Prognostic Value of C-Reactive Protein in Primary Total Hip Arthroplasty
Abstract
1. Introduction
- Do cases with and without later PJI differ in terms of preoperative CRP values? How predictive is the preoperative CRP value?
- Do cases with and without later PJI differ in terms of postoperative CRP levels? How predictive is the postoperative CRP value?
- Do cases with and without later PJI differ in terms of the perioperative course of CRP (absolute and relative CRP increase)?
- What is the optimal cut-off value in this context and how high is the sensitivity and specificity of this CRP value?
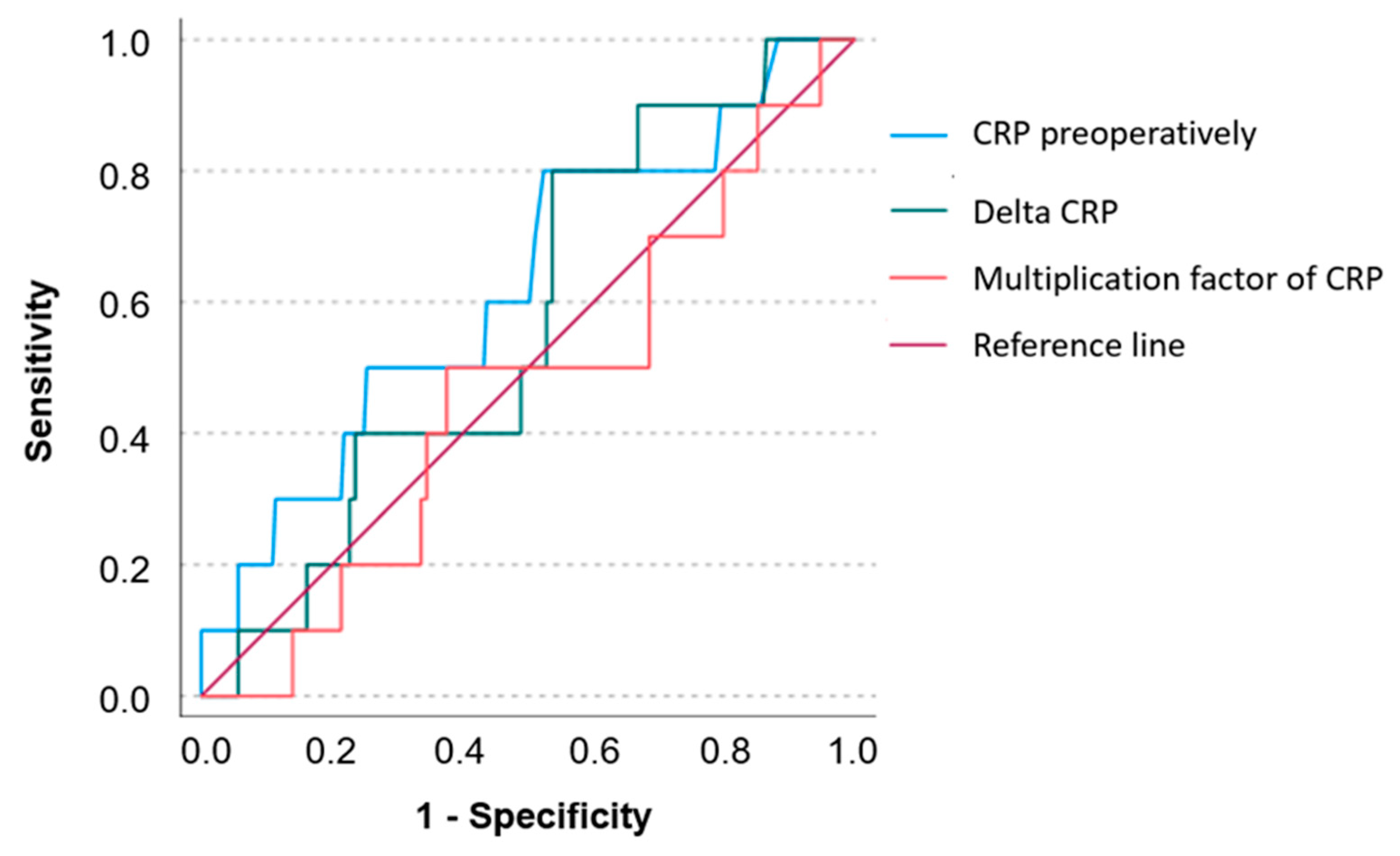
2. Results
3. Discussion
4. Materials and Methods
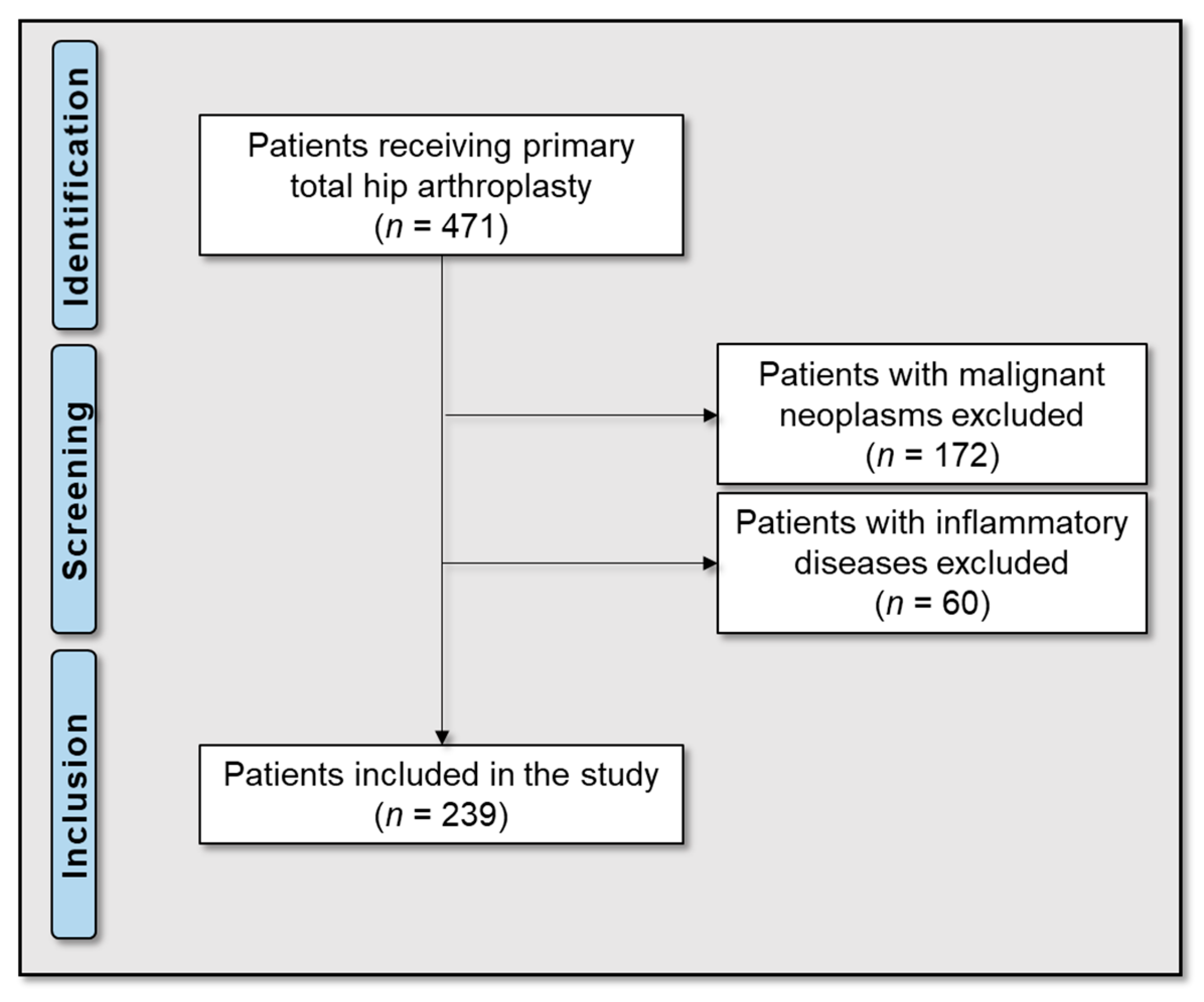
4.1. Patients and Research Workflow
4.2. Treatment Protocol
4.3. Laboratory Parameters
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Learmonth, I.D.; Young, C.; Rorabeck, C. The operation of the century: Total hip replacement. Lancet 2007, 370, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Popov, V.L.; Poliakov, A.M.; Pakhaliuk, V.I. Improving the Endoprosthesis Design and the Postoperative Therapy as a Means of Reducing Complications Risks after Total Hip Arthroplasty. Lubricants 2022, 10, 38. [Google Scholar] [CrossRef]
- Morscher, E. Failures and successes in total hip replacement-why good ideas may not work. Scand. J. Surg. 2003, 92, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Zmistowski, B.; Karam, J.A.; Durinka, J.B.; Casper, D.S.; Parvizi, J. Periprosthetic joint infection increases the risk of one-year mortality. JBJS 2013, 95, 2177–2184. [Google Scholar] [CrossRef]
- Gundtoft, P.H.; Overgaard, S.; Schønheyder, H.C.; Møller, J.K.; Kjærsgaard-Andersen, P.; Pedersen, A.B. The “true” incidence of surgically treated deep prosthetic joint infection after 32,896 primary total hip arthroplasties: A prospective cohort study. Acta Orthop. 2015, 86, 326–334. [Google Scholar] [CrossRef]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. Jbjs 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Ong, K.L.; Kurtz, S.M.; Lau, E.; Bozic, K.J.; Berry, D.J.; Parvizi, J. Prosthetic joint infection risk after total hip arthroplasty in the Medicare population. J. Arthroplast. 2009, 24, 105–109. [Google Scholar] [CrossRef]
- Otto-Lambertz, C.; Yagdiran, A.; Wallscheid, F.; Eysel, P.; Jung, N. Periprosthetic infection in joint replacement: Diagnosis and treatment. Dtsch. Ärzteblatt Int. 2017, 114, 347. [Google Scholar]
- Bozic, K.J.; Kurtz, S.M.; Lau, E.; Ong, K.; Chiu, V.; Vail, T.P.; Rubash, H.E.; Berry, D.J. The epidemiology of revision total knee arthroplasty in the United States. Clin. Orthop. Relat. Res.® 2010, 468, 45–51. [Google Scholar] [CrossRef]
- Akindolire, J.; Morcos, M.W.; Marsh, J.D.; Howard, J.L.; Lanting, B.A.; Vasarhelyi, E.M. The economic impact of periprosthetic infection in total hip arthroplasty. Can. J. Surg. 2020, 63, E52–E56. [Google Scholar] [CrossRef]
- Haenle, M.; Skripitz, C.; Mittelmeier, W.; Skripitz, R. Economic impact of infected total hip arthroplasty in the German diagnosis-related groups system. Orthopade 2012, 41, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, A.; Kolin, D.A.; Farley, K.X.; Wilson, J.M.; McLawhorn, A.S.; Cross, M.B.; Sculco, P.K. Projected Economic Burden of Periprosthetic Joint Infection of the Hip and Knee in the United States. J. Arthroplast. 2021, 36, 1484–1489.e1483. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Zmistowski, B.; Berbari, E.F.; Bauer, T.W.; Springer, B.D.; Della Valle, C.J.; Garvin, K.L.; Mont, M.A.; Wongworawat, M.D.; Zalavras, C.G. New definition for periprosthetic joint infection: From the Workgroup of the Musculoskeletal Infection Society. Clin. Orthop. Relat. Res.® 2011, 469, 2992–2994. [Google Scholar] [CrossRef] [PubMed]
- Alijanipour, P.; Bakhshi, H.; Parvizi, J. Diagnosis of periprosthetic joint infection: The threshold for serological markers. Clin. Orthop. Relat. Res.® 2013, 471, 3186–3195. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, E.; Antoci Jr, V.; Pulido, L.; Joshi, A.; Hozack, W.; Parvizi, J. The use of receiver operating characteristics analysis in determining erythrocyte sedimentation rate and C-reactive protein levels in diagnosing periprosthetic infection prior to revision total hip arthroplasty. Int. J. Infect. Dis. 2009, 13, e444–e449. [Google Scholar] [CrossRef]
- Bauer, T.W.; Parvizi, J.; Kobayashi, N.; Krebs, V. Diagnosis of periprosthetic infection. JBJS 2006, 88, 869–882. [Google Scholar]
- Parvizi, J.; Ghanem, E.; Menashe, S.; Barrack, R.L.; Bauer, T.W. Periprosthetic infection: What are the diagnostic challenges? JBJS 2006, 88, 138–147. [Google Scholar] [CrossRef]
- Clyne, B.; Olshaker, J.S. The C-reactive protein11Clinical Laboratory in Emergency Medicine is coordinated by Jonathan S. Olshaker, MD, of the University of Maryland Medical Center, Baltimore, Maryland. J. Emerg. Med. 1999, 17, 1019–1025. [Google Scholar] [CrossRef]
- Sanzén, L.; Sundberg, M. Periprosthetic low-grade hip infections erythrocyte sedimentation rate and C-reactive protein in 23 cases. Acta Orthop. Scand. 1997, 68, 461–465. [Google Scholar] [CrossRef]
- Baré, J.; MacDonald, S.J.; Bourne, R.B. Preoperative evaluations in revision total knee arthroplasty. Clin. Orthop. Relat. Res. (1976–2007) 2006, 446, 40–44. [Google Scholar]
- Della Valle, C.J.; Sporer, S.M.; Jacobs, J.J.; Berger, R.A.; Rosenberg, A.G.; Paprosky, W.G. Preoperative testing for sepsis before revision total knee arthroplasty. J. Arthroplast. 2007, 22, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Godoy, G.; Sumarriva, G.; Ochsner, J.L.; Chimento, G.; Schmucker, D.; Dasa, V.; Meyer, M. Preoperative acute inflammatory markers as predictors for postoperative complications in primary total knee arthroplasty. Ochsner J. 2016, 16, 481–485. [Google Scholar] [PubMed]
- Benda, S.; Mederake, M.; Schuster, P.; Fink, B. Diagnostic Value of C-Reactive Protein and Serum White Blood Cell Count during Septic Two-Stage Revision of Total Knee Arthroplasties. Antibiotics 2022, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Mederake, M.; Hofmann, U.K.; Benda, S.; Schuster, P.; Fink, B. Diagnostic Value of CRP and Serum WBC Count during Septic Two-Stage Revision of Total Hip Arthroplasties. Antibiotics 2022, 11, 1098. [Google Scholar] [CrossRef]
- Wixted, C.M.; Charalambous, L.T.; Kim, B.I.; Case, A.; Hendershot, E.F.; Seidelman, J.L.; Seyler, T.M.; Jiranek, W.A. D-Dimer, Erythrocyte Sedimentation Rate, and C-Reactive Protein Sensitivities for Periprosthetic Joint Infection Diagnosis. J. Arthroplast. 2023, 38, 914–917. [Google Scholar] [CrossRef]
- Xu, C.; Guo, H.; Qu, P.; Fu, J.; Kuo, F.-C.; Chen, J.-Y. Preoperatively elevated serum inflammatory markers increase the risk of periprosthetic joint infection following total knee arthroplasty in patients with osteoarthritis. Ther. Clin. Risk Manag. 2018, 14, 1719–1724. [Google Scholar] [CrossRef]
- Rohe, S.; Bohle, S.; Matziolis, G.; Jacob, B.; Wassilew, G.; Brodt, S. C-reactive protein during the first 6 postoperative days after total hip arthroplasty cannot predict early periprosthetic infection. Arch. Orthop. Trauma. Surg. 2023, 143, 3495–3503. [Google Scholar] [CrossRef]
- Inoue, D.; Kabata, T.; Kajino, Y.; Yanagi, Y.; Ima, M.; Iyobe, T.; Fujimaru, N.; Demura, S. Do elevated preoperative serum inflammatory markers influence surgical site or periprosthetic joint infections following primary total hip arthroplasty? J. Orthop. Sci. 2024. [Google Scholar] [CrossRef] [PubMed]
- Dugdale, E.M.; Uvodich, M.E.; Osmon, D.R.; Pagnano, M.W.; Berry, D.J.; Abdel, M.P. Laboratory Value Effectiveness in Predicting Early Postoperative Periprosthetic Joint Infection After Total Hip Arthroplasty. J. Arthroplast. 2022, 37, 574–580. [Google Scholar] [CrossRef]
- Pfitzner, T.; Krocker, D.; Perka, C.; Matziolis, G. Das C-reaktive Protein. Ein unabhängiger Risikofaktor für die Entwicklung eines Infekts nach Primärendoprothetik. Orthopade 2008, 37, 1116–1120. [Google Scholar] [CrossRef]
- Ghosh, S.; Paul, S.; Bhattacharjee, D.; Ghosh, P.; Chatterjee, N. Prognostic value of baseline high-sensitivity C-reactive protein in patients undergoing replacement arthroplasty. J. Nepal. Med. Assoc. 2009, 48, 144–148. [Google Scholar] [CrossRef]
- Domecky, P.; Rejman Patkova, A.; Mala-Ladova, K.; Maly, J. Inflammatory blood parameters as prognostic factors for implant-associated infection after primary total hip or knee arthroplasty: A systematic review. BMC Musculoskelet. Disord. 2023, 24, 383. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Ampuero, J.; de Dios, M. What are the risk factors for infection in hemiarthroplasties and total hip arthroplasties? Clin. Orthop. Relat. Res. 2010, 468, 3268–3277. [Google Scholar] [CrossRef] [PubMed]
- Cumming, D.; Parker, M.J. Urinary catheterisation and deep wound infection after hip fracture surgery. Int. Orthop. 2007, 31, 483–485. [Google Scholar] [CrossRef]
- Ridgeway, S.; Wilson, J.; Charlet, A.; Kafatos, G.; Pearson, A.; Coello, R. Infection of the surgical site after arthroplasty of the hip. J. Bone Jt. Surg. Br. Vol. 2005, 87, 844–850. [Google Scholar] [CrossRef]
- Rodriguez-Bano, J.; del Toro, M.D.; Lupion, C.; Suárez, A.I.; Silva, L.; Nieto, I.; Muniain, M.A. Arthroplasty-related infection: Incidence, risk factors, clinical features, and outcome. Enfermedades Infecc. Y Microbiol. Clin. 2008, 26, 614–620. [Google Scholar]
- Patel, R. Periprosthetic Joint Infection. Reply. N. Engl. J. Med. 2023, 388, 1439. [Google Scholar] [CrossRef]
- Garvin, K.L.; Hanssen, A.D. Infection after total hip arthroplasty. Past, present, and future. J. Bone Jt. Surg. Am. 1995, 77, 1576–1588. [Google Scholar] [CrossRef]
- Rodriguez-Merchan, E.C.; Delgado-Martinez, A.D. Risk Factors for Periprosthetic Joint Infection after Primary Total Knee Arthroplasty. J. Clin. Med. 2022, 11, 6128. [Google Scholar] [CrossRef]

| Type | n | % |
|---|---|---|
| Cementless | 35 | 14.6 |
| Hybrid | 97 | 40.6 |
| Cemented | 107 | 44.8 |
| n | % | |
|---|---|---|
| Arterial hypertension | 144 | 60 |
| Coronary heart disease | 45 | 19 |
| Type 2 diabetes mellitus | 41 | 17 |
| Atrial fibrillation | 25 | 10 |
| Obesity | 22 | 9 |
| Hyperlipidemia | 11 | 5 |
| Cardiac infarction | 11 | 5 |
| Peripheral arterial disease | 8 | 3 |
| Renal insufficiency | 7 | 3 |
| Apoplexy | 6 | 3 |
| Chronic obstructive pulmonary disease | 3 | 1 |
| Pulmonary embolism | 2 | 1 |
| Deep vein thrombosis | 1 | 1 |
| CRP Preoperatively [mg/dL] (Median/Range) | CRP Postoperatively [mg/dL] (Median/Range) | Absolute ΔCRP [mg/dL] (Median/Range) | Multiplication Factor of CRP (Post-/Preoperative) (Median/Range) | |
|---|---|---|---|---|
| Without infection | 0.26 (0.01–5.5) | 8.86 (0.02–28.67) | 8.5 (−0.29–27.81) | 29.38 (0.15–1369) |
| With infection | 0.45 (0.05–12.58) | 11.3 (2.85–19.46) | 8.37 (2.28–18.07) | 29.39 (1.47–157.4) |
| p-Value | 0.182 | 0.167 | 0.456 | 0.684 |
| Statistical power | 0.08 | 0.08 | 0.06 | 0.05 |
| CRP Preoperatively | CRP Postoperatively | Absolute ΔCRP | Multiplication Factor of CRP | |
|---|---|---|---|---|
| Effect size Cohen’s d | 0.173 | 0.18 | 0.096 | 0.053 |
| Sample size | 1298 | 1198 | 4206 | 13,792 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mederake, M.; Hofmann, U.K.; Eleftherakis, G. Prognostic Value of C-Reactive Protein in Primary Total Hip Arthroplasty. Antibiotics 2025, 14, 205. https://doi.org/10.3390/antibiotics14020205
Mederake M, Hofmann UK, Eleftherakis G. Prognostic Value of C-Reactive Protein in Primary Total Hip Arthroplasty. Antibiotics. 2025; 14(2):205. https://doi.org/10.3390/antibiotics14020205
Chicago/Turabian StyleMederake, Moritz, Ulf Krister Hofmann, and Georgios Eleftherakis. 2025. "Prognostic Value of C-Reactive Protein in Primary Total Hip Arthroplasty" Antibiotics 14, no. 2: 205. https://doi.org/10.3390/antibiotics14020205
APA StyleMederake, M., Hofmann, U. K., & Eleftherakis, G. (2025). Prognostic Value of C-Reactive Protein in Primary Total Hip Arthroplasty. Antibiotics, 14(2), 205. https://doi.org/10.3390/antibiotics14020205






