58 | n-Heneicosane | 2099 | 2100 | 1.02 | 0.048 | - | - | C21H44 |
| 59 | Phytol | 2117 | 2116 | 3.77 | 0.006 | - | - | C20H40O |
| 60 | 1-Docosene | 2195 | 2189 | 0.53 | 0.006 | - | - | C22H44 |
| 61 | n-Docosane | 2199 | 2200 | 0.78 | 0.007 | - | - | C22H46 |
| 62 | n-Tricosane | 2300 | 2300 | 3.24 | 0.121 | - | - | C23H48 |
| 63 | n-Tetracosane | 2404 | 2400 | 1.86 | 0.058 | - | - | C24H50 |
| 64 | n-Pentacosane | 2535 | 2500 | 3.37 | 0.013 | - | - | C25H22 |
| Monoterpenes hydrocarbons | 0.60 | 20.37 | ||||||
| Oxygenated monoterpenes | 2.74 | 71.93 | ||||||
| Sesquiterpenes hydrocarbons | 31.09 | 0.00 | ||||||
| Oxigenated sesquiterpenes | 28.69 | 0.00 | ||||||
| Others | 27.74 | 1.29 | ||||||
| Total identified | 90.86 | 93.59 | ||||||
| Microorganism | Leaves | Fruits | Positive Control a |
|---|---|---|---|
| MIC (μg/mL) | |||
| Gram-positive cocci | |||
| Enterococcus faecalis (ATCC ® 19433) | 4000 | 4000 | 0.7812 |
| Enterococcus faecium (ATCC ® 27270) | 4000 | 4000 | <0.3906 |
| Staphylococcus aureus (ATCC ® 25923) | - | - | <0.3906 |
| Gram-negative bacilli | |||
| Escherichia coli O157:H7 (ATCC ® 43888) | - | - | 1.5625 |
| Pseudomonas aeruginosa (ATCC ® 10145) | - | - | <0.3906 |
| Salmonella enterica subs enterica serovar Thypimurium WDCM 00031, derived (ATCC ® 14028) | - | - | <0.3906 |
| Gram-positive bacilli | |||
| Lysteria monocytogenes (ATTC ® 19115) | - | - | 1.5625 |
| Yeasts and sporulated fungi | |||
| Candida albicans (ATTC ® 10231) | - | - | <0.098 |
| Aspergillus niger (ATCC ® 6275) | 4000 | 1000 | <0.098 |
| Sample | ABTS | TEAC | DPPH |
|---|---|---|---|
| SC50 (µg/mL—µM *) ± SD | |||
| Z.mantaro leaves EO | 2798.85 ± 15.69 | 10.96 ± 1.64 | - |
| Z. mantaro fruit EO | 274.14 ± 1.06 | 150.47 ± 27.37 | - |
| Trolox * | 29.09 ± 1.05 | 35.54 ± 1.04 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morocho, V.; Eras, O.; Rojas, T.; Jiménez, B.; Roa, M.F.; Cartuche, L. Biological Activity and Chemical Composition of Essential Oil from Leaves and Fruits of Zanthoxylum mantaro (J.F.Macbr.) J.F.Macbr. Antibiotics 2025, 14, 216. https://doi.org/10.3390/antibiotics14030216
Morocho V, Eras O, Rojas T, Jiménez B, Roa MF, Cartuche L. Biological Activity and Chemical Composition of Essential Oil from Leaves and Fruits of Zanthoxylum mantaro (J.F.Macbr.) J.F.Macbr. Antibiotics. 2025; 14(3):216. https://doi.org/10.3390/antibiotics14030216
Chicago/Turabian StyleMorocho, Vladimir, Odalis Eras, Teresa Rojas, Britany Jiménez, María Fernanda Roa, and Luis Cartuche. 2025. "Biological Activity and Chemical Composition of Essential Oil from Leaves and Fruits of Zanthoxylum mantaro (J.F.Macbr.) J.F.Macbr" Antibiotics 14, no. 3: 216. https://doi.org/10.3390/antibiotics14030216
APA StyleMorocho, V., Eras, O., Rojas, T., Jiménez, B., Roa, M. F., & Cartuche, L. (2025). Biological Activity and Chemical Composition of Essential Oil from Leaves and Fruits of Zanthoxylum mantaro (J.F.Macbr.) J.F.Macbr. Antibiotics, 14(3), 216. https://doi.org/10.3390/antibiotics14030216








