Seventeen Years of an Antibiotic Stewardship Programme: Trends in Antibiotic Prescribing and Gram-Negative Bacilli Susceptibility at a Quaternary Healthcare Institution
Abstract
1. Introduction
1.1. Initiation Phase (2008–2014)
1.2. Advancement Phase (2015–2018)
1.3. Optimisation Phase (2019–2023)
1.4. Innovation Phase (2023 to Date)
1.5. Study Objectives
2. Methods
2.1. Data Collection
2.2. Statistical Analysis
3. Results
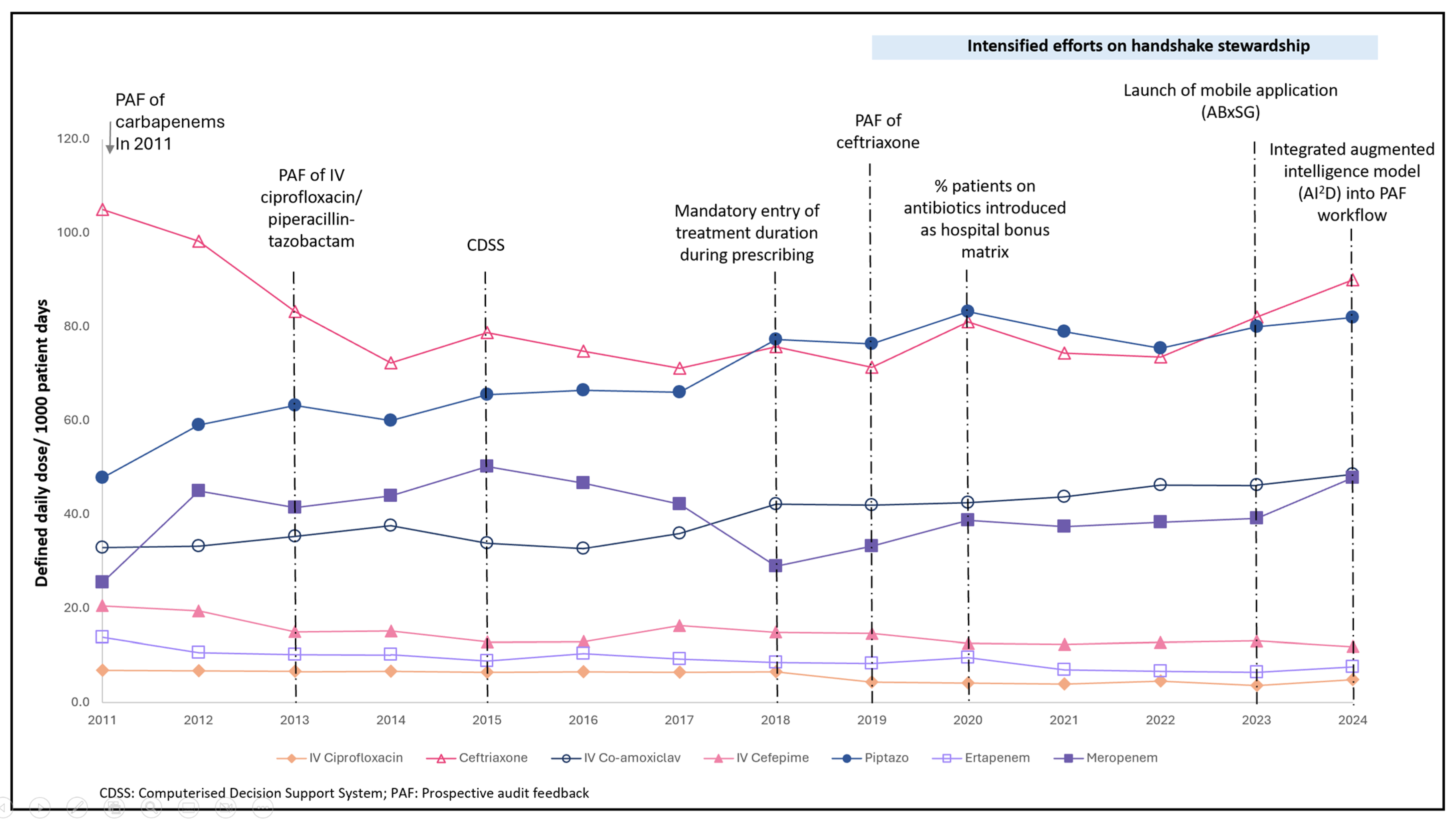
3.1. Antibiotic Consumption
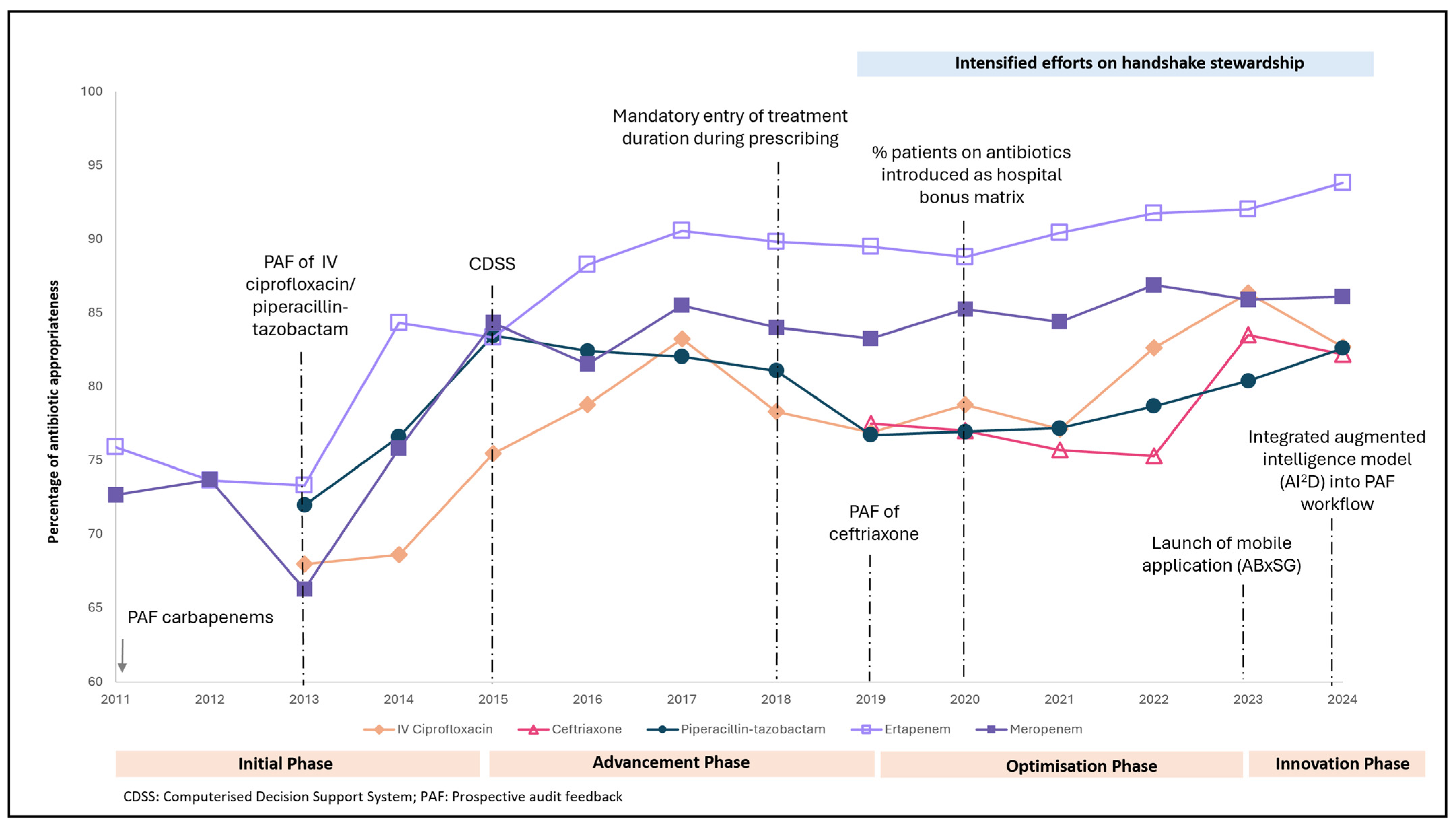
3.2. Antibiotic Appropriateness
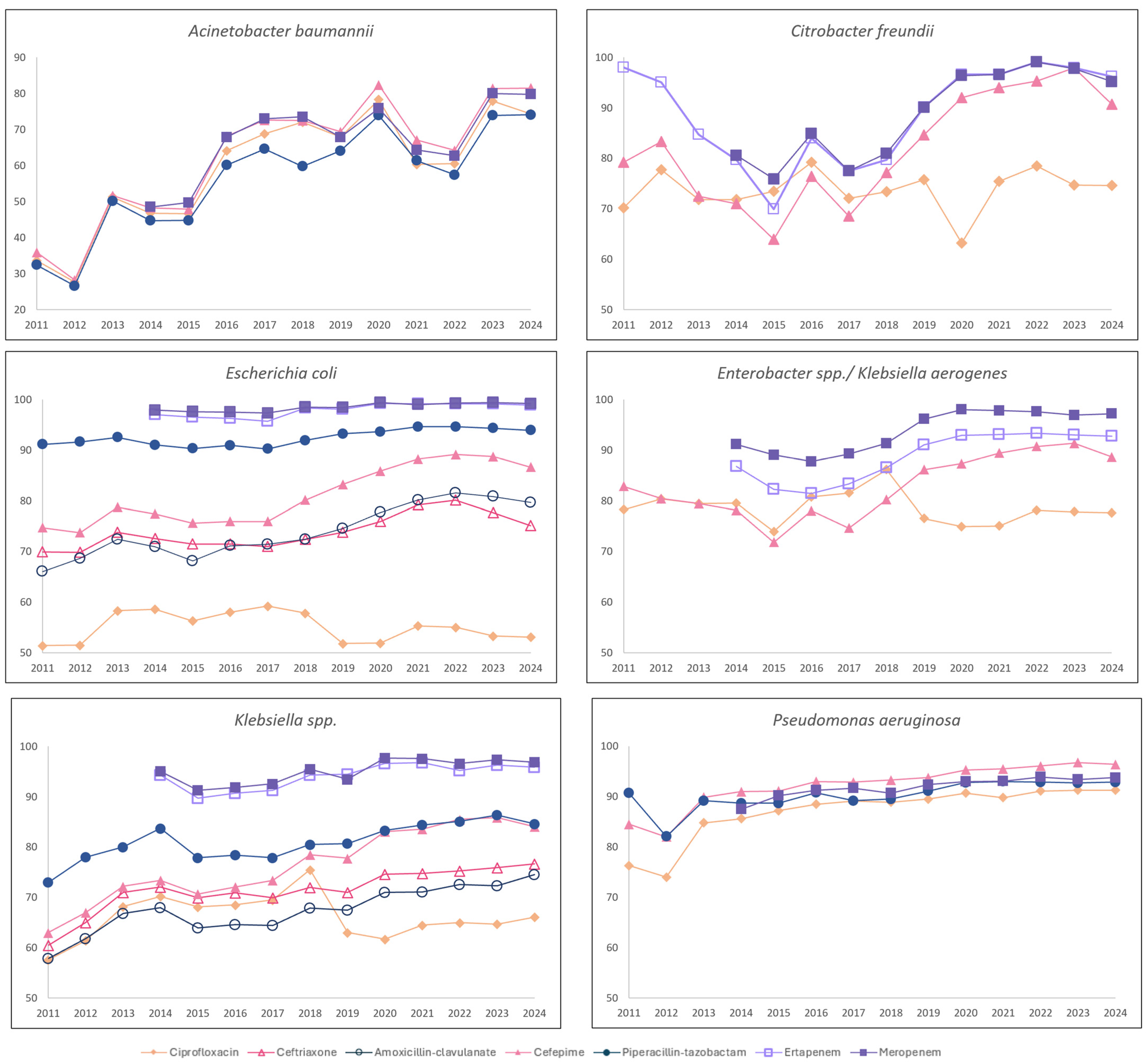
3.3. GNB Susceptibility
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef]
- Pollack, L.A.; Srinivasan, A. Core elements of hospital antibiotic stewardship programs from the Centers for Disease Control and Prevention. Clin. Infect. Dis. 2014, 59 (Suppl. 3), S97–S100. [Google Scholar] [CrossRef]
- Chua, A.Q.; Kwa, A.; Tan, T.Y.; Legido-Quigley, H.; Hsu, L.Y. Ten-year narrative review on antimicrobial resistance in Singapore. Singap. Med. J. 2019, 60, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.M.; Ang, L.W.; Heng, S.T.; Kwa, A.L.-H.; Wu, J.E.; Seah, X.F.V.; Lee, S.Y.; Seah, J.; Choo, R.; Lim, P.L.; et al. Antibiotic utilisation and resistance over the first decade of nationally funded antimicrobial stewardship programmes in Singapore acute-care hospitals. Antimicrob. Resist. Infect. Control. 2023, 12, 82. [Google Scholar] [CrossRef]
- Ababneh, M.A.; Nasser, S.A.; Rababa’h, A.M. A systematic review of Antimicrobial Stewardship Program implementation in Middle Eastern countries. Int. J. Infect. Dis. 2021, 105, 746–752. [Google Scholar] [CrossRef]
- Karanika, S.; Paudel, S.; Grigoras, C.; Kalbasi, A.; Mylonakis, E. Systematic Review and Meta-analysis of Clinical and Economic Outcomes from the Implementation of Hospital-Based Antimicrobial Stewardship Programs. Antimicrob. Agents Chemother. 2016, 60, 4840–4852. [Google Scholar] [CrossRef]
- Strazzulla, A.; Adrien, V.; Houngnandan, S.R.; Devatine, S.; Bahmed, O.; Abroug, S.; Hamrouni, S.; Monchi, M.; Diamantis, S. Characteristics of Pseudomonas aeruginosa infection in intensive care unit before (2007–2010) and after (2011–2014) the beginning of an antimicrobial stewardship program. Antimicrob. Steward. Healthc. Epidemiol. 2024, 4, e60. [Google Scholar] [CrossRef]
- Mahmoudi, L.; Sepasian, A.; Firouzabadi, D.; Akbari, A. The Impact of an Antibiotic Stewardship Program on the Consumption of Specific Antimicrobials and Their Cost Burden: A Hospital-wide Intervention. Risk Manag. Healthc. Policy 2020, 13, 1701–1709. [Google Scholar] [CrossRef]
- Chrysou, K.; Zarkotou, O.; Kalofolia, S.; Papagiannakopoulou, P.; Mamali, V.; Chrysos, G.; Themeli-Digalaki, K.; Sypsas, N.; Tsakris, A.; Pournaras, S. Impact of a 4-year antimicrobial stewardship program implemented in a Greek tertiary hospital. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Liew, Y.X.; Lee, W.; Loh, J.C.Z.; Cai, Y.; Tang, S.S.L.; Lim, C.L.L.; Teo, J.; Ong, R.W.Q.; Kwa, A.L.-H.; Chlebicki, M.P. Impact of an antimicrobial stewardship programme on patient safety in Singapore General Hospital. Int. J. Antimicrob. Agents 2012, 40, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Loo, L.W.; Liew, Y.X.; Lee, W.; Lee, L.W.; Chlebicki, P.; Kwa, A.L.-H. Discontinuation of antibiotic therapy within 24 hours of treatment initiation for patients with no clinical evidence of bacterial infection: A 5-year safety and outcome study from Singapore General Hospital Antimicrobial Stewardship Program. Int. J. Antimicrob. Agents 2019, 53, 606–611. [Google Scholar] [CrossRef]
- Teo, J.; Kwa, A.L.H.; Loh, J.; Chlebicki, M.P.; Lee, W. The effect of a whole-system approach in an antimicrobial stewardship programme at the Singapore General Hospital. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Versporten, A.; Zarb, P.; Caniaux, I.; Gros, M.-F.; Drapier, N.; Miller, M.; Jarlier, V.; Nathwani, D.; Goossens, H.; Koraqi, A.; et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: Results of an internet-based global point prevalence survey. Lancet Glob. Health 2018, 6, e619–e629. [Google Scholar] [CrossRef] [PubMed]
- Loo, L.W.; Zhou, Y.P.; Wang, Y.B.; Lee, L.W.; Chung, J.S. Antimicrobial Stewardship in Cardiac Device Surgery: Impact of Behavioural Change Interventions on Extended Prophylaxis Practices. Antibiotics 2025, 14, 754. [Google Scholar] [CrossRef]
- Lee, L.W.; Lim, S.Y.C.; Zhou, Y.P.; Chung, S.J.; Chin, D.Z.; Kwa, A.L.H.; Lee, W.H.L. Impact of the ABxSG Mobile Application on Antibiotic Prescribing: An Interrupted Time Series Study. Antibiotics 2025, 14, 933. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Lim, J.L.; Lee, L.X.T.; Yii, Y.C.D.; Zhou, Y.P.; Wang, Y.; Cherng, P.Z.B.; Chlebicki, P.M.; Gan, L.S.C.; Thien, S.Y.; et al. Augmented Intelligence in Infectious Diseases (AI2D) as an Antimicrobial Stewardship Tool for Early Antibiotic Discontinuation in Suspected Lower Respiratory Tract Infections [Abstract]. In Proceedings of the Congress of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), 11–15 April 2025; Vienna, Austria. Available online: https://registration.escmid.org/AbstractList.aspx?e=30&header=0&preview=1&aig=-1&ai=29130 (accessed on 27 October 2025).
- Sumi, C.D.; Heffernan, A.J.; Lipman, J.; Roberts, J.A.; Sime, F.B. What Antibiotic Exposures Are Required to Suppress the Emergence of Resistance for Gram-Negative Bacteria? A systematic review. Clin. Pharmacokinet. 2019, 58, 1407–1443. [Google Scholar] [CrossRef]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.-M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining antibiotic levels in intensive care unit patients: Are current β-lactam antibiotic doses Sufficient for critically ill patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef]
- WHO Collaborating Centre for Drug Statistics Methodology. ATC Classification Index with DDDs, 2024; WHO: Oslo, Norway, 2024. [Google Scholar]
- CLSI. Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data, 5th ed.; CLSI guideline M39; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022. [Google Scholar]
- CLSI. Piperacillin-Tazobactam Breakpoints for Pseudomonas aeruginosa; CLSI rationale document MR15; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024. [Google Scholar]
- Tamma, P.D.; A Harris, P.N.; Mathers, A.J.; Wenzler, E.; Humphries, R.M. Breaking Down the Breakpoints: Rationale for the 2022 Clinical and Laboratory Standards Institute Revised Piperacillin-Tazobactam Breakpoints Against Enterobacterales. Clin. Infect. Dis. 2023, 77, 1585–1590. [Google Scholar] [CrossRef]
- Bork, J.T.; Heil, E.L.; Leekha, S.; Fowler, R.C.; Hanson, N.D.; Majumdar, A.; Johnson, J.K. Impact of CLSI and EUCAST Cefepime breakpoint changes on the susceptibility reporting for Enterobacteriaceae. Diagn. Microbiol. Infect. Dis. 2017, 89, 328–333. [Google Scholar] [CrossRef]
- Mihalov, P.; Hodosy, J.; Koščálová, A.; Čaprnda, M.; Kachlíková, M.; Jurenka, J.; Bendžala, M.; Sabaka, P. Antimicrobial Therapy as a Risk Factor of Multidrug-Resistant Acinetobacter Infection in COVID-19 Patients Admitted to the Intensive Care Unit. Can. J. Infect. Dis. Med. Microbiol. 2023, 2023, 4951273. [Google Scholar] [CrossRef]
- Livermore, D.M.; Hope, R.; Reynolds, R.; Blackburn, R.; Johnson, A.P.; Woodford, N. Declining cephalosporin and fluoroquinolone non-susceptibility among bloodstream Enterobacteriaceae from the UK: Links to prescribing change? J. Antimicrob. Chemother. 2013, 68, 2667–2674. [Google Scholar] [CrossRef]
- Moosdeen, F. The evolution of resistance to cephalosporins. Clin. Infect. Dis. 1997, 24, 487–493. [Google Scholar] [CrossRef]
- Aldeyab, M.A.; Harbarth, S.; Vernaz, N.; Kearney, M.P.; Scott, M.G.; Elhajji, F.W.D.; Aldiab, M.A.; McElnay, J.C. The impact of antibiotic use on the incidence and resistance pattern of extended-spectrum beta-lactamase-producing bacteria in primary and secondary healthcare settings. Br. J. Clin. Pharmacol. 2012, 74, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health. One Health Report on Antimicrobial Utilisation and Resistance 2019; Ministry of Health: Singapore, 2019. Available online: https://isomer-user-content.by.gov.sg/3/1be4b872-672a-4ea5-a417-e1c679a241d6/2019-one-health-report-on-antimicrobial-utilisation-and-resistance.pdf (accessed on 21 September 2024).
- Medina Presentado, J.C.; Paciel López, D.; Berro Castiglioni, M.; Gerez, J. Ceftriaxone and ciprofloxacin restriction in an intensive care unit: Less incidence of Acinetobacter spp. and improved susceptibility of Pseudomonas aeruginosa. Rev. Panam. Salud Publica 2011, 30, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Oh, C.E.; Choi, E.H.; Lee, H.J. The impact of the increased use of piperacillin/tazobactam on the selection of antibiotic resistance among invasive Escherichia coli and Klebsiella pneumoniae isolates. Int. J. Infect. Dis. 2013, 17, e638–e643. [Google Scholar] [CrossRef] [PubMed]
- Marquet, A.; Vibet, M.-A.; Caillon, J.; Javaudin, F.; Chapelet, G.; Montassier, E.; Batard, E. Is There an Association Between Use of Amoxicillin-Clavulanate and Resistance to Third-Generation Cephalosporins in Klebsiella pneumoniae and Escherichia coli at the Hospital Level? Microb. Drug Resist. 2018, 24, 987–994. [Google Scholar] [CrossRef]
- Payne, L.E.; Gagnon, D.J.; Riker, R.R.; Seder, D.B.; Glisic, E.K.; Morris, J.G.; Fraser, G.L. Cefepime-induced neurotoxicity: A systematic review. Crit. Care 2017, 21, 276. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, Y.; Zhou, Y.; Ye, C. Ertapenem-Induced Neurotoxicity: A Literature Review of Clinical Characteristics and Treatment Outcomes. Infect. Drug Resist. 2023, 16, 3649–3658. [Google Scholar] [CrossRef]
- Alobaid, A.S.; Wallis, S.C.; Jarrett, P.; Starr, T.; Stuart, J.; Lassig-Smith, M.; Mejia, J.L.; Roberts, M.S.; Roger, C.; Udy, A.A.; et al. Population Pharmacokinetics of Piperacillin in Nonobese, Obese, and Morbidly Obese Critically Ill Patients. Antimicrob. Agents Chemother. 2017, 61, e01276-16. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. Aminopenicillin Breakpoints for Enterobacterales. General Consultation. 2022. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Consultation/2021/Aminopenicillins_and_Enterobacterales_General_consultation_November_2021.pdf (accessed on 21 September 2024).
- Davey, P.; A Marwick, C.; Scott, C.L.; Charani, E.; McNeil, K.; Brown, E.; Gould, I.M.; Ramsay, C.R.; Michie, S. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst. Rev. 2017, CD003543. [Google Scholar] [CrossRef]
- Nachtigall, I.; Tafelski, S.; Deja, M.; Halle, E.; Grebe, M.C.; Tamarkin, A.; Rothbart, A.; Uhrig, A.; Meyer, E.; Musial-Bright, L.; et al. Long-term effect of computer-assisted decision support for antibiotic treatment in critically ill patients: A prospective ‘before/after’ cohort study. BMJ Open 2014, 4, e005370. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Andreassen, S.; Tacconelli, E.; Nielsen, A.D.; Almanasreh, N.; Frank, U.; Cauda, R.; Leibovici, L.; TREAT Study Group. Improving empirical antibiotic treatment using TREAT, a computerized decision support system: Cluster randomized trial. J. Antimicrob. Chemother. 2006, 58, 1238–1245. [Google Scholar] [CrossRef]
- Poline, J.; Postaire, M.; Parize, P.; Pilmis, B.; Bille, E.; Zahar, J.R.; Frange, P.; Cohen, J.F.; Lortholary, O.; Toubiana, J. Stewardship program on carbapenem prescriptions in a tertiary hospital for adults and children in France: A cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1039–1048. [Google Scholar] [CrossRef]
- Ishtiaq, U.; Acosta, K.; Akabusi, C.; Noble, K.; Gujadhur, N.; Cluzet, V. Appropriateness of Empiric Initiation of Meropenem in the Intensive Care Unit as Determined by Internal Medicine Residents. Antimicrob. Steward. Healthc. Epidemiol. 2024, 4, e185. [Google Scholar] [CrossRef]
- Zhang, D.; Cui, K.; Lu, W.; Bai, H.; Zhai, Y.; Hu, S.; Li, H.; Dong, H.; Feng, W.; Dong, Y. Evaluation of carbapenem use in a tertiary hospital: Antimicrobial stewardship urgently needed. Antimicrob. Resist. Infect. Control. 2019, 8, 5. [Google Scholar] [CrossRef]
- National Infection Prevention and Control Committee. The National Infection Prevention and Control Standards for Acute Healthcare Facilities, Revised 2022; Ministry of Health: Singapore, 2022. Available online: https://isomer-user-content.by.gov.sg/95/a6666058-124b-46fb-80a5-e0a9692c0bb1/revised-national-ipc-standards-for-acute-healthcare-facilities_2022_on-moh-website.pdf (accessed on 28 November 2025).
- Zakhour, J.; Haddad, S.F.; Kerbage, A.; Wertheim, H.; Tattevin, P.; Voss, A.; Ünal, S.; Ouedraogo, A.S.; Kanj, S.S. International Society of Antimicrobial Chemotherapy (ISAC) and the Alliance for the Prudent Use of Antibiotics (APUA). Diagnostic stewardship in infectious diseases: A continuum of antimicrobial stewardship in the fight against antimicrobial resistance. Int. J. Antimicrob. Agents 2023, 62, 106816. [Google Scholar] [CrossRef]
- Claeys, K.C.; Trautner, B.W.; Leekha, S.; Coffey, K.C.; Crnich, C.J.; Diekema, D.J.; Fakih, M.G.; Goetz, M.B.; Gupta, K.; Jones, M.M.; et al. Optimal Urine Culture Diagnostic Stewardship Practice-Results from an Expert Modified-Delphi Procedure. Clin. Infect. Dis. 2022, 75, 382–389. [Google Scholar] [CrossRef]
- Pinto-de-Sá, R.; Sousa-Pinto, B.; Costa-de-Oliveira, S. Brave New World of Artificial Intelligence: Its Use in Antimicrobial Stewardship—A Systematic Review. Antibiotics 2024, 13, 307. [Google Scholar] [CrossRef]
- Tang, S.; Chang, D.; Zhi Chin, D.; Piotr Chlebicki, M.; Jasmine Chung, S.; Wei Lee, L.; Lee, W.; Zhou, P.Y.; Kwa, A. 163. Can Machine Learning Guide Antibiotic Initiation for Lower Respiratory Tract Infections? Open Forum Infect. Dis. 2023, 10 (Suppl. 2), ofad500.236. [Google Scholar] [CrossRef]
- E Hanson, K.; Tsalik, E.L. Host Immune Response Profiling for the Diagnosis of Infectious Diseases. J. Infect. Dis. 2025, 232, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
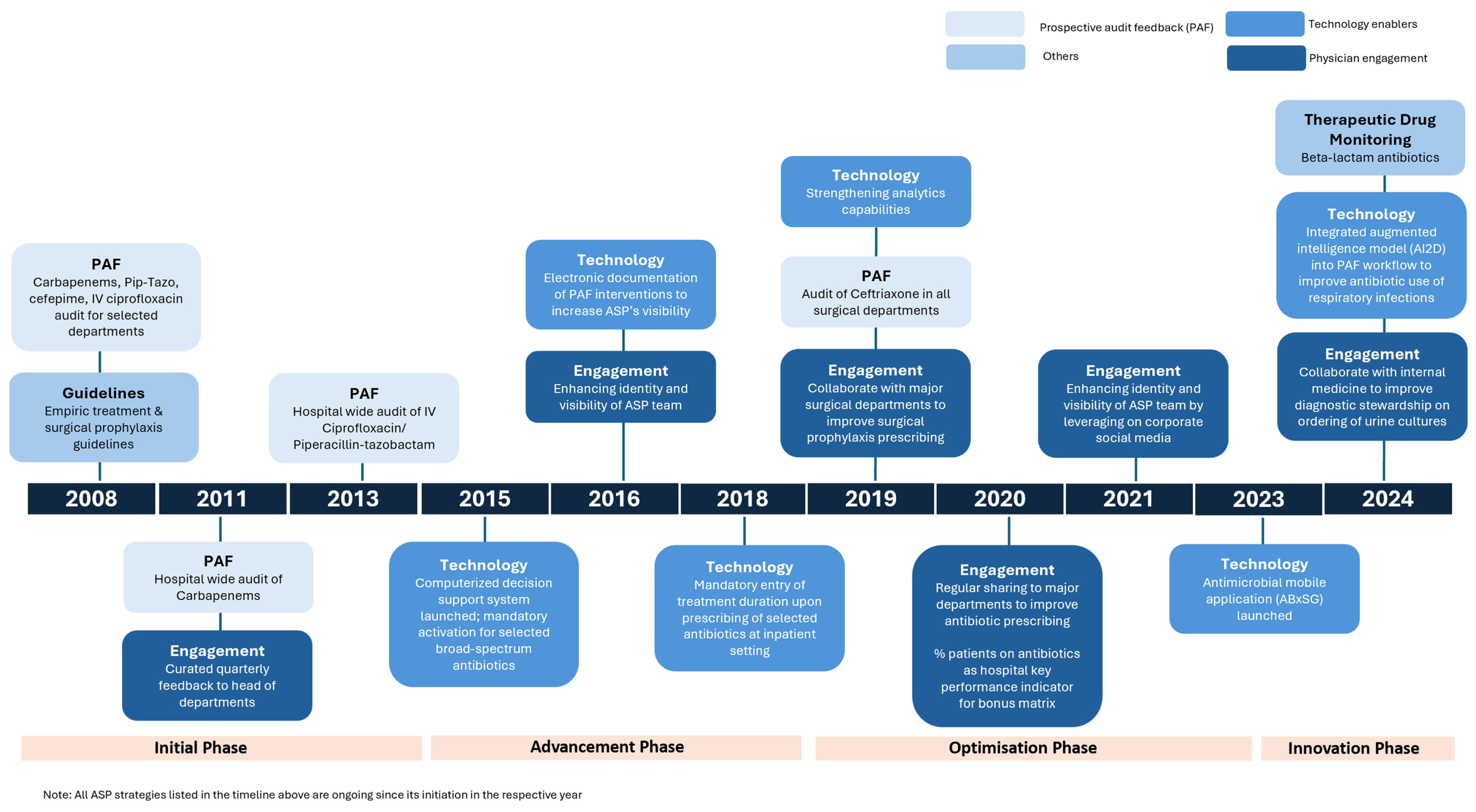
| Phase | Years | Key Stewardship Strategies |
|---|---|---|
| Initiation | 2008–2014 |
|
| Advancement | 2015–2018 |
|
| Optimisation | 2019–2023 |
|
| Innovation | 2023–date |
|
| Antibiotic | DDD/1000 PD (2011) | DDD/1000 PD (2024) | DDD | Trend (2011–2024) | Kendall tau Coefficient | p-Value |
|---|---|---|---|---|---|---|
| IV Ciprofloxacin | 11.0 | 4.9 | 7.8 (4.4–9.4) | Decreasing | −0.853 | 0.000 |
| Ceftriaxone | 105.0 | 90.0 | 77.2 (73.7–82.9) | Stable | −0.165 | 0.412 |
| IV Amoxicillin–clavulanate | 32.9 | 48.5 | 39.8 (34.2–43.4) | Increasing | 0.780 | 0.000 |
| Cefepime | 41.1 | 11.9 | 25.8 (12.8–30.2) | Decreasing | −0.641 | 0.001 |
| Piperacillin-tazobactam | 47.8 | 82.0 | 70.9 (63.8–78.5) | Increasing | 0.780 | 0.000 |
| Ertapenem | 13.8 | 7.5 | 9.0 (7.7–10.2) | Decreasing | −0.751 | 0.000 |
| Meropenem | 38.3 | 47.8 | 40.3 (38.3–44.7) | Stable | −0.011 | 0.956 |
| Gram- Negative Bacilli | No. of Isolates a Year ◊ | Antibiotic | % Susceptibility * (as of 2011) | % Susceptibility (as of 2024) | Susceptibility Trend ** | Kendall’s tau Coefficient | p-Value |
|---|---|---|---|---|---|---|---|
| Acinetobacter baumannii | 254 (216–378) | Ciprofloxacin | 33.6 | 74.4 | Increasing | 0.58 | <0.01 |
| Cefepime | 35.9 | 81.5 | Increasing | 0.56 | <0.01 | ||
| Piperacillin– tazobactam | 32.4 | 74.1 | Increasing | 0.66 | <0.01 | ||
| Meropenem | 48.5 | 79.8 | Increasing | 0.48 | <0.05 | ||
| Citrobacter freundii | 99 (88–110) | Ciprofloxacin | 70.2 | 74.6 | stable | 0.22 | 0.273 |
| Cefepime | 79.2 | 90.7 | Increasing | 0.53 | <0.05 | ||
| Ertapenem | 79.7 | 96.2 | Increasing | 0.65 | <0.01 | ||
| Meropenem | 80.6 | 99.2 | Increasing | 0.67 | <0.01 | ||
| Enterobacter spp. (including Klebsiella aerogenes) | 805 (747–885) | Ciprofloxacin | 78.2 | 77.6 | Stable | −0.17 | 0.412 |
| Cefepime | 82.8 | 88.6 | Increasing | 0.47 | <0.05 | ||
| Ertapenem | 86.7 | 92.7 | Increasing | 0.60 | <0.05 | ||
| Meropenem | 91.1 | 97.2 | Increasing | 0.52 | <0.05 | ||
| E. Coli | 4282 (3924–4738) | Ciprofloxacin | 51.4 | 53.1 | Stable | −0.10 | 0.622 |
| Ceftriaxone | 69.9 | 75.1 | Increasing | 0.59 | <0.01 | ||
| Amoxicillin–clavulanate | 66.0 | 79.6 | Increasing | 0.77 | <0.01 | ||
| Cefepime | 74.7 | 86.6 | Increasing | 0.73 | <0.01 | ||
| Piperacillin– tazobactam | 91.1 | 93.9 | Increasing | 0.49 | <0.05 | ||
| Ertapenem | 97.0 | 98.9 | Stable | 0.45 | 0.059 | ||
| Meropenem | 97.9 | 99.2 | Increasing | 0.55 | <0.05 | ||
| Klebsiella spp. | 2323 (2180–2767) | Ciprofloxacin | 57.6 | 66.1 | Stable | 0.08 | 0.702 |
| Ceftriaxone | 60.5 | 76.7 | Increasing | 0.77 | <0.01 | ||
| Amoxicillin–clavulanate | 57.9 | 74.5 | Increasing | 0.76 | <0.01 | ||
| Cefepime | 63.0 | 84.1 | Increasing | 0.84 | <0.01 | ||
| Piperacillin– tazobactam | 73.0 | 84.6 | Increasing | 0.69 | <0.01 | ||
| Ertapenem | 94.3 | 95.9 | Increasing | 0.62 | <0.01 | ||
| Meropenem | 95.1 | 96.9 | Increasing | 0.53 | <0.05 | ||
| Pseudomonas aeruginosa | 1522 (1380–1831) | Ciprofloxacin | 76.3 | 91.3 | Increasing | 0.93 | <0.01 |
| Cefepime | 84.5 | 96.4 | Increasing | 0.93 | <0.01 | ||
| Piperacillin– tazobactam | 90.7 | 92.9 | Increasing | 0.65 | <0.01 | ||
| Meropenem | 87.5 | 93.8 | Increasing | 0.86 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.P.; Chung, S.J.; Lee, W.H.L.; Wang, Y.; Lim, S.Y.C.; Tan, Y.E.; Kwa, A.L.H. Seventeen Years of an Antibiotic Stewardship Programme: Trends in Antibiotic Prescribing and Gram-Negative Bacilli Susceptibility at a Quaternary Healthcare Institution. Antibiotics 2025, 14, 1239. https://doi.org/10.3390/antibiotics14121239
Zhou YP, Chung SJ, Lee WHL, Wang Y, Lim SYC, Tan YE, Kwa ALH. Seventeen Years of an Antibiotic Stewardship Programme: Trends in Antibiotic Prescribing and Gram-Negative Bacilli Susceptibility at a Quaternary Healthcare Institution. Antibiotics. 2025; 14(12):1239. https://doi.org/10.3390/antibiotics14121239
Chicago/Turabian StyleZhou, Yvonne Peijun, Shimin Jasmine Chung, Winnie Hui Ling Lee, Yibo Wang, Shena Yun Chun Lim, Yen Ee Tan, and Andrea Lay Hoon Kwa. 2025. "Seventeen Years of an Antibiotic Stewardship Programme: Trends in Antibiotic Prescribing and Gram-Negative Bacilli Susceptibility at a Quaternary Healthcare Institution" Antibiotics 14, no. 12: 1239. https://doi.org/10.3390/antibiotics14121239
APA StyleZhou, Y. P., Chung, S. J., Lee, W. H. L., Wang, Y., Lim, S. Y. C., Tan, Y. E., & Kwa, A. L. H. (2025). Seventeen Years of an Antibiotic Stewardship Programme: Trends in Antibiotic Prescribing and Gram-Negative Bacilli Susceptibility at a Quaternary Healthcare Institution. Antibiotics, 14(12), 1239. https://doi.org/10.3390/antibiotics14121239






