Inventory of Survey Instruments for Monitoring Antimicrobial Use in Primary Care Settings in Low- and Middle-Income Countries: A Narrative Review
Abstract
1. Introduction
2. Methods
2.1. Design
2.2. Definition
2.3. Search Strategy
2.4. Extraction of Instruments from Studies
2.5. Inclusion Criteria
- (a)
- Research undertaken in hospital inpatient settings, hospital entry surveys assessing medicine use prior to admission, or studies where inpatient and outpatient use could not be differentiated;
- (b)
- Focus group discussions that only measured knowledge of or attitudes towards antimicrobial use or resistance, or used hypothetical clinical scenarios or simulated patients;
- (c)
- Tools where the primary purpose was to measure access to medicines or their prices.
2.6. Data Extraction
2.7. Data Analysis
3. Results
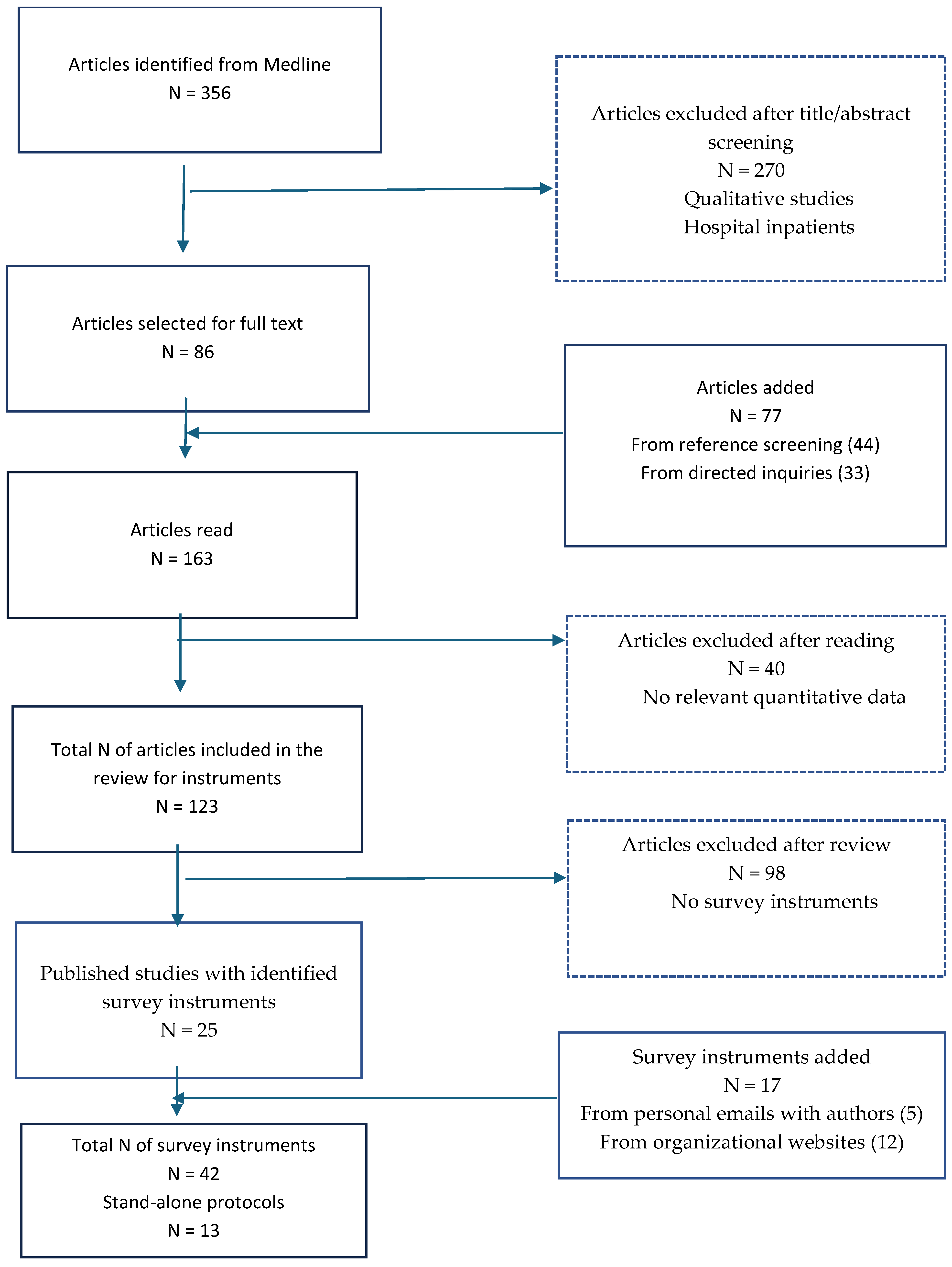
3.1. Search Results
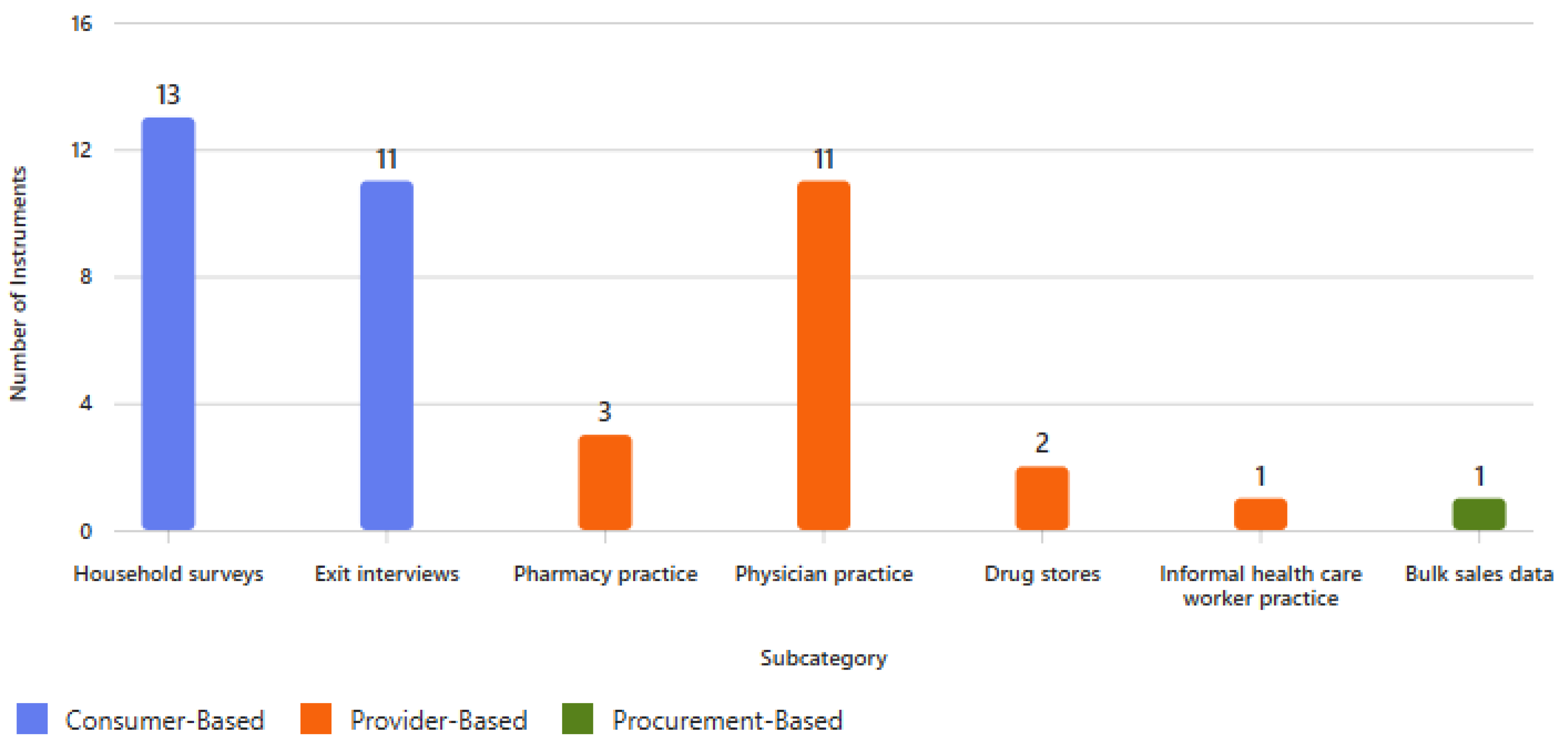
3.2. Overall Characteristics of the Survey Instruments
3.3. Consumer-Directed Surveys
3.3.1. Household Surveys
3.3.2. Exit Interviews
3.4. Health Facility Surveys and Data Collection Forms
3.4.1. Pharmacy Audit Surveys
3.4.2. Physician/Primary Care-Directed Surveys
3.4.3. Corner Store/Drug Store- and Informal Health Care Worker-Directed Surveys
3.5. Aggregated Data
Bulk Sales Data
3.6. Stand-Alone Protocols
4. Discussion
4.1. Consumer and Household Surveys
4.2. Pharmacy, Physician, and Informal Practice Audits
4.3. Routinely Collected and Electronic Data Sources
4.4. Methodological Limitations of Existing Tools
4.5. Implications for Future Research and Policy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| ADILA | Antibiotic Data to Inform Local Action |
| AMR | Antimicrobial resistance |
| AMS | Antimicrobial stewardship |
| AMU | Antimicrobial use |
| AWaRe | Access, Watch, and Reserve (WHO antibiotic classification) |
| DDD | Defined daily dose |
| FIP | International Pharmaceutical Federation |
| GLASS | Global Antimicrobial Resistance and Use Surveillance System |
| GLASS-AMU | GLASS Antimicrobial Use module |
| HICs | High-income countries |
| HiTAP | Health Information and Technology Assessment Program, Thailand |
| ISIUM | International Society for Improving Use of Medicines |
| LMICs | Low- and middle-income countries |
| LSHTM | London School of Hygiene and Tropical Medicine |
| MORU | Mahidol Oxford Tropical Medicine Research Unit, Thailand |
| MURIA | Medicines Utilisation Research In Africa network |
| PHC | Primary health care |
| REDCIMLAC | Red de Centros de Información de Medicamentos de Latinoamérica y el Caribe |
| UNICEF | United Nations International Children’s Emergency Fund |
| USAID | United States Agency for International Development |
| WHO | World Health Organization |
References
- WHO Regional Office for Europe. Antimicrobial Stewardship Interventions: A Practical Guide; WHO Regional Office for Europe: Copenhagen, Denmark, 2021. [Google Scholar]
- Sulis, G.; Sayood, S.; Gandra, S. Antimicrobial resistance in low- and middle-income countries: Current status and future directions. Expert. Rev. Anti Infect. Ther. 2022, 20, 147–160. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report; Antibiotic use data for 2022; World Health Organization: Geneva, Switzerland, 2025. [Google Scholar]
- World Health Organization. The WHO AWaRe (Access, Watch, Reserve) Antibiotic Book; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- United Nations General Assembly. Resolution on Antimicrobial Resistance (A/RES/74/2). 2024. Available online: https://undocs.org/A/RES/74/2 (accessed on 10 November 2024).
- Michalsen, B.O.; Xu, A.X.T.; Alderson, S.L.; Bjerrum, L.; Brehaut, J.; Bucher, H.C.; Clarkson, J.; Duncan, E.; Grimshaw, J.; Gunnarsson, R.; et al. Regional and national antimicrobial stewardship activities: A survey from the Joint Programming Initiative on Antimicrobial Resistance—Primary Care Antibiotic Audit and Feedback Network (JPIAMR-PAAN). JAC Antimicrob. Resist. 2023, 5, dlad048. [Google Scholar] [CrossRef] [PubMed]
- Ebell, M.H.; Radke, T. Antibiotic use for viral acute respiratory tract infections remains common. Am. J. Manag. Care 2015, 21, e567–e575. [Google Scholar] [PubMed]
- Ndaki, P.M.; Mwanga, J.R.; Mushi, M.F.; Konje, E.T.; Mwita, S.M.; Mshana, S.E. Drivers of inappropriate use of antibiotics among community members in low- and middle-income countries: A systematic review of qualitative studies. BMC Public Health 2025, 25, 705. [Google Scholar] [CrossRef]
- Queenan, K.; Chandler, C.; Goodman, C. A Review of Methods and Metrics for Studying Human and Livestock Antibiotic Use at the Granular Level; A Pre-Read for Roundtable Discussion in London; Working Paper; London School of Hygiene and Tropical Medicine: London, UK, 2017. [Google Scholar]
- Centers for Disease Control and Prevention. Antibiotic Use in the United States: Antibiotic Prescribing and Use. 2025. Available online: https://www.cdc.gov/antibiotic-use/hcp/data-research/antibiotic-prescribing.html (accessed on 10 June 2025).
- Cook, D.A. Systematic and Nonsystematic Reviews: Choosing an Approach. In Healthcare Simulation Research; Nestel, D., Hui, J., Kunkler, K., Scerbo, M., Calhoun, A., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Sulis, G.; Adam, P.; Nafade, V.; Patiño, L.; Ghazaryan, L.; Koyuncu, A.; Kuehne, T.; Ahmad, R.; Islam, M.A.; Nair, M.; et al. Antibiotic prescription practices in primary care in low- and middle-income countries: A systematic review and meta-analysis. PLoS Med. 2020, 17, e1003139. [Google Scholar] [CrossRef]
- World Bank Group Country Classifications by Income Level for FY24 (1 July 2023–30 June 2024). World Bank Blogs n.d. Available online: https://blogs.worldbank.org/en/opendata/new-world-bank-group-country-classifications-income-level-fy24 (accessed on 21 April 2025).
- World Health Organization. Global Report on Infection Prevention and Control; WHO: Geneva, Switzerland, 2024; ISBN 978-92-4-010398-6. [Google Scholar]
- Al-Azzam, S.I.; Al-Husein, B.A.; Alzoubi, F.; Masadeh, M.M.; Mukattash, T.L. Self-medication with antibiotics in Jordanian population. Int. J. Occup. Med. Environ. Health 2007, 20, 373–380. [Google Scholar] [CrossRef]
- Wertheim, H.F.L.; Chuc, N.T.K.; Punpuing, S.; Pham, H.V.; Khamphaphongphane, B.; Limato, R.; Villegas, E.; Pradipta, I.S.; Ahmad, R.A.; Kurniawan, A.; et al. Community-level antibiotic access and use (ABACUS) in low- and middle-income countries: Finding targets for social interventions to improve appropriate antimicrobial use—An observational multi-centre study. Wellcome Open Res. 2017, 2, 58. [Google Scholar] [CrossRef]
- Hardon, A.; Hodgkin, C.; Fresle, D. How to Investigate the Use of Medicines by Consumers; World Health Organization: Geneva, Switzerland; University of Amsterdam: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Singh-Phulgenda, S.; Antoniou, P.; Wong, D.L.F.; Charani, E.; Holmes, A.H.; Huttner, B.; Monnet, D.L.; Pulcini, C.; Vlahović-Palčevski, V.; Zarb, P.; et al. Knowledge, attitudes and behaviors on antimicrobial resistance among general public across 14 member states in the WHO European region: Results from a cross-sectional survey. Front. Public Health 2023, 11, 1274818. [Google Scholar] [CrossRef]
- Khare, S.; Pathak, A.; Purohit, M.R.; Sharma, A.; Mahadik, V.K.; Sharma, M.; Diwan, V. Determinants and pathways of healthcare-seeking behaviours in under-5 children for common childhood illnesses and antibiotic prescribing: A cohort study in rural India. BMJ Open. 2021, 11, e052435. [Google Scholar] [CrossRef]
- kotwani, E.T.; Platts-Mills, J.A.; Seidman, J.C.; John, S.; Mahfuz, M.; Ulak, M.; Shrestha, S.K.; Ahmed, T.; Alam, D.; Mduma, E.; et al. Use of antibiotics in children younger than two years in eight countries: A prospective cohort study. Bull. World Health Organ. 2017, 95, 49–61. [Google Scholar]
- Padget, M.; Tamarelle, J.; Herindrainy, P.; Ndir, A.; Diene Sarr, F.; Richard, V.; Piola, P.; Guillemot, D.; Delarocque-Astagneau, E.; EMAE Team. A community survey of antibiotic consumption among children in Madagascar and Senegal: The importance of healthcare access and care quality. J. Antimicrob. Chemother. 2017, 72, 564–573. [Google Scholar] [CrossRef]
- Lanyero, H.; Eriksen, J.; Obua, C.; Stålsby Lundborg, C.; Nanzigu, S.; Katureebe, A.; Kalyango, J.N.; Ocan, M. Use of antibacterials in the management of symptoms of acute respiratory tract infections among children under five years in Gulu, northern Uganda: Prevalence and determinants. PLoS ONE 2020, 15, e0235164. [Google Scholar] [CrossRef]
- Zhu, Y.; Tang, X.; Yan, R.; Shao, Z.; Zhou, Y.; Deng, X.; Luo, S.; He, H. Non-prescription antibiotic use for cough among Chinese children under 5 years of age: A community-based cross-sectional study. BMJ Open 2021, 11, e051372. [Google Scholar] [CrossRef]
- Chai, J.; Coope, C.; Cheng, J.; Wang, Y.; Cui, D.; Ren, X.; Sun, J.; Yin, X.; Zhang, C.; Wei, X. Cross-sectional study of the use of antimicrobials following common infections by rural residents in Anhui, China. BMJ Open 2019, 9, e024856. [Google Scholar] [CrossRef]
- Cheng, J.; Coope, C.; Chai, J.; Wang, Y.; Cui, D.; Ren, X.; Sun, J.; Yin, X.; Zhang, C.; Wei, X. Knowledge and behaviors in relation to antibiotic use among rural residents in Anhui, China. Pharmacoepidemiol. Drug Saf. 2018, 27, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, Y.; Hulth, A.; Xiao, Y.; Nilsson, L.E.; Zhou, X.; Hu, X.; Lundborg, C.S. Study protocol for One Health data collections, analyses and intervention of the Sino-Swedish integrated multisectoral partnership for antibiotic resistance containment (IMPACT). BMJ Open. 2018, 8, e017832. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization. Estimation of the Use, Quality and Cost of Antibiotics; Pan American Health Organization: Washington, DC, USA, 2005. [Google Scholar]
- Kotwani, A.; Holloway, K. Trends in antibiotic use among outpatients in New Delhi, India. BMC Infect. Dis. 2011, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.V.; Do, N.T.T.; Nguyen, C.T.K.; Tran, T.T.; Hoang, T.H.; Vu, T.H.; Pham, C.V.; Wertheim, H.F.L. Community-level consumption of antibiotics according to the AWaRe (Access, Watch, Reserve) classification in rural Vietnam. JAC Antimicrob. Resist. 2020, 2, dlaa048. [Google Scholar] [CrossRef]
- Moise, K.; Bernard, J.J.; Henrys, J.H. Evaluation of antibiotic self-medication among outpatients of the state university hospital of Port-Au-Prince, Haiti: A cross-sectional study. Pan Afr. Med. J. 2017, 28, 4. [Google Scholar] [CrossRef]
- Figueras, A. Survey on the Use of Antibiotics in Pharmacies Study Protocol; Catalan Foundation Institute of Pharmacology: Barcelona, Spain, 2017. [Google Scholar]
- Donadel, M.; Karimova, G.; Nabiev, R.; Khodjamurodov, G.; Rechel, B.; Habibov, N.; Mirzoev, T.; Sautenkova, N.; Huseynov, S.; Farrington, J. Drug prescribing patterns at primary health care level and related out-of-pocket expenditures in Tajikistan. BMC Health Serv. Res. 2016, 16, 556. [Google Scholar] [CrossRef]
- Mekuria, L.A.; de Wit, T.F.R.; Spieker, N.; Wubie, M.; Abegaz, T.; Taye, G.; van der Velden, A.; van der Meer, J.W. Analyzing data from the digital healthcare exchange platform for surveillance of antibiotic prescriptions in primary care in urban Kenya: A mixed-methods study. PLoS ONE 2019, 14, e0222651. [Google Scholar]
- Adeyemi, O.O.; Alabi, A.S.; Adeyemi, O.A.; Olayinka, A.T.; Olonitola, O.S.; Okonko, I.O.; Olayinka, B.O.; Olayinka, F.O. Acute gastroenteritis and the usage pattern of antibiotics and traditional herbal medications for its management in a Nigerian community. PLoS ONE 2021, 16, e0257837. [Google Scholar] [CrossRef] [PubMed]
- Kotwani, A.; Chaudhury, R.R.; Holloway, K. Antibiotic-prescribing practices of primary care prescribers for acute diarrhea in New Delhi, India. Value Health 2012, 15 (Suppl. 1), S116–S119. [Google Scholar] [CrossRef] [PubMed]
- Kotwani, A.; Holloway, K. Antibiotic prescribing practice for acute, uncomplicated respiratory tract infections in primary care settings in New Delhi, India. Trop. Med. Int. Health 2014, 19, 761–768. [Google Scholar] [CrossRef]
- Sabry, N.A.; Farid, S.F.; Dawoud, D.M. Antibiotic dispensing in Egyptian community pharmacies: An observational study. Res. Social. Adm. Pharm. 2014, 10, 168–184. [Google Scholar] [CrossRef]
- WHO Regional Office for Europe. Antimicrobials Supplied in Community Pharmacies in Eastern Europe and Central Asia in the Early Phases of the COVID-19 Pandemic; WHO Regional Office for Europe: Copenhagen, Denmark, 2022. [Google Scholar]
- Pathak, D.; Pathak, A.; Marrone, G.; Diwan, V.; Lundborg, C.S. Adherence to treatment guidelines for acute diarrhoea in children up to 12 years in Ujjain, India--a cross-sectional prescription analysis. BMC Infect. Dis. 2011, 11, 32. [Google Scholar] [CrossRef]
- Robles, Y.R.; Langit, M.R.G.; Jose, M.L.S.; Villanueva, R.M.; Mendoza, M.A.; Cruz, J.M.; Santos, R.A.; Ramos, E. Manual of Procedures for Implementing Antimicrobial Stewardship in Primary Health Care Setting; Department of Health—Pharmaceutical Division: Quezon, Philippines, 2019. [Google Scholar]
- Sivasampu, S.; Wahab, Y.F.; Ong, S.M.; Ismail, S.A.; Goh, P.P.; Jeyaindran, S. National Medical Care Statistics (NMCS) 2014; Report No.: NCRC/HSU/2016.1; NMRR Approval No. NMRR-09-842-4718; Ministry of Health Malaysia: Kuala Lumpur, Malaysia, 2016. [Google Scholar]
- Pathak, A.; Mahadik, K.; Dhaneria, S.P.; Sharma, A.; Eriksson, B.; Lundborg, C.S. Antibiotic prescribing in outpatients: Hospital and seasonal variations in Ujjain, India. Scand. J. Infect. Dis. 2011, 43, 479–488. [Google Scholar] [CrossRef]
- World Health Organization Action Programme on Essential Drugs and Vaccines. How to Investigate Drug Use in Health Facilities: Selected Drug Use Indicators; World Health Organization: Geneva, Switzerland, 1993. [Google Scholar]
- Mandal, P.; Asad, M.; Kayal, A.; Saha, S.; Ghosh, A.; Das, A.; Banerjee, S.; Chatterjee, S. Assessment of use of World Health Organization access, watch, reserve antibiotics and core prescribing indicators in pediatric outpatients in a tertiary care teaching hospital in Eastern India. Perspect. Clin. Res. 2023, 14, 61–67. [Google Scholar] [CrossRef]
- Global-PPS the Outpatient Module: Global-PPS. 2024. Available online: https://www.global-pps.com/ (accessed on 10 November 2024).
- Cook, A.; Goelen, J.; Moore, C.E.; Ashley, E.A.; Turner, P.; Sharland, M.; Huttner, B.; Zanichelli, V.; Versporten, A.; Bielicki, J. A pilot protocol for surveillance of infection and antibiotic prescribing in primary healthcare across the globe: Antibiotic Prescribing in Primary Healthcare Point Prevalence Survey (APC-PPS). Wellcome Open Res. 2025, 10, 26. [Google Scholar] [CrossRef]
- Abdou, E.; Hayder, R.; Salaheldin, M.; Ahmed, A.; Osman, H.; Elhassan, M.; Elamin, A.; Babiker, A. Over-prescription of Watch antibiotics in primary healthcare settings in Sudan: Results from routinely collected prescription data. J. Infect. Dev. Ctries. 2025, 19, 91–97. [Google Scholar] [CrossRef]
- South East Asia Regional Office of the World Health Organization. Medicines Management in Health Care Delivery; South East AsiaRegional Office ofthe World Health Organization: Delhi, India, 2016. [Google Scholar]
- World Health Organization. WHO Operational Package for Assessing, Monitoring and Evaluating Country Pharmaceutical Situations: Guide for Coordinators and Data Collectors; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Cordoba, G.; Caballero, L.; Sandholdt, H.; Holm, A.; Bjerrum, L. Antibiotic prescriptions for suspected respiratory tract infection in primary care in South America. J. Antimicrob. Chemother. 2017, 72, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Mbonye, A.K.; Buregyeya, E.; Rutebemberwa, E.; Clarke, S.E.; Chandler, C.I.; Hansen, K.S.; Magnussen, P.; LaRussa, P.; Staedke, S.G. Prescription for antibiotics at drug shops and strategies to improve quality of care and patient safety: A cross-sectional survey in the private sector in Uganda. BMJ Open 2016, 6, e010632. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moreno, P.; Cerón, A.; Sosa, K.; López, A.; García, M.; Rodríguez, J.; Hernández, L.; Pérez, R. Availability of over-the-counter antibiotics in Guatemalan corner stores. PLoS ONE 2020, 15, e0239873. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.; Purohit, M.; Sharma, M.; Gaur, A.; Jain, S.; Diwan, V.; Pathak, A.; Marrone, G.; Lundborg, C.S. Antibiotic Prescribing by Informal Healthcare Providers for Common Illnesses: A Repeated Cross-Sectional Study in Rural India. Antibiotics 2019, 8, 139. [Google Scholar] [CrossRef]
- Department of Health Republic of South Africa. Guidelines on Implementation of the Antimicrobial Strategy in South Africa: One Health Approach & Governance; Department of Health Republic of South Africa: Pretoria, South Africa, 2017. [Google Scholar]
- Department of Health Republic of South Africa. Surveillance for Antimicrobial Resistance and Consumption of Antimicrobials in South Africa, 2021; Department of Health Republic of South Africa: Pretoria, South Africa, 2022. [Google Scholar]
- Burkina Faso National Order of Pharmacies. Protocol: Collection of Data on the Consumption of Antibacterials in Pharmacies in the City of Ouagadougou; Burkina Faso National Order of Pharmacies: Ouagadougou, Burkina Faso, 2017. [Google Scholar]
- Lubell, Y.; Greer, R.; Nedsuwan, S.; Blacksell, S.D.; Dondorp, A.M.; Day, N.P.J. A Retrospective Service Evaluation of the Management of Acutely Ill Patients and the Indicators for Antibiotic Prescription in Primary Care in Northern Thailand; Mahidol University: Bangkok, Thailand, 2017. [Google Scholar]
- Zhao, L.; Kwiatkowska, R.M.; Chai, J.; Cabral, C.; Chen, M.; Bowker, K.; Coope, C.; Shen, J.; Shen, X.; Cheng, J.; et al. Pathways to optimising antibiotic use in rural China: Identifying key determinants in community and clinical settings, a mixed methods study protocol. BMJ Open. 2019, 9, e027819. [Google Scholar] [CrossRef]
- Stalsby Lundborg, C.; Diwan, V.; Pathak, A.; Purohit, M.; Sharma, M.; Tamhankar, A.J.; Marrone, G.; Khare, S.; Gaur, A.; Jain, S. Protocol: A ‘One health’ two year follow-up, mixed methods study on antibiotic resistance, focusing children under 5 and their environment in rural India. BMC Public Health 2015, 15, 1321. [Google Scholar] [CrossRef]
- Hopkins, H.; Bassat, Q.; Chandler, C.I.; Crump, J.A.; Feasey, N.A.; Ferrand, R.A.; Gibb, D.M.; Gonzalez, R.; Green, N.; Guiraud, I.; et al. Febrile Illness Evaluation in a Broad Range of Endemicities (FIEBRE): Protocol for a multisite prospective observational study of the causes of fever in Africa and Asia. BMJ Open 2020, 10, e035632. [Google Scholar] [CrossRef]
- Dixon, J.; MacPherson, E.; Manyau, S.; Nayiga, S.; Gaur, A.; Singh, P.; Nabirye, C.; Kayendeke, M.; Denyer Willis, L.; Staedke, S.G.; et al. The ‘Drug Bag’ method: Lessons from anthropological studies of antibiotic use in Africa and South-East Asia. Glob. Health Action. 2019, 12, 1639388. [Google Scholar] [CrossRef]
- Managament Sciences for Health. Chapter 20. Investigating medicine use. In MDS-3: Managing Access to Medicines and Health Technologies; Management Sciences for Health: Arlington, VA, USA, 2012; p. 28. [Google Scholar]
- Bethlehem, J.G. Applied Survey Methods: A Statistical Perspective; Wiley: New Jersey, NJ, USA, 2009. [Google Scholar]
- Gualano, M.R.; Gili, R.; Scaioli, G.; Bert, F.; Siliquini, R. General population’s knowledge and attitudes about antibiotics: A systematic review and meta-analysis. Pharmacoepidemiol. Drug Saf. 2015, 24, 2–10. [Google Scholar] [CrossRef]
- Kosiyaporn, H.; Chanvatik, S.; Issaramalai, T.; Kaewkhankhaeng, W.; Kulthanmanusorn, A.; Saengruang, N.; Witthayapipopsakul, W.; Viriyathorn, S.; Kirivan, S.; Patcharanarumol, W.; et al. Surveys of knowledge and awareness of antibiotic use and antimicrobial resistance in general population: A systematic review. PLoS ONE 2020, 15, e0227973. [Google Scholar] [CrossRef]
- Mabirizi, D.; Phulu, B.; Churfo, W.; Mwinga, S.; Mazibuko, G.; Sagwa, E.; Indongo, L.; Hafner, T. Implementing an Integrated Pharmaceutical Management Information System for Antiretrovirals and Other Medicines: Lessons From Namibia. Glob. Health Sci. Pract. 2018, 6, 723–735. [Google Scholar] [CrossRef]
- Zhao, H.; Wei, L.; Li, H.; Zhang, M.; Cao, B.; Bian, J.; Zhan, S. Appropriateness of antibiotic prescriptions in ambulatory care in China: A nationwide descriptive database study. Lancet Infect. Dis. 2021, 21, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Tangcharoensathien, V.; Sommanustweechai, A.; Chanthong, B.; Sumpradit, N.; Kiatying-Angsulee, N.; Janejai, N.; Patcharanarumol, W. Surveillance of antimicrobial consumption: Methodological review for systems development in Thailand. J. Glob. Health 2017, 7, 010307. [Google Scholar]
- Sanchez Choez, X.; Armijos Acurio, M.L.; Jimbo Sotomayor, R.E. Appropriateness and adequacy of antibiotic prescription for upper respiratory tract infections in ambulatory health care centers in Ecuador. BMC Pharmacol. Toxicol. 2018, 19, 46. [Google Scholar] [CrossRef] [PubMed]
- Burkina Faso National Order of Pharmacies. Report of the Consumption of Antibiotics in the Pharmaceutical Offices of the City of Ouagadougou; Burkina Faso National Order of Pharmacies: Ouagadougou, Burkina Faso, 2017. [Google Scholar]
- Matuz, M.; Benko, R.; Doro, P.; Hajdu, E.; Nagy, G.; Nagy, E.; Monnet, D.L.; Soos, G. Non-prescription antibiotic use in Hungary. Pharm. World Sci. 2007, 29, 695–698. [Google Scholar] [CrossRef]
- Bozic, B.; Bajcetic, M. Use of antibiotics in paediatric primary care settings in Serbia. Arch. Dis. Childhood. 2015, 100, 966–969. [Google Scholar] [CrossRef] [PubMed]
- Machado-Alba, J.E.; Gonzalez-Santos, D.M. Dispensing antibiotics to outpatients in a Colombian population. Rev. Salud Publica 2009, 11, 734–744. [Google Scholar] [CrossRef]
- Machado-Alba, J.E.; Valladales-Restrepo, L.F.; Gaviria-Mendoza, A.; Machado-Duque, M.E.; Machado-Velez, A. Patterns of Antibiotic Prescription in Colombia: Are There Differences between Capital Cities and Municipalities? Antibiotics 2020, 9, 389. [Google Scholar] [CrossRef]
- National Department of Health. Surveillance for Antimicrobial Resistance and Consumption of Antibiotics in South Africa 2018–2022; National Department of Health: Pretoria, South Africa, 2024. [Google Scholar]
- Soleymani, F.; Taheri, F.; Roughead, E.; Nikfar, S.; Abdollahi, M. Pattern of Antidepressant Utilization and Cost in Iran from 2006 to 2013 in Comparison with Other Countries. J. Epidemiol. Glob. Health 2018, 8, 213–219. [Google Scholar] [CrossRef]
- Pharmaceutical Services Programme. Malaysian Statistics on Medicines 2015–2016; Ministry of Health Malaysia: Kuala Lumpur, Malaysian, 2020. [Google Scholar]
| International Society for Improving Use of Medicines (ISIUM) | www.isium.org (accessed on 10 April 2024) |
| International Pharmaceutical Federation (FIP) | www.fip.org (accessed on 10 April 2024) |
| Medicines Utilisation Research In Africa (MURIA) network | https://muria.mandela.ac.za/ (accessed on 10 April 2024) |
| REDCIMLAC (Red de Centros de Información de Medicamentos de Latinoamérica y el Caribe | www.redcimlac.org (accessed on 10 April 2024) |
| London School of Hygiene and Tropical Medicine (LSHTM) Antimicrobial Resistance Centre | www.lshtm.ac.uk (accessed on 10 April 2024) |
| ReACT, action on antibiotic resistance | www.reactgroup.org (accessed on 10 April 2024) |
| Mahidol Oxford Tropical Medicine Research Unit, Thailand (MORU) | www.tropmedres.ac (accessed on 10 April 2024) |
| HiTAP (Health Information and Technology Assessment Program, Thailand) | www.hitap.net (accessed on 10 April 2024) |
| Management Sciences for Health | www.msh.org (accessed on 10 April 2024) |
| WHO Headquarters and Regional Offices | www.who.int (accessed on 10 April 2024) |
| Bill and Melinda Gates Foundation | www.gatesfoundation.org (accessed on 10 April 2024) |
| UNICEF | www.unicef.org (accessed on 10 April 2024) |
| Save the Children | www.savethechildren.org (accessed on 10 April 2024) |
| USAID | www.usaid.gov (accessed on 10 April 2024) |
| Antimicrobial use tracker | https://antimicrobialsinsociety.org/antimicrobial-use-tracker/ (accessed on 10 April 2024) |
| Antibiotic Data to Inform Local Action (ADILA) | https://cnpi-amr.org/research/adila/ (accessed on 10 April 2024) |
| Category | Subcategory | Number of Survey Instruments (%) |
|---|---|---|
| By time period | ||
| Year of conduct | 1993–2000 | 1 (2.4) |
| 2001–2010 | 4 (9.5) | |
| 2011–2017 | 18 (42.9) | |
| 2018–2023 (post-AWaRe) | 19 (45.2) | |
| By geographic region | ||
| WHO region * | Global (multinational) | 7 (16.7) |
| Americas | 6 (14.3) | |
| African | 7 (16.7) | |
| Eastern Mediterranean | 4 (9.5) | |
| European | 3 (7.1) | |
| South-East Asian | 8 (19.0) | |
| Western Pacific | 7 (16.7) |
| Ref. | Target Group | Disease | Where Used | Country | No. and Type of Questions | Main Measure | Reported or Possible Measures |
|---|---|---|---|---|---|---|---|
| Household survey: Generic | |||||||
| [15] | Generic | Generic | Home | Jordan | 14 closed questions Structured responses; medicine name is text entry | Self-reported, 1-month period prevalence |
|
| [16] | Generic | Generic | Home | Multi-country | 65 closed questions with structured responses; medicine name is text entry | Self-reported 1-month period prevalence |
|
| [27] + Sm | Generic | Generic | Electronic survey | Latin America | 37 closed questions Structured responses; medicine name is text entry | Self-reported 15-day period prevalence |
|
| [17] | Generic | Generic | Generic (home or exit) | Global | 3 example surveys 5–15 open questions with unstructured responses | % of illness episodes where antimicrobial used |
|
| [18] | Generic | Generic | Interviews at bus stations, shopping malls, or health facilities | WHO EUR | 15 structured questions plus demographic information | Self-reported use of at least once in last year |
|
| Household surveys: Child-focused | |||||||
| [19] | Children under five years | Generic | Home Illness self-report diary | India | 30 questions Majority open questions with unstructured responses | % of illness episodes where antimicrobial used |
|
| [20] + Sm | Children under 2 years | Generic | Home Biweekly illness report form | Multi-country | 11 closed questions Structured responses | Courses per child per year |
|
| [21] | Children under 2 | Generic | Home | Senegal, Madagascar | 35 closed questions Mostly structured responses; medication name is text entry; visual cues provided | Self-reported 3-month period prevalence |
|
| [22] | Children under 5 | Diarrhea or acute respiratory tract infection | Home | Uganda | 22 closed questions Mostly structured responses; medication name is text entry | Self-reported 2-week period prevalence |
|
| Household surveys: Disease-focused | |||||||
| [23] | Children <5 years | Cough | Household | China | 30 closed questions with structured responses | Self-reported 1-month period prevalence |
|
| [24] | Generic | Sneeze/cough; diarrhea, gastroenteritis; urethritis | Home | China | 62 questions Structured responses | Self-reported 3- or 12-month period |
|
| [25] | Generic | Sneeze/cough; diarrhea and gastroenteritis; urethritis | Home | China | 68 questions Structured responses | Almost identical to [23] | Refer to [23] |
| Household surveys: One Health | |||||||
| [26] | Generic | Generic | Home | China | >100 questions Majority structured responses; includes animal contact | Self-reported One-week, one-month period prevalence |
|
| Ref. | Target Group | Disease | Where Used | Country | Number and Type of Questions | Main Measure | Reported or Possible Measures |
|---|---|---|---|---|---|---|---|
| Exit interviews: Generic | |||||||
| [27] + Sm | Generic | Generic | Pharmacy | Latin America | 37 closed questions with structured responses; medicine name is text entry | Point prevalence |
|
| [28] + Sm | Generic | Generic | Public and private facilities: GPs, retail pharmacy shops | India | 16 closed questions with unstructured responses | Point prevalence |
|
| [16] | Generic | Generic | Medicine suppliers | Multi-country | 15 closed questions with structured responses; medicine name is text entry | Point prevalence |
|
| [29] | Generic | Generic | Vietnam | Same as [14] | Same as [16] |
| |
| [30] | Generic | Generic | Outpatients | Haiti | 7 questions Open ended questions; unstructured responses | Self-reported two-week prevalence of self-medicated use |
|
| [31] + Sm | Generic | Generic | Pharmacies | Latin America | 14 closed questions Structured and unstructured responses | Point prevalence Self-reported six-month prevalence |
|
| [27] + Sm | Generic | Generic | Pharmacy | Latin America | 37 closed questions with structured responses; medicine name is text entry | Point prevalence |
|
| [32] | Generic | Generic | Primary health facility (post visit at home) | Tajikistan | 64 closed questions with structured responses | % of encounters where antimicrobial prescribed | Possible to stratify measure by source, demographics, and indication |
| Exit interviews: Disease-focused | |||||||
| [33] | Generic | Acute respiratory illness | Primary care clinics | Kenya | Semi-structured, open-ended; unstructured responses | Point prevalence |
|
| [34] | Adults (14 years and over) | Gastroenteritis | Primary care facilities | Nigeria | 14 closed questions with structured responses; medicine name is text entry | Point prevalence |
|
| [35] + Sm | Generic | Acute diarrhea | Public and private facilities: GPs, retail pharmacy shops | India | 16 closed questions Unstructured responses | Point prevalence |
|
| [36] + Sm | Generic | Acute respiratory illness/upper respiratory illness | Public and private facilities: GPs, retail pharmacy shops | India | 16 closed questions Unstructured responses. | Point prevalence |
|
| Ref. | Target Group | Disease | Where Used | Country | Number and Type of Questions | Main Measure | Reported or Possible Measures |
|---|---|---|---|---|---|---|---|
| Pharmacy records | |||||||
| [37] | Generic | Generic | Pharmacies | Egypt | ~20 questions 3 different forms dependent on source of request Structured and unstructured responses. | Antimicrobial prescriptions are the denominator |
|
| [38] | Generic | Generic | Pharmacies | Europe and Central Asia | 13 questions Structured and unstructured responses | Antimicrobial prescriptions are the denominator |
|
| [39] | Generic | Diarrhea | Primary Care | India | 8 questions Structured and unstructured responses | % of encounters where antimicrobial prescribed | Limited to antimicrobial yes or no |
| Ref. | Target Group | Disease | Where Used | Country | Type of Questions | Main Measure | Reported or Possible Measures |
|---|---|---|---|---|---|---|---|
| Physician records: Generic | |||||||
| [40] | Generic | Generic | Public or private primary health care facilities | Philippines | Case report form, unstructured text Summary report form | Antimicrobial prescriptions are the denominator |
|
| [41] | Generic | Generic | Public or private primary care | Malaysia | Case report form: unstructured text Demographics, reason for encounter, diagnoses, and interventions | % of encounters where antimicrobial prescribed |
|
| [42] | Generic | Generic | Outpatient clinics at hospitals | India | Semi-structured case report form | % of encounters where antimicrobial prescribed |
|
| [43] | Generic | Generic | Health Facility | Generic | Case report form Unstructured Demographics, reason for encounter, pharmaceutical treatments | % of encounters where antimicrobial prescribed |
|
| [44] + Sm | Generic | Generic | Health Facility | India | Case report form Unstructured Demographics, reason for encounter, pharmaceutical treatments | % of encounters where antimicrobial prescribed |
|
| [45] | Generic | Generic | Health Facility | Global | Case report form Structured and unstructured Medicines: unstructured | % of encounters where antimicrobial prescribed |
|
| [46] | Generic | Generic | Health Facility | Egypt | Case report from Structured Medicine names: unstructured | % of encounters where antimicrobial prescribed |
|
| [47] + Sm | Generic | Generic | Health Facility | Sudan | Case report form Unstructured | % of encounters where antimicrobial prescribed |
|
| Generic records: Disease-focused | |||||||
| [48] + Sm | Generic | Upper respiratory tract infection | Health Facility | South East Asia | Unstructured text | % of encounters where antimicrobial prescribed |
|
| [49] | Children under 5y | Diarrhea, pneumonia, acute respiratory infection | Health Facilities | Multi-country | Structured case report form Use of medicine: yes/no | % of encounters where antimicrobial prescribed | Did not collect antimicrobial type |
| [50] | Generic | URTI | Primary care | Latin America | Structured Demographics, reason for encounter, diagnoses, and interventions | % of encounters where antimicrobial prescribed |
|
| Ref. | Target Group | Disease | Where Used | Country | Number and Type of Questions | Main Measure | Reported or Possible Measures |
|---|---|---|---|---|---|---|---|
| Corner stores (in stock surveys only) | |||||||
| [51] | Generic | Generic | Drug store | Uganda | 89 questions Structured responses | Limited to stock of antimicrobials; no volume measures |
|
| [52] | Generic | Generic | Corner store | Guatemala | 15 questions—dependent on number of antimicrobials sold Structured responses | Limited to stock of antimicrobials; no volume measures |
|
| Informal health care providers | |||||||
| [53] | Generic | Generic | Informal health care provider practice | India | 12 questions Unstructured response | % of antimicrobials as a proportion of total prescriptions |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanovska, V.; Laba, T.-L.; Lim, R.; Kotwani, A.; Muller, A.; Escher, M.; Huttner, B.; Roughead, E. Inventory of Survey Instruments for Monitoring Antimicrobial Use in Primary Care Settings in Low- and Middle-Income Countries: A Narrative Review. Antibiotics 2025, 14, 1159. https://doi.org/10.3390/antibiotics14111159
Ivanovska V, Laba T-L, Lim R, Kotwani A, Muller A, Escher M, Huttner B, Roughead E. Inventory of Survey Instruments for Monitoring Antimicrobial Use in Primary Care Settings in Low- and Middle-Income Countries: A Narrative Review. Antibiotics. 2025; 14(11):1159. https://doi.org/10.3390/antibiotics14111159
Chicago/Turabian StyleIvanovska, Verica, Tracey-Lea Laba, Renly Lim, Anita Kotwani, Arno Muller, Martina Escher, Benedikt Huttner, and Elizabeth Roughead. 2025. "Inventory of Survey Instruments for Monitoring Antimicrobial Use in Primary Care Settings in Low- and Middle-Income Countries: A Narrative Review" Antibiotics 14, no. 11: 1159. https://doi.org/10.3390/antibiotics14111159
APA StyleIvanovska, V., Laba, T.-L., Lim, R., Kotwani, A., Muller, A., Escher, M., Huttner, B., & Roughead, E. (2025). Inventory of Survey Instruments for Monitoring Antimicrobial Use in Primary Care Settings in Low- and Middle-Income Countries: A Narrative Review. Antibiotics, 14(11), 1159. https://doi.org/10.3390/antibiotics14111159






