Genomic Insights into Colistin and Tigecycline Resistance in ESBL-Producing Escherichia coli and Klebsiella pneumoniae Harboring blaKPC Genes in Ecuador
Abstract
1. Introduction
2. Results
2.1. Antibiotic Susceptibility Profile
2.2. Acquired Resistance Genetic Determinants and Mutations Related to Antibiotic Resistance Profiles
2.3. Phylogenetic Analysis
2.4. Serotypes and Virulence of the E. coli Isolates
2.5. Plasmid Analysis of the mcr-1 Gene
2.6. Genetic Environment of Carbapenem Resistance Genes
3. Discussion
Limitations
4. Materials and Methods
4.1. Initial Characterization of the Isolates
- All isolates from poultry were identified in 2014 in a previous study conducted by our group [22].
- The clinical isolates were selected from a 2016 sample collection of 4000 Enterobacteriaceae gathered by Zurita&Zurita Laboratories.
- The compost isolate was identified during an analysis of the prevalence of blaCTX-M in an agricultural productive unit in Quito, Ecuador, conducted in 2016 (unpublished data).
4.2. Sample Collection and Preparation for Sequencing
4.3. Antibiotic Susceptibility Testing
4.4. Whole-Genome Sequencing
4.5. Mating Assay and PCR-Based Replicon Typing (PBRT)
4.6. Plasmid Analysis
4.7. Data Management and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doi, Y. Treatment Options for Carbapenem-resistant Gram-negative Bacterial Infections. Clin. Infect. Dis. 2019, 69 (Suppl. 7), S565–S575. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lutgring, J.D.; Limbago, B.M. The Problem of Carbapenemase-Producing-Carbapenem-Resistant Enterobacteriaceae. J. Infect. Dis. 2019, 215, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Mączyńska, B.; Frej-Mądrzak, M.; Sarowska, J.; Woronowicz, K.; Choroszy-Król, I.; Jama-Kmiecik, A. Evolution of Antibiotic Resistance in Escherichia coli and Klebsiella pneumoniae Clinical Isolates in a Multi-Profile Hospital over 5 Years (2017–2021). J. Clin. Med. 2023, 12, 2414. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peirano, G.; Pitout, J.D.D. Molecular Epidemiology of Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae in Canada. Front. Microbiol. 2019, 10, 1640. [Google Scholar]
- Wise, M.G.; Karlowsky, J.A.; Mohamed, N.; Hermsen, E.D.; Kamat, S.; Townsend, A.; Brink, A.; Soriano, A.; Paterson, D.L.; Moore, L.S.P.; et al. Global trends in carbapenem- and difficult-to-treat-resistance among World Health Organization priority bacterial pathogens: ATLAS surveillance program 2018–2022. J. Glob. Antimicrob. Resist. 2024, 37, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Latifi, F.; Pakzad, R.; Asadollahi, P.; Hematian, A.; Pakzad, I. Worldwide Prevalence of Colistin Resistance among Enterobacteriaceae: A Systematic Review and Meta-Analysis. Clin. Lab. 2023, 69, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Gharaibeh, S.; Shatnawi, A. The Role of Polymyxins in the Treatment of Gram-Negative Infections. Antibiotics 2019, 8, 116. [Google Scholar]
- Mondal, A.H.; Khare, K.; Saxena, P.; Debnath, P.; Mukhopadhyay, K.; Yadav, D. A Review on Colistin Resistance: An Antibiotic of Last Resort. Microorganisms 2024, 12, 772. [Google Scholar] [CrossRef] [PubMed]
- Stefaniuk, E.; Tyski, S. Epidemiology of Polymyxin Resistance in Enterobacteriaceae. J. Clin. Microbiol. 2019, 57, e00523–e00619. [Google Scholar]
- Gostyńska, A.; Piwowarczyk, L.; Nadolna, M.; Jelińska, A.; Dettlaff, K.; Ogrodowczyk, M.; Popielarz-Brzezińska, M.; Stawny, M. Toward Safe Pharmacotherapy: The Interplay between Meropenem and Parenteral Nutrition Admixtures. Antibiotics 2021, 10, 217. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Kritsotakis, E.I.; Gikas, A. Treatment options for K. pneumoniae, P. aeruginosa and A. baumannii co-resistant to carbapenems, aminoglycosides, polymyxins and tigecycline: An approach based on the mechanisms of resistance to carbapenems. Infection 2020, 48, 835–851. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anyanwu, M.U.; Nwobi, O.C.; Okpala, C.O.R.; Ezeonu, I.M. Mobile Tigecycline Resistance: An Emerging Health Catastrophe Requiring Urgent One Health Global Intervention. Front. Microbiol. 2022, 13, 808744. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yaghoubi, S.; Zekiy, A.O.; Krutova, M.; Gholami, M.; Kouhsari, E.; Sholeh, M.; Ghafouri, Z.; Maleki, F. Tigecycline antibacterial activity, clinical effectiveness, and mechanisms and epidemiology of resistance: Narrative review. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1003–1022. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhai, W.; Tian, Y.; Lu, M.; Zhang, M.; Song, H.; Fu, Y.; Ma, T.; Sun, C.; Bai, L.; Wang, Y.; et al. Presence of Mobile Tigecycline Resistance Gene tet(X4) in Clinical Klebsiella pneumoniae. Microbiol. Spectr. 2022, 10, e0108121. [Google Scholar] [CrossRef]
- Ortega-Paredes, D.; de Janon, S.; Villavicencio, F.; Ruales, K.J.; De La Torre, K.; Villacís, J.E.; Wagenaar, J.A.; Matheu, J.; Bravo-Vallejo, C.; Fernández-Moreira, E.; et al. Broiler Farms and Carcasses Are an Important Reservoir of Multi-Drug Resistant Escherichia coli in Ecuador. Front. Vet. Sci. 2020, 7, 547843. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zurita, J.; Sevillano, G.; Paz YMiño, A.; Haro, N.; Larrea-Álvarez, M.; Alcocer, I.; Ortega-Paredes, D. Dominance of ST131, B2, blaCTX-M-15, and papA-papC-kpsMII-uitA among ESBL Escherichia coli isolated from bloodstream infections in Quito, Ecuador: A 10-year surveillance study (2009–2019). J. Appl. Microbiol. 2023, 134, lxad269. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Paredes, D.; Barba, P.; Mena-López, S.; Espinel, N.; Crespo, V.; Zurita, J. High quantities of multidrug-resistant Escherichia coli are present in the Machángara urban river in Quito, Ecuador. J. Water Health 2020, 18, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Zurita, J.; Yánez, F.; Sevillano, G.; Ortega-Paredes, D.; Paz, Y.; Miño, A. Ready-to-eat street food: A potential source for dissemination of multidrug-resistant Escherichia coli epidemic clones in Quito, Ecuador. Lett. Appl. Microbiol. 2020, 70, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Zurita, J.; Solís, M.B.; Ortega-Paredes, D.; Barba, P.; Paz YMiño, A.; Sevillano, G. High prevalence of B2-ST131 clonal group among extended-spectrum β-lactamase-producing Escherichia coli isolated from bloodstream infections in Quito, Ecuador. J. Glob. Antimicrob. Resist. 2019, 19, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Paredes, D.; Barba, P.; Zurita, J. Colistin-resistant Escherichia coli clinical isolate harbouring the mcr-1 gene in Ecuador. Epidemiol. Infect. 2016, 144, 2967–2970. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Zurita, J.; Ortega-Paredes, D.; Barba, P. First Description of Shigella sonnei Harboring blaCTX-M-55 Outside Asia. J. Microbiol. Biotechnol. 2016, 26, 2224–2227. [Google Scholar] [CrossRef] [PubMed]
- Vinueza-Burgos, C.; Ortega-Paredes, D.; Narváez, C.; De Zutter, L.; Zurita, J. Characterization of cefotaxime resistant Escherichia coli isolated from broiler farms in Ecuador. PLoS ONE 2019, 14, e0207567. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ortega-Paredes, D.; Haro, M.; Leoro-Garzón, P.; Barba, P.; Loaiza, K.; Mora, F.; Fors, M.; Vinueza-Burgos, C.; Fernández-Moreira, E. Multidrug-resistant Escherichia coli isolated from canine faeces in a public park in Quito, Ecuador. J. Glob. Antimicrob. Resist. 2019, 18, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Paredes, D.; Barba, P.; Mena-López, S.; Espinel, N.; Zurita, J. Escherichia coli hyperepidemic clone ST410-A harboring blaCTX-M-15 isolated from fresh vegetables in a municipal market in Quito-Ecuador. Int. J. Food Microbiol. 2018, 280, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Barba, P.; Ortega-Paredes, D.; Molina Cuasapaz, G.; Zurita, J. Occurrence of extended-spectrum beta-lactamase producing Escherichia coli in a dairy farm from Quito, Ecuador. API 2017. [Google Scholar] [CrossRef]
- Ortega-Paredes, D.; Larrea-Álvarez, C.M.; Torres-Elizalde, L.; de Janon, S.; Vinueza-Burgos, C.; Hidalgo-Arellano, L.; Šefcová, M.A.; Molina-Cuasapaz, G.; Fernandez-Moreira, E.; Larrea-Álvarez, M. Antibiotic Resistance Awareness among Undergraduate Students in Quito, Ecuador. Antibiotics 2022, 11, 197. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zurita, J.; Alcocer, I.; Ortega-Paredes, D.; Barba, P.; Yauri, F.; Iñiguez, D.; Mora, M. Carbapenemhydrolysing β-lactamase KPC-2 in Klebsiella pneumoniae isolated in Ecuadorian hospitals. J. Glob. Antimicrob. Resist. 2013, 1, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Bastidas-Caldes, C.; Guerrero-Freire, S.; Ortuño-Gutiérrez, N.; Sunyoto, T.; Gomes-Dias, C.A.; Ramírez, M.S.; Calero-Cáceres, W.; Harries, A.D.; Rey, J.; de Waard, J.H.; et al. Colistin resistance in Escherichia coli and Klebsiella pneumoniae in humans and backyard animals in Ecuador. Rev. Panam. Salud Publica 2023, 47, e48. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lagerstrom, K.M.; Hadly, E.A. Under-Appreciated Phylogroup Diversity of Escherichia coli within and between Animals at the Urban-Wildland Interface. Appl. Environ. Microbiol. 2023, 89, e0014223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ovi, F.; Zhang, L.; Nabors, H.; Jia, L.; Adhikari, P. A compilation of virulence-associated genes that are frequently reported in avian pathogenic Escherichia coli (APEC) compared to other E. coli. J. Appl. Microbiol. 2023, 134, lxad014. [Google Scholar] [CrossRef] [PubMed]
- Riley, L.W. Distinguishing Pathovars from Nonpathovars: Escherichia coli. Microbiol. Spectr. 2020, 8, 10–128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mendes, G.; Santos, M.L.; Ramalho, J.F.; Duarte, A.; Caneiras, C. Virulence factors in carbapenem-resistant hypervirulent Klebsiella pneumoniae. Front. Microbiol. 2023, 14, 1325077. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cejas, D.; Elena, A.; Guevara Nuñez, D.; Sevilla Platero, P.; De Paulis, A.; Magariños, F.; Alfonso, C.; Berger, M.A.; Fernández-Canigia, L.; Gutkind, G.; et al. Changing epidemiology of KPC-producing Klebsiella pneumoniae in Argentina: Emergence of hypermucoviscous ST25 and high-risk clone ST307. J. Glob. Antimicrob. Resist. 2019, 18, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Ernst, C.M.; Braxton, J.R.; Rodriguez-Osorio, C.A.; Zagieboylo, A.P.; Li, L.; Pironti, A.; Manson, A.L.; Nair, A.V.; Benson, M.; Cummins, K.; et al. Adaptive evolution of virulence and persistence in carbapenem-resistant Klebsiella pneumoniae. Nat. Med. 2020, 26, 705–711. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, X.; Li, Y.; Yang, Y.; Shen, Z.; Wu, Y.; Wang, S. Global impact of mcr-1-positive Enterobacteriaceae bacteria on “one health”. Crit. Rev. Microbiol. 2020, 46, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Agrocalidad. Agrocalidad Prohíbe El Uso Del Antibiótico Colistina En Animales 2019. Available online: http://www.agrocalidad.gob.ec/agrocalidad-prohibe-el-uso-del-antibiotico-colistina-en-animales/ (accessed on 18 January 2023).
- Liu, J.H.; Liu, Y.Y.; Shen, Y.B.; Yang, J.; Walsh, T.R.; Wang, Y.; Shen, J. Plasmid-mediated colistin-resistance genes: mcr. Trends Microbiol. 2024, 32, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Hussein, N.H.; Al-Kadmy, I.M.S.; Taha, B.M.; Hussein, J.D. Mobilized colistin resistance (mcr) genes from 1 to 10: A comprehensive review. Mol. Biol. Rep. 2021, 48, 2897–2907. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Santiago, J.; Cornejo-Juárez, P.; Silva-Sánchez, J.; Garza-Ramos, U. Polymyxin resistance in Enterobacterales: Overview and epidemiology in the Americas. Int. J. Antimicrob. Agents. 2021, 58, 106426. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, R.; Xia, Q.; Yu, J.; Yi, L.X.; Huang, Y.; Deng, M.; He, W.Y.; Bai, Y.; Lv, L.; et al. The evolution of infectious transmission promotes the persistence of mcr-1 plasmids. mBio 2023, 14, e0044223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tietgen, M.; Sedlaczek, L.; Higgins, P.G.; Kaspar, H.; Ewers, C.; Göttig, S. Colistin Resistance Mechanisms in Human and Veterinary Klebsiella pneumoniae Isolates. Antibiotics 2022, 11, 1672. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Lin, Y.; Wang, Z.; Hu, N.; Liu, Q.; Zhou, W.; Li, X.; Hu, L.; Guo, J.; Huang, X.; et al. Molecular Mechanisms of Colistin Resistance in Klebsiella pneumoniae in a Tertiary Care Teaching Hospital. Front. Cell. Infect. Microbiol. 2021, 11, 673503. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, Q.; Cheung, Y.; Liu, C.; Xiao, Q.; Sun, B.; Zhou, J.; Chan, E.W.C.; Zhang, R.; Chen, S. Structural and mechanistic basis of the high catalytic activity of monooxygenase Tet(X4) on tigecycline. BMC Biol. 2021, 19, 262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Korczak, L.; Majewski, P.; Iwaniuk, D.; Sacha, P.; Matulewicz, M.; Wieczorek, P.; Majewska, P.; Wieczorek, A.; Radziwon, P.; Tryniszewska, E. Molecular mechanisms of tigecycline-resistance among Enterobacterales. Front. Cell. Infect. Microbiol. 2024, 14, 1289396. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.; Wu, H.; Mei, C.Y.; Wang, Y.; Wang, Z.Y.; Lu, M.J.; Pan, Z.M.; Jiao, X. Multiple Mechanisms of Tigecycline Resistance in Enterobacteriaceae from a Pig Farm, China. Microbiol. Spectr. 2021, 9, e0041621. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sundaramoorthy, N.S.; Sivasubramanian, A.; Nagarajan, S. Simultaneous inhibition of MarR by salicylate and efflux pumps by curcumin sensitizes colistin resistant clinical isolates of Enterobacteriaceae. Microb. Pathog. 2020, 148, 104445. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Sheng, Z.; Hao, M.; Jiang, J.; Ye, M.; Chen, Y.; Xu, X.; Guo, Q.; Wang, M. RamA upregulates multidrug resistance efflux pumps AcrAB and OqxAB in Klebsiella pneumoniae. Int. J. Antimicrob. Agents 2021, 57, 106251. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hu, X.; Zhao, Y.; Shi, Y.; Ding, H.; Wu, R.; Zhao, Z.; Ji, J. Comparative Analysis of blaKPC Expression in Tn4401 Transposons and the Tn3-Tn4401 Chimera. Antimicrob. Agents Chemother. 2019, 63, e02434–e02518. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.A.; Melano, R.; Cárdenas, P.A.; Trueba, G. Mobile genetic elements associated with carbapenemase genes in South American Enterobacterales. Braz. J. Infect. Dis. 2020, 24, 231–238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lentz, S.A.M.; Dalmolin, T.V.; Barth, A.L.; Martins, A.F. mcr-1 Gene in Latin America: How Is It Disseminated Among Humans, Animals, and the Environment? Front. Public Health 2021, 9, 648940. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De La Cadena, E.; Mahecha, M.; Velandia, A.M.; García-Betancur, J.C.; Rojas, L.J.; Porras, J.; Pallares, C.; Villegas, M.V. Identification of mcr-1 Genes and Characterization of Resistance Mechanisms to Colistin in Escherichia coli Isolates from Colombian Hospitals. Antibiotics 2023, 12, 488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, R.; van Dorp, L.; Shaw, L.P.; Bradley, P.; Wang, Q.; Wang, X.; Jin, L.; Zhang, Q.; Liu, Y.; Rieux, A.; et al. The global distribution and spread of the mobilized colistin resistance gene mcr-1. Nat. Commun. 2018, 9, 1179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- WHO. WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
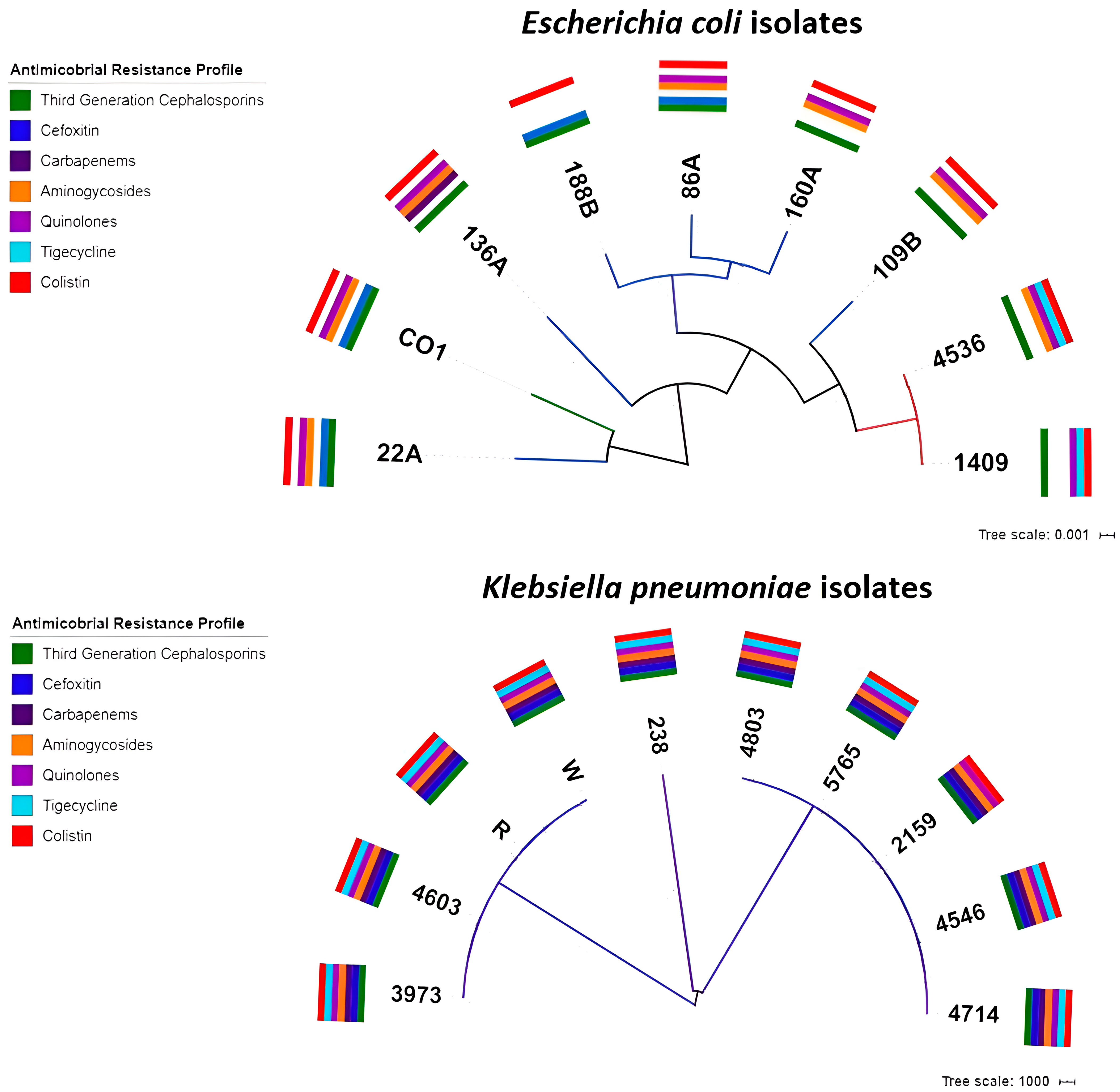
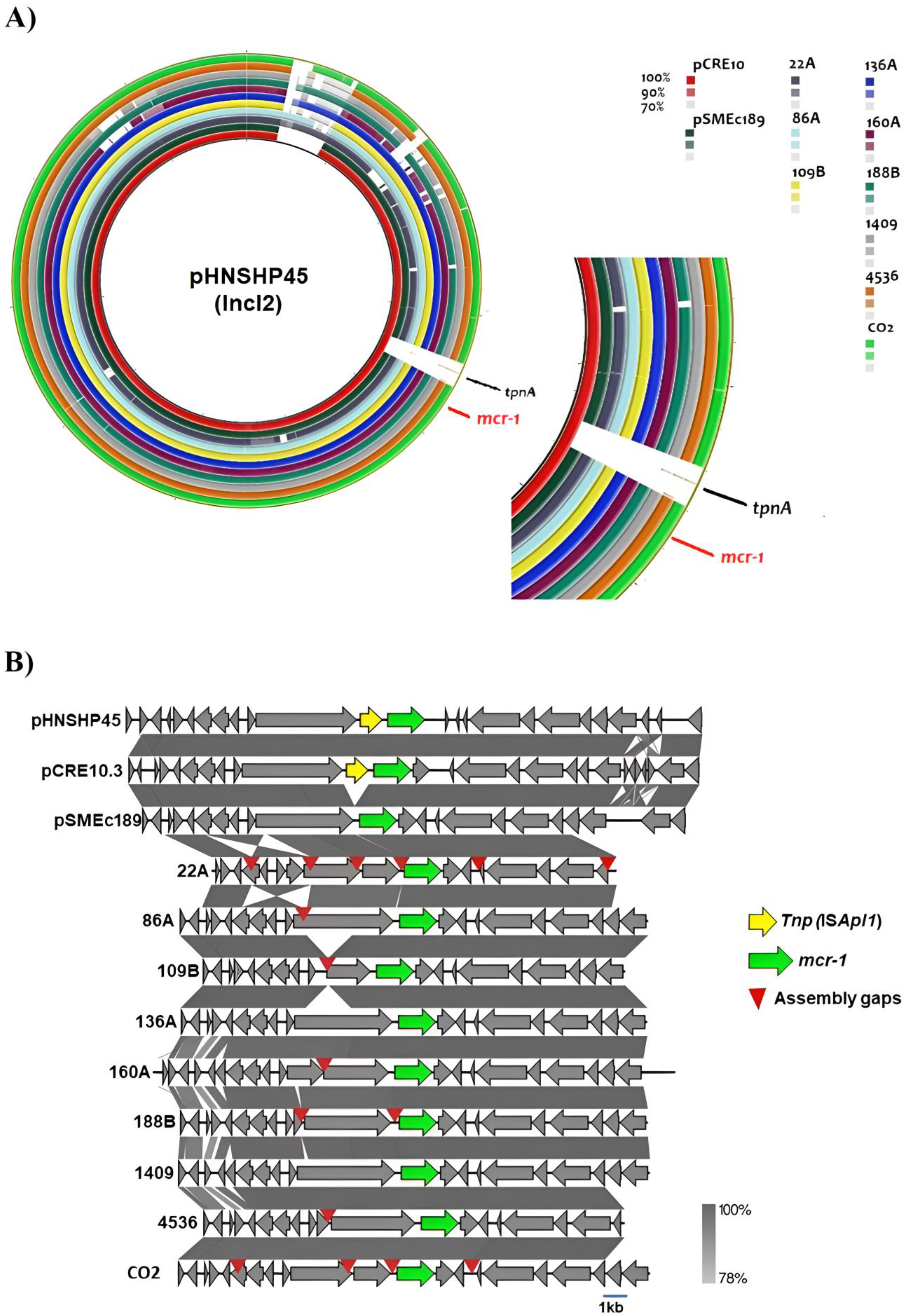
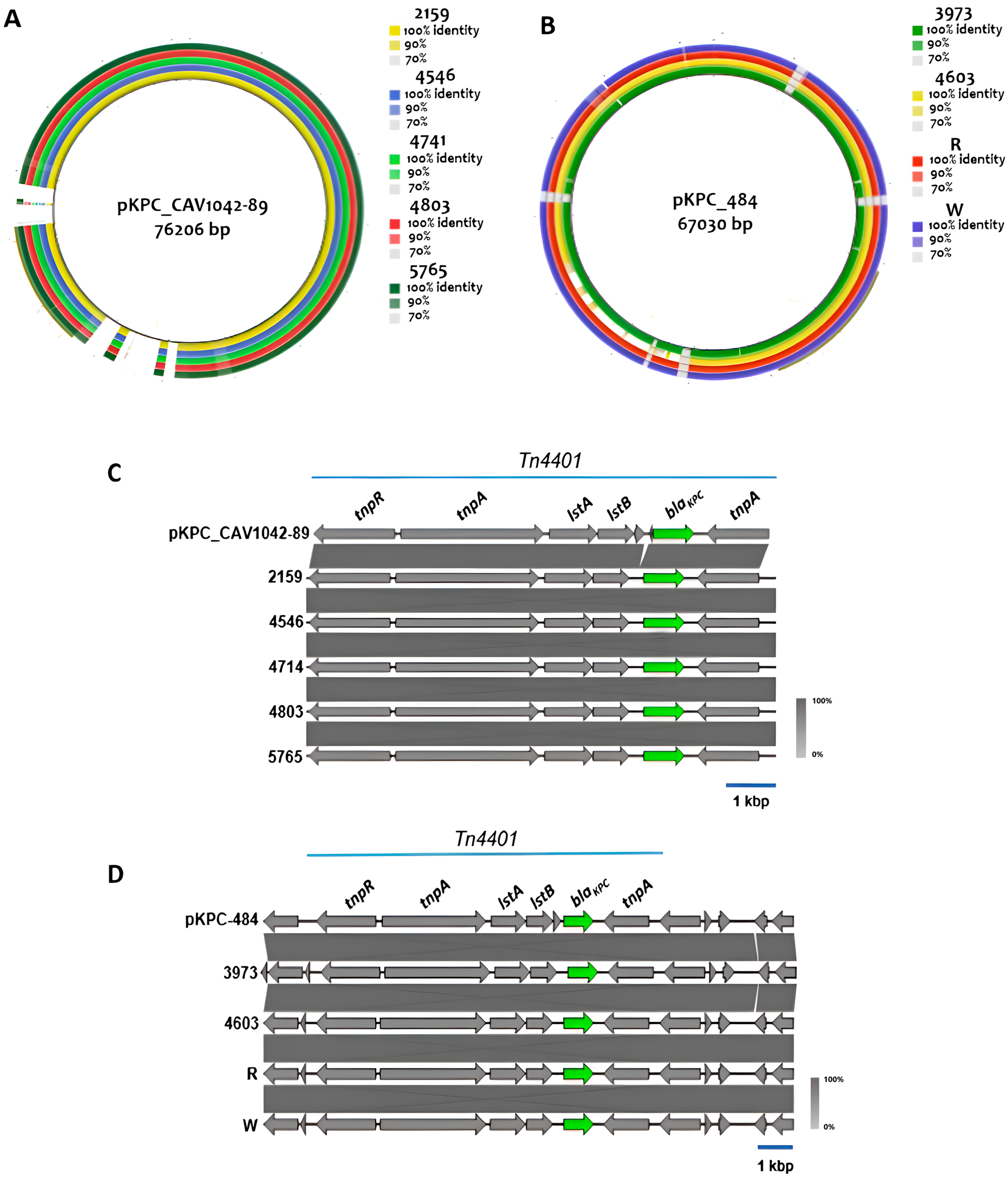
| Isolate | Species | Date | Origin | Age | Location | AMP | TPZ | FOX | CAZ | CRO | FEP | DOR | ERT | IMI | MEM | AK | GEN | CIP | TIG | COL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1409 | E. coli | 10 February 2016 | Peritoneal liquid | 14 | Quito | 16 | ≤4 | 8 | 16 | ≥64 | 2 | ≤0.12 | ≤0.5 | ≤0.25 | ≤0.25 | ≤2 | ≤1 | ≥4 | 2 | 4 |
| 4536 | E. coli | 10 June 2016 | Wound secretion | 72 | Quito | 32 | 64 | ≤4 | 16 | ≥64 | 2 | ≤0.12 | ≤0.5 | ≤0.25 | ≤0.25 | 8 | ≥16 | ≥4 | 1 | 4 |
| CO2 (CO1) | E. coli | 2016 | Compost dairy farm | - | Quito | ≥32 | ≤4 | 16 | 16 | ≥64 | ≥64 | ≤0.12 | ≤0.5 | ≤0.25 | ≤0.25 | ≤2 | ≥16 | ≥4 | ≤0.5 | 4 |
| 86A | E. coli | 2014 | Poultry | - | Yaruquí | ≥32 | ≤4 | 16 | 4 | ≥64 | ≥64 | ≤0.12 | ≤0.5 | ≤0.25 | ≤0.25 | ≤2 | ≥16 | ≥4 | ≤0.5 | 8 |
| 109B | E. coli | 2014 | Poultry | - | El Chota | ≥32 | ≤4 | 8 | ≤1 | ≥64 | 2 | ≤0.12 | ≤0.5 | ≤0.25 | ≤0.25 | ≤2 | ≥16 | ≥4 | ≤0.5 | 4 |
| 136A | E. coli | 2014 | Poultry | - | Santo Domingo | 16 | ≤4 | ≤4 | ≤1 | 16 | ≤1 | ≤0.12 | ≤0.5 | ≤0.25 | ≤0.25 | ≤2 | ≥16 | ≥4 | ≤0.5 | 8 |
| 22A | E. coli | 2014 | Poultry | - | Ascazubi | ≥32 | ≥128 | ≥64 | 16 | 16 | ≤1 | ≤0.12 | ≤0.5 | ≤0.25 | ≤0.25 | ≤2 | ≥16 | ≥4 | ≤0.5 | 8 |
| 160A | E. coli | 2014 | Poultry | - | Guayllabamba | ≥32 | ≤4 | 8 | 32 | ≥64 | 4 | 0.5 | ≤0.5 | ≤0.25 | ≤0.25 | 4 | ≥16 | ≥4 | ≤0.5 | ≥16 |
| 188B | E. coli | 2014 | Poultry | - | Ibarra | 16 | ≤4 | ≥64 | 4 | 8 | ≤1 | ≤0.12 | ≤0.5 | ≤0.25 | ≤0.25 | ≤2 | ≤1 | ≤0.25 | ≤0.5 | 8 |
| 1220672 (W) | K. pneumoniae | 12 February 2016 | Urine | 61 | Quito | ≥32 | ≥128 | ≥64 | ≥64 | ≥64 | ≥64 | ≥8 | ≥8 | ≥16 | ≥16 | ≥64 | ≥16 | ≥4 | 4 | ≥16 |
| 1204191 (R) | K. pneumoniae | 12 February 2016 | Blood | 52 | Quito | ≥32 | ≥128 | ≥64 | ≥64 | ≥64 | ≥64 | ≥8 | ≥8 | ≥16 | ≥16 | ≥64 | ≥16 | ≥4 | 4 | ≥16 |
| 238 | K. pneumoniae | 2 June 2016 | Cervical abscess | 54 | Quito | 16 | ≤4 | ≤4 | ≤1 | ≤1 | ≤1 | ≤0.12 | ≤0.5 | ≤0.25 | ≤0.25 | ≤2 | ≤1 | ≤0.5 | 2 | 4 |
| 2159 | K. pneumoniae | 3 March 2016 | Wound secretion | 37 | Quito | ≥32 | ≥128 | 32 | ≥64 | ≥64 | ≥64 | ≥8 | ≥8 | ≥16 | ≥16 | 16 | ≥16 | 2 | 2 | ≥16 |
| 3973 | K. pneumoniae | 19 May 2016 | Tracheal secretion | 55 | Quito | ≥32 | ≥128 | ≥64 | ≥64 | ≥64 | ≥64 | ≥8 | ≥8 | ≥16 | ≥16 | ≥64 | ≥16 | ≥4 | 4 | ≥16 |
| 4546 | K. pneumoniae | 10 June 2016 | Surgical wound discharge | 56 | Quito | ≥32 | ≥128 | ≥64 | ≥64 | ≥64 | ≥64 | ≥8 | ≥8 | 8 | ≥16 | ≥64 | ≥16 | ≥4 | 2 | ≥16 |
| 4603 | K. pneumoniae | 13 June 2016 | Tracheal secretion | 64 | Quito | ≥32 | ≥128 | ≥64 | ≥64 | ≥64 | ≥64 | ≥8 | ≥8 | ≥16 | ≥16 | ≥64 | ≥16 | ≥4 | ≥8 | ≥16 |
| 4714 | K. pneumoniae | 17 June 2016 | Blood | 52 | Quito | ≥32 | ≥128 | ≥64 | ≥64 | ≥64 | ≥64 | ≥8 | ≥8 | 8 | ≥16 | ≥64 | ≥16 | ≥4 | 2 | ≥16 |
| 4803 | K. pneumoniae | 21 June 2016 | Tracheal secretion | 95 | Quito | ≥32 | ≥128 | ≥64 | ≥64 | ≥64 | ≥64 | ≥8 | ≥8 | ≥16 | ≥16 | ≥64 | ≥16 | ≥4 | 2 | ≥16 |
| 5765 | K. pneumoniae | 26 July 2016 | Sputum | 71 | Quito | ≥32 | ≥128 | ≥64 | ≥64 | ≥64 | ≥64 | ≥8 | ≥8 | 8 | ≥16 | ≥64 | ≥16 | ≥4 | 2 | ≥16 |
| Isolate | Serotype | gad | iss | mchF | cma | lpfA | iroN | air | eilA | iha | celB | ccl | ireA | cba | astA | Total per Isolate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1409 ͳ | O9:H4 | X | X | 2 | ||||||||||||
| 4536 ͳ | O9:H4 | X | X | X | 3 | |||||||||||
| CO2 α | H25 | X | X | X | X | X | X | X | 7 | |||||||
| 86A * | 0115:H28 | X | X | X | X | X | 5 | |||||||||
| 109B * | 02:H4 | X | X | X | X | X | 5 | |||||||||
| 136A * | H28 | X | X | X | X | X | X | X | X | X | X | 10 | ||||
| 22A * | H34 | X | X | X | X | X | 5 | |||||||||
| 160A * | 0157:H21 | X | X | X | X | X | X | 6 | ||||||||
| 188A * | H20 | X | X | 2 | ||||||||||||
| Total per gene | 8 | 7 | 5 | 4 | 4 | 2 | 3 | 3 | 2 | 1 | 1 | 1 | 1 | 1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Paredes, D.; Del Canto, F.; Rios, R.; Diaz, L.; Reyes, J.; Arias, C.A.; Zurita, J. Genomic Insights into Colistin and Tigecycline Resistance in ESBL-Producing Escherichia coli and Klebsiella pneumoniae Harboring blaKPC Genes in Ecuador. Antibiotics 2025, 14, 206. https://doi.org/10.3390/antibiotics14020206
Ortega-Paredes D, Del Canto F, Rios R, Diaz L, Reyes J, Arias CA, Zurita J. Genomic Insights into Colistin and Tigecycline Resistance in ESBL-Producing Escherichia coli and Klebsiella pneumoniae Harboring blaKPC Genes in Ecuador. Antibiotics. 2025; 14(2):206. https://doi.org/10.3390/antibiotics14020206
Chicago/Turabian StyleOrtega-Paredes, David, Felipe Del Canto, Rafael Rios, Lorena Diaz, Jinnethe Reyes, Cesar A. Arias, and Jeannete Zurita. 2025. "Genomic Insights into Colistin and Tigecycline Resistance in ESBL-Producing Escherichia coli and Klebsiella pneumoniae Harboring blaKPC Genes in Ecuador" Antibiotics 14, no. 2: 206. https://doi.org/10.3390/antibiotics14020206
APA StyleOrtega-Paredes, D., Del Canto, F., Rios, R., Diaz, L., Reyes, J., Arias, C. A., & Zurita, J. (2025). Genomic Insights into Colistin and Tigecycline Resistance in ESBL-Producing Escherichia coli and Klebsiella pneumoniae Harboring blaKPC Genes in Ecuador. Antibiotics, 14(2), 206. https://doi.org/10.3390/antibiotics14020206






