Insights and Lessons from Chilean Salmon Aquaculture on Antimicrobial Use
Abstract
1. Introduction
- AMR Prevention: Strategies were discussed to better understand, detect, and disrupt the emergence of AMR in salmon aquaculture.
- Communication and Education: Effective communication and education programs were designed to promote responsible AMU among all stakeholders within the salmon industry.
- Therapeutic Alternatives and Efficacy: The exploration of alternative therapeutic strategies and their efficacy in controlling infectious diseases in salmon was identified as a central focus.
- Environmental Impact Assessment: A comprehensive evaluation of the environmental consequences of AMU in aquatic ecosystems was planned.
2. Results
2.1. Participants
2.2. Roundtables
3. Discussion
3.1. AMR Prevention
3.1.1. Understanding the Role of Aquaculture in the Development of AMR
3.1.2. Proposing Public Policies to Prevent the Development of AMR in Aquaculture
3.1.3. Identifying Key Stakeholders Responsible for Preventing and/or Mitigating AMR Risks
3.1.4. Identifying Effective Strategies for Communicating AMR Risks to Aquaculture and Public Health Stakeholders
3.2. Communication and Education on AMU
- -
- Developing a comprehensive project with the overarching goal of designing and implementing a targeted communication strategy.
- -
- Establishing an interdisciplinary team that includes communication professionals to ensure that dissemination efforts are guided by robust scientific and expert knowledge.
- -
- Identifying specific target audiences to ensure effective education and awareness-raising, as well as determining the most appropriate information channels for each audience.
- -
- Training communication platforms, including the press and media channels, to ensure that messages are accurate, consistent, and help to prevent misinformation.
3.3. Alternatives and Therapeutic Efficacy
Exploring Effective Alternatives and Preventive Tools Against Bacterial Infections in Aquaculture
3.4. Environmental Impacts of Antibiotic Use in Aquaculture
3.4.1. Assessing the Environmental Impact of AMU in Aquatic Ecosystems
3.4.2. Methodologies for Environmental Impact Assessments and Existing Knowledge Gaps
3.4.3. The Necessity of Conducting an Environmental Risk Assessment
3.4.4. Identifying Regulatory Gaps and Acceptable Standards
3.5. The Roadmap
3.6. Challenges and Limitations
4. Materials and Methods
4.1. Participants
4.2. Roundtables
- ROUNDTABLE 1—AMR PREVENTION Goals:
- 1.
- To describe the role of aquaculture in the development of AMR.
- 2.
- To propose public policies aimed at preventing the emergence and spread of AMR in aquaculture.
- 3.
- To identify key stakeholders responsible for preventing and/or mitigating AMR-related risks.
- 4.
- To identify effective methods for communicating AMR risk to aquaculture stakeholders and public health authorities.
- ROUNDTABLE 2—COMMUNICATION AND EDUCATION OF AMU Goals:
- 1.
- To identify effective mechanisms for disseminating information within the aquaculture industry and to the broader community.
- 2.
- To propose key topics to be included in veterinary medicine curricula.
- 3.
- To identify available technologies that can be leveraged for communication and education on AMU.
- ROUNDTABLE 3—ALTERNATIVES AND THERAPEUTIC EFFICACY Goals:
- 1.
- To identify alternative treatments for bacterial diseases in aquaculture and evaluate their efficacy.
- 2.
- To review and describe preventive measures, such as natural products and vaccines, currently in use or under development for the control of bacterial diseases.
- 3.
- To propose methodologies for efficacy testing.
- 4.
- To analyze the current regulatory framework for the registration of pharmaceutical and biological products.
- ROUNDTABLE 4—ENVIRONMENTAL IMPACTS OF AMU IN AQUACULTURE Goals:
- 1.
- To determine the potential impacts of AMU on the aquatic environment.
- 2.
- To identify methodologies for evaluating environmental impacts in the aquatic environment, including, for example, technique sensitivity and available analytical capacity.
- 3.
- To identify existing knowledge gaps.
- 4.
- To discuss the need for conducting an environmental risk assessment.
- 5.
- To identify regulatory gaps and define acceptable standards.
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- SERNAPESCA. Informe Sobre el uso de Antimicrobianos y Antiparasitarios en la Salmonicultura Nacional. Año 2024; Servicio Nacional de Pesca y Acuicultura: Valparaíso, Chile, 2025.
- Toonen, H.M.; Bush, S.R.; Ibarra, R.; O’Sullivan, C.; Hudson, E.; Asif, F.; Bridson, P.; Corsini, F.; Fitzsimmons, K.; Kruk, S.R.L.; et al. Aquaculture Governance Indicators: A diagnostic framework for steering towards sustainability. PLoS Sustain. Transform. 2025, 4, e0000165. [Google Scholar] [CrossRef]
- Flores-Kossack, C.; Montero, R.; Köllner, B.; Maisey, K. Chilean aquaculture and the new challenges: Pathogens, immune response, vaccination and fish diversification. Fish Shellfish. Immunol. 2020, 98, 52–67. [Google Scholar] [CrossRef]
- Avendaño-Herrera, R.; Mancilla, M.; Miranda, C. Use of antimicrobials in Chilean Salmon farming: Facts, myths and perspectives. Rev. Aquac. 2023, 15, 89–111. [Google Scholar] [CrossRef]
- Farías, D.R.; Ibarra, R.; Estévez, R.A.; Tlusty, M.F.; Nyberg, O.; Troell, M.; Avendaño-Herrera, R.; Norden, W. Towards Sustainable Antibiotic Use in Aquaculture and Antimicrobial Resistance: Participatory Experts’ Overview and Recommendations. Antibiotics 2024, 13, 887. [Google Scholar] [CrossRef] [PubMed]
- Mardones, F.O.; Paredes, F.; Medina, M.; Tello, A.; Valdivia, V.; Ibarra, R.; Correa, J.; Gelcich, S. Identification of research gaps for highly infectious diseases in Aquaculture: The case of the endemic Piscirickettsia salmonis in the Chilean salmon farming industry. Aquaculture 2018, 482, 211–220. [Google Scholar] [CrossRef]
- Meyer, A.; Sadler, R.; Bannister-Tyrrell, M.; Gallardo Lagno, A.L.; Stegeman, A.; Cameron, A. Is between-farm water-borne pathogen dissemination an important driver in the epidemiology of salmonid rickettsial septicaemia in Chile? Aquaculture 2021, 530, 735751. [Google Scholar] [CrossRef]
- Quiñones, R.A.; Fuentes, M.; Montes, R.M.; Soto, D.; León-Muñoz, J. Environmental issues in Chilean salmon farming: A review. Rev. Aquac. 2019, 11, 375–402. [Google Scholar] [CrossRef]
- Bondad-Reantaso, M.; MacKinnon, B.; Karunasagar, I.; Fridman, S.; Alday-Sanz, V.; Brun, E.; Groumellec, M.L.; Li, A.; Surachetpong, W.; Karunasagar, I.; et al. Review of alternatives to antibiotic use in aquaculture. Rev. Aquac. 2022, 15, 1421–1451. [Google Scholar] [CrossRef]
- Garlock, T.M.; Asche, F.; Anderson, J.L.; Eggert, H.; Anderson, T.M.; Che, B.; Chávez, C.A.; Chu, J.; Chukwuone, N.; Dey, M.M.; et al. Environmental, economic, and social sustainability in aquaculture: The aquaculture performance indicators. Nat. Commun. 2024, 15, 5274. [Google Scholar] [CrossRef] [PubMed]
- SERNAPESCA. Informe Sobre el uso de Antimicrobianos y Antiparasitarios en la Salmonicultura Nacional 2023; Servicio Nacional de Pesca y Acuicultura: Valparaíso, Chile, 2024.
- Miranda, C.D.; Godoy, F.A.; Lee, M.R. Current Status of the Use of Antibiotics and the Antimicrobial Resistance in the Chilean Salmon Farms. Front. Microbiol. 2018, 9, 1284. [Google Scholar] [CrossRef]
- One Health High-Level Expert Panel (OHHLEP); Adisasmito, W.B.; Almuhairi, S.; Behravesh, C.B.; Bilivogui, P.; Bukachi, S.A.; Casas, N.; Cediel Becerra, N.; Charron, D.F.; Chaudhary, A.; et al. One Health: A new definition for a sustainable and healthy future. PLoS Pathog. 2022, 18, e1010537. [Google Scholar] [CrossRef]
- Bondad-Reantaso, M.G.; Fejzic, N.; MacKinnon, B.; Huchzermeyer, D.; Seric-Haracic, S.; Mardones, F.O.; Mohan, C.V.; Taylor, N.; Jansen, M.D.; Tavornpanich, S.; et al. A 12-point checklist for surveillance of diseases of aquatic organisms: A novel approach to assist multidisciplinary teams in developing countries. Rev. Aquac. 2021, 13, 1469–1487. [Google Scholar] [CrossRef]
- Mardones, F.O. Preventive medicine of aquatic animals. In Aquaculture Health Management; Academic Press: Cambridge, MA, USA, 2020; pp. 163–186. [Google Scholar]
- Salgado-Caxito, M.; Zimin-Veselkoff, N.; Adell, A.D.; Olivares-Pacheco, J.; Mardones, F.O. Qualitative Risk Assessment for Antimicrobial Resistance among Humans from Salmon Fillet Consumption Due to the High Use of Antibiotics against Bacterial Infections in Farmed Salmon. Antibiotics 2022, 11, 662. [Google Scholar] [CrossRef]
- Annual Report. ANIMUSE: Monitoring Antimicrobial Use in Animals [Press Release]. WOAH2022. Available online: https://www.woah.org/en/article/animuse-monitoring-antimicrobial-use-in-animals/ (accessed on 28 February 2025).
- Alvarado-Flores, C.; Encina-Montoya, F.; Tucca, F.; Vega-Aguayo, R.; Nimptsch, J.; Oberti, C.; Carmona, E.R.; Luders, C. Assessing the ecological risk of active principles used currently by freshwater fish farms. Sci. Total Environ. 2021, 775, 144716. [Google Scholar] [CrossRef] [PubMed]
- Cravedi, J.; Choubert, G.; Delous, G. Digestibility of chloramphenicol, oxolinic acid and oxytetracycline in rainbow trout and influence of these antibiotics on lipid digestibility. Aquaculture 1987, 60, 133–141. [Google Scholar] [CrossRef]
- Kemper, N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol. Indic. 2008, 8, 1–13. [Google Scholar] [CrossRef]
- Cairney, D.; Morrisey, D. Estimation of Feed Loss from Two Salmon Cage Sites in Queen Charlotte Sound; NEL2011-026; Elaborado por NIWA (National Institute of Water & Atmospheric Research Ltd.): Auckland, New Zealand, 2011.
- MINSEGPRES. Revisión de la Norma de Emisión de Descargas Residuos Líquidos a Aguas Marinas y Continentales Superficiales (d.s. 90/2000); Ministerio Secretaría General de la Presidencia: Santiago, Chile, 2021.
- Happold, J.; Meyer, A.; Sadler, R.; Cowled, B.; Mackenzie, C.; Stevenson, M.; Ward, M.P.; Gallardo Lagno, A.L.; Cameron, A. Effectiveness of antimicrobial treatment of salmonid rickettsial septicaemia in commercial salmon and trout farms in Chile. Aquaculture 2020, 525, 735323. [Google Scholar] [CrossRef]
- SAG. Productos Biológicos Inmmunológicos con Registro Previosional, uso en Salmonideos Santiago, Chile: Servicio Agrícola y Ganadero, Ministerio de Agricultura, Gobierno de Chile. 2025. Available online: https://www.sag.gob.cl/sites/default/files/lista_salmonidos_registro_provisional_17122024.pdf (accessed on 8 August 2025).
- Rozas, M.; Enríquez, R. Piscirickettsiosis and Piscirickettsia salmonis in fish: A review. J. Fish Dis. 2014, 37, 25. [Google Scholar] [CrossRef]
- San Martin, B.; Fresno, M.; Cornejo, J.; Godoy, M.; Ibarra, R.; Vidal, R.; Araneda, M.; Anadón, A.; Lapierre, L. Optimization of florfenicol dose against Piscirickettsia salmonis in Salmo salar through PK/PD studies. PLoS ONE 2019, 14, e0215174. [Google Scholar] [CrossRef]
- Canon-Jones, H.; Cortes, H.; Castillo-Ruiz, M.; Schlotterbeck, T.; San Martin, R. Quillaja saponaria (Molina) Extracts Inhibits In Vitro Piscirickettsia salmonis Infections. Animals 2020, 10, 2286. [Google Scholar] [CrossRef] [PubMed]
- Carrizo, J.C.; Griboff, J.; Bonansea, R.I.; Nimptsch, J.; Valdés, M.E.; Wunderlin, D.A.; Amé, M.V. Different antibiotic profiles in wild and farmed Chilean salmonids. Which is the main source for antibiotic in fish? Sci. Total Environ. 2021, 800, 149516. [Google Scholar] [CrossRef]
- Rico, A.; Vighi, M.; Van den Brink, P.J.; Horst, M.; Macken, A.; Lillicrap, A.; Falconer, L.; Telfer, T.C. Use of models for the environmental risk assessment of veterinary medicines in European aquaculture: Current situation and future perspectives. Rev. Aquac. 2019, 11, 969–988. [Google Scholar] [CrossRef]
- Barra, R.O.; Cardenas-Soraca, D.; Campos-Garagay, M.; McMaster, M.E.; Hewitt, L.M. Integrated approaches for detecting the occurrence and effects of endocrine disrupting substances in surface waters. Curr. Opin. Environ. Sci. Health 2020, 18, 20–25. [Google Scholar] [CrossRef]
- Brack, W.; Aissa, S.A.; Backhaus, T.; Dulio, V.; Escher, B.I.; Faust, M.; Hilscherova, K.; Hollender, J.; Hollert, H.; Müller, C.; et al. Effect-based methods are key. The European Collaborative Project SOLUTIONS recommends integrating effect-based methods for diagnosis and monitoring of water quality. Environ. Sci. Eur. 2019, 31, 10. [Google Scholar] [CrossRef]
- WOAH. OIE Annual Report on Antimicrobial Agents Intended for Use in Animals. Better Understanding of the Global Situation. Fifth Report; World Organization for Animal Health (OIE): Paris, France, 2021. [Google Scholar]
- Newman, M.; Unger, M. Fundamentals of Ecotoxicology, 2nd ed.; Lewis Publisher: Lakeland, FL, USA, 2003; Chapter 13 ; pp. 279–299. [Google Scholar]
- VICH. Topic GL38: Environmental Impact Assessment (EIAs) for Veterinary Medicinal Products (VMPs)—Phase II; CVMP/VICH/790/03; European Medicines Agency: London, UK, 2004. [Google Scholar]
- Jara, B.; Tucca, F.; Srain, B.M.; Mejanelle, L.; Aranda, M.; Fernández, C.; Pantoja-Gutiérrez, S. Antibiotics florfenicol and flumequine in the water column and sediments of Puyuhuapi Fjord, Chilean Patagonia. Chemosphere 2021, 275, 130029. [Google Scholar] [CrossRef] [PubMed]


| ROUNDTABLE 1—AMR PREVENTION | |
|---|---|
| Question | Comments |
| Is aquaculture a source or receptor of AMR? Are there tools to demonstrate AMR and/or how can it be measured? | Aquaculture plays a dual role as both a source and a recipient of AMR, depending on antibiotic use and aquaculture type. |
| Have public–private programs to reduce AMU and the risk of AMR been effective, or what should be changed? |
|
| What should an AMR monitoring program be like in aquaculture? | AMR surveillance programs should be comprehensive, adopting a “One Health” approach, and be supported by both public and private financing and participation, should feature supra-institutional governance and be guided by an expert advisory committee. |
| If more knowledge about AMR in aquatic environments is required, how long could it take and what costs could it entail? | The salmon industry has implemented a range of effective strategies to reduce AMU, including smolt quality management, biosecurity measures, proper vaccine use, early treatments, health surveillance, sea lice control, fish-welfare bioactive additives, functional diets, oxygen management, predator control, genetic improvements, and shorter time spent at sea. |
| What are the minimum elements that a regulation should have for responsible AMU? |
|
| ROUNDTABLE 2—COMMUNICATION and EDUCATION OF AM USE | |
| How can the training of human capital in aquaculture be improved? What gaps in professional training in animal health exist or should be improved for better management of AMU? | Veterinary training provides adequate knowledge for professionals to thrive in the aquaculture industry. However, as with any professional, continuous education and specialization are essential for ongoing development. |
| How should stakeholders and communities be informed about aspects related to the AMU in aquaculture? What technological or communication tools can be used to communicate about AMU at different levels? |
|
| What are the aspects of greatest concern regarding management, use, and impacts from the point of view of the salmon value chain? | Increased production costs, environmental impact, AMR, repercussions on human health, consumer perception, and effects on the industry’s image. |
| ROUNDTABLE 3—ALTERNATIVES AND THERAPEUTIC EFFICACY | |
| What alternatives to AMU as a treatment for fish infections are foreseen in the short term? | Standardized testing of functional diets is urgently needed. Although new vaccines show promise, they are not expected to be available in the near future. Maintaining animal welfare must remain a top priority. |
| How should these tools be made effective and available to fish farmers? | Feeding strategies during treatment should be further studied. Experimental marine centers could play a key role in advancing this research. |
| What practices have been shown to reduce AMs consumption without compromising mortality? |
|
| How could AM dosages be optimized in a treatment? |
|
| What are the necessary elements for successful treatment? |
|
| ROUNDTABLE 4 —ENVIRONMENTAL IMPACTS OF ANTIMICROBIAL USE IN AQUACULTURE | |
| What are the specific environmental impacts of AMU in freshwater and marine aquaculture on aquatic ecosystems? | Potential negative effects of AMU in aquaculture on the overall health and balance of aquatic ecosystems. |
| What are the most suitable methods and tools for evaluating the environmental impacts of AMU in aquaculture? |
|
| What additional information or studies are needed to have a more complete understanding of these long-term impacts? |
|
| Considering the environmental impacts of AMU in aquaculture, what key elements should an effective environmental regulation include to minimize risks? | Environmental regulations regarding AMU should be regularly updated to incorporate new scientific findings and improve our understanding of the impact of these compounds. |
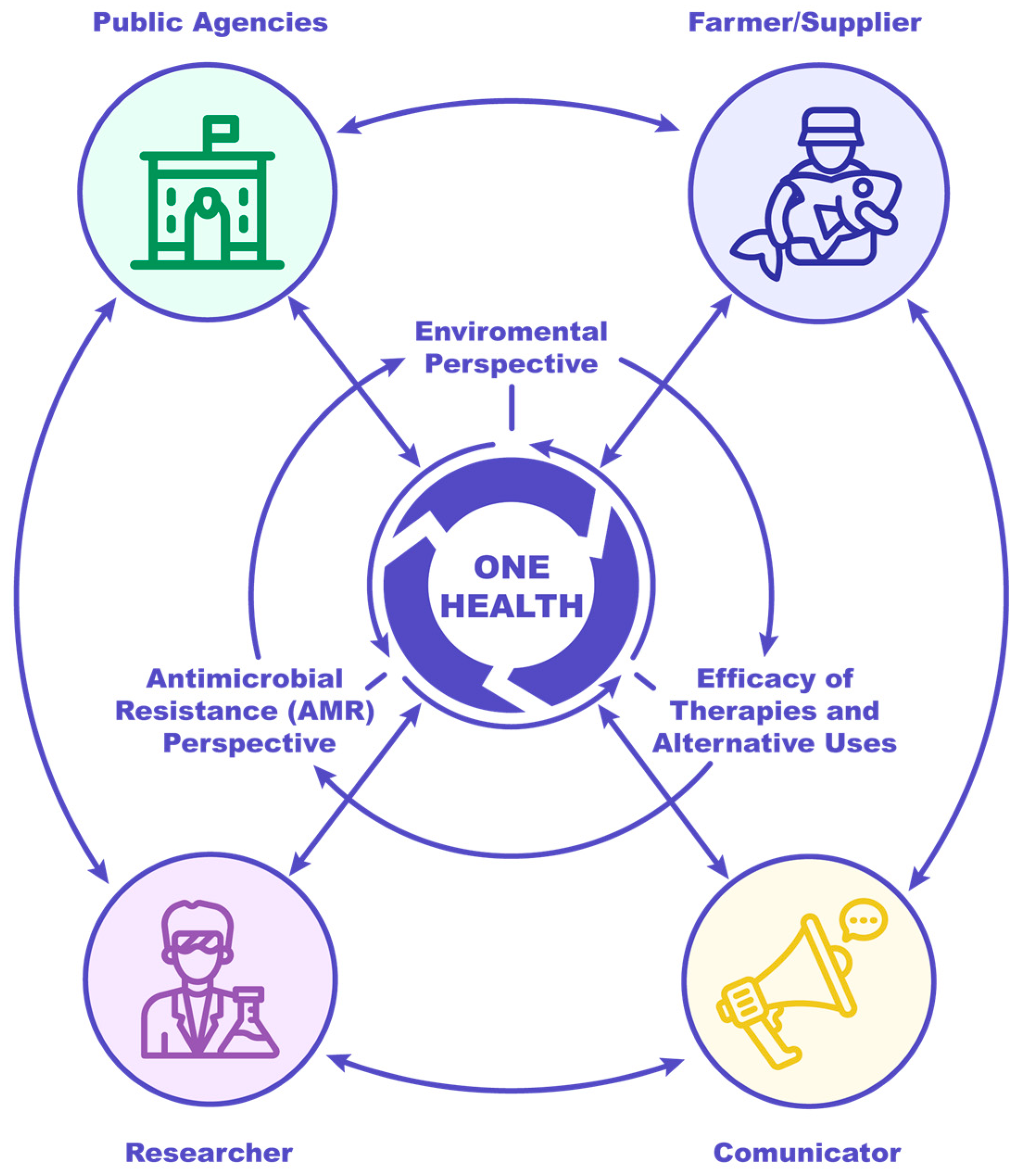
| Domain | Stakeholder | Prioritized Action |
|---|---|---|
| ENVIRONMENTAL PERSPECTIVE | Public Agencies | Establish an integrated “One Health” governance framework for environmental health policy. |
| Mandate Environmental Risk Assessments (ERAs) within AM product authorization processes, ensuring transparency and reliable reporting. | ||
| Delineate and implement ecosystemic measurement protocols that account for the spatial and temporal variability of xenobiotics, encouraging collaboration and continuous improvements. | ||
| Farmer/Supplier | Implement protocols for minimizing AM environmental dissemination and ensure preparedness for ERA compliance. | |
| Researcher | Develop and validate rigorous ecological impact assessment methodologies specific to intensive aquaculture systems. | |
| Quantify the cumulative environmental effects of AMU across relevant spatial and temporal scales. | ||
| Communicator | Disseminate information consistently to promote the intersectoral “One Health” paradigm. | |
| ANTIMICROBIAL RESISTANCE (AMR) PERSPECTIVE | Public Agencies | Mandates and resources integrated into “One Health” AMR surveillance programs. |
| Modify legislation to explicitly differentiate aquaculture as both a source and recipient of AMR determinants. | ||
| Mandate the incorporation of quantifiable efficacy metrics (e.g., decrease in resistance prevalence) into AMR reduction initiatives. | ||
| Farmer/Supplier | Ensure active participation and transparent data submission to “One Health” AMR surveillance initiatives. | |
| Implement systematic monitoring and timely reporting of operational AMR prevalence and antibiotic consumption data. | ||
| Researcher | Refine and integrate methodologies to accurately distinguish AMR origin and assess potential public health relevance. | |
| Design and validate objective efficacy indicators for assessing the performance of AMR mitigation strategies. | ||
| Communicator | Synthesize and communicate complex AMR data effectively, differentiating between sources for public understanding. | |
| EFFICACY OF THERAPIES AND ALTERNATIVE USES | Establish positive financial and regulatory incentives to promote the development and adoption of non-antibiotic prophylactic and therapeutic agents. | |
| Public Agencies | Resource and establish on-site demonstration and experimental facilities for real-world validation of innovative strategies. | |
| Require the submission of robust, validated efficacy data for the renewal and registration of veterinary medicinal products. | ||
| Farmer/Supplier | Integrate and enforce evidence-based management practices proven to minimize antibiotic consumption (e.g., biosecurity optimization). | |
| Proactively evaluate and adopt validated non-AM alternatives (e.g., functional feeds, contemporary vaccines). | ||
| Optimize the inclusion and delivery mechanisms of veterinary therapeutics via feed to ensure appropriate dosage administration. | ||
| Researcher | Conduct rigorous, real-world field validation studies on the efficacy of non-antibiotic interventions. | |
| Prioritize research on AM population pharmacokinetics and the optimization of therapeutic dosing regimens. | ||
| Communicator | Facilitate the knowledge transfer of scientific efficacy data and alternative therapeutic options to stakeholders. |
| Domain | Prioritized Action |
|---|---|
| Develop and implement a comprehensive communication strategy. Initiate a project to create and roll out a detailed communication strategy that involves forming an interdisciplinary team with communication specialists and subject-matter experts to craft messaging based on expert insights. Finally, identify specific target audiences for practical education and awareness. |
| Identify consistent distribution channels for information that aligns with each target audience. Messages should be coherent, clear, and focused, utilizing appropriate tools to effectively transmit AMU information in aquaculture and its risks. |
| Highlight the critical importance of collaborative work between industry and universities. This partnership is key to understanding industry needs and enabling universities to contribute solutions, both in professional training and scientific research. | |
| Strengthen veterinary education in aquaculture by advocating for and supporting the development of specialized modules or programs in aquaculture. Emphasize the importance of continuous education and specialization (i.e., diplomas, MSc, PhD) for veterinary professionals already in the field. | |
| Develop a continuous communication roadmap with long-term goals and consistent messaging to achieve a generational shift. |
| Establish mentorship programs that connect experienced professionals with new graduates to facilitate knowledge transfer and integration into the industry, with best practices over the long term. | |
| Design communication campaigns with specific evaluation indicators to measure effectiveness and impact. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farias, D.R.; Ibarra, R.; Tucca, F.; Jaramillo-Torres, A.; Cornejo, J.; Ibieta, P.; Mardones, F.O.; Avendaño-Herrera, R. Insights and Lessons from Chilean Salmon Aquaculture on Antimicrobial Use. Antibiotics 2025, 14, 1177. https://doi.org/10.3390/antibiotics14121177
Farias DR, Ibarra R, Tucca F, Jaramillo-Torres A, Cornejo J, Ibieta P, Mardones FO, Avendaño-Herrera R. Insights and Lessons from Chilean Salmon Aquaculture on Antimicrobial Use. Antibiotics. 2025; 14(12):1177. https://doi.org/10.3390/antibiotics14121177
Chicago/Turabian StyleFarias, Daniela R., Rolando Ibarra, Felipe Tucca, Alexander Jaramillo-Torres, Javiera Cornejo, Pablo Ibieta, Fernando O. Mardones, and Ruben Avendaño-Herrera. 2025. "Insights and Lessons from Chilean Salmon Aquaculture on Antimicrobial Use" Antibiotics 14, no. 12: 1177. https://doi.org/10.3390/antibiotics14121177
APA StyleFarias, D. R., Ibarra, R., Tucca, F., Jaramillo-Torres, A., Cornejo, J., Ibieta, P., Mardones, F. O., & Avendaño-Herrera, R. (2025). Insights and Lessons from Chilean Salmon Aquaculture on Antimicrobial Use. Antibiotics, 14(12), 1177. https://doi.org/10.3390/antibiotics14121177








