Molecular Epidemiology, Antimicrobial Resistance, and Clinical Characteristics of Streptococcus pneumoniae Isolated from Adult Patients with Invasive Pneumococcal Disease
Abstract
1. Introduction
2. Results
2.1. Clinical–Demographic Characteristics of IPD Patients
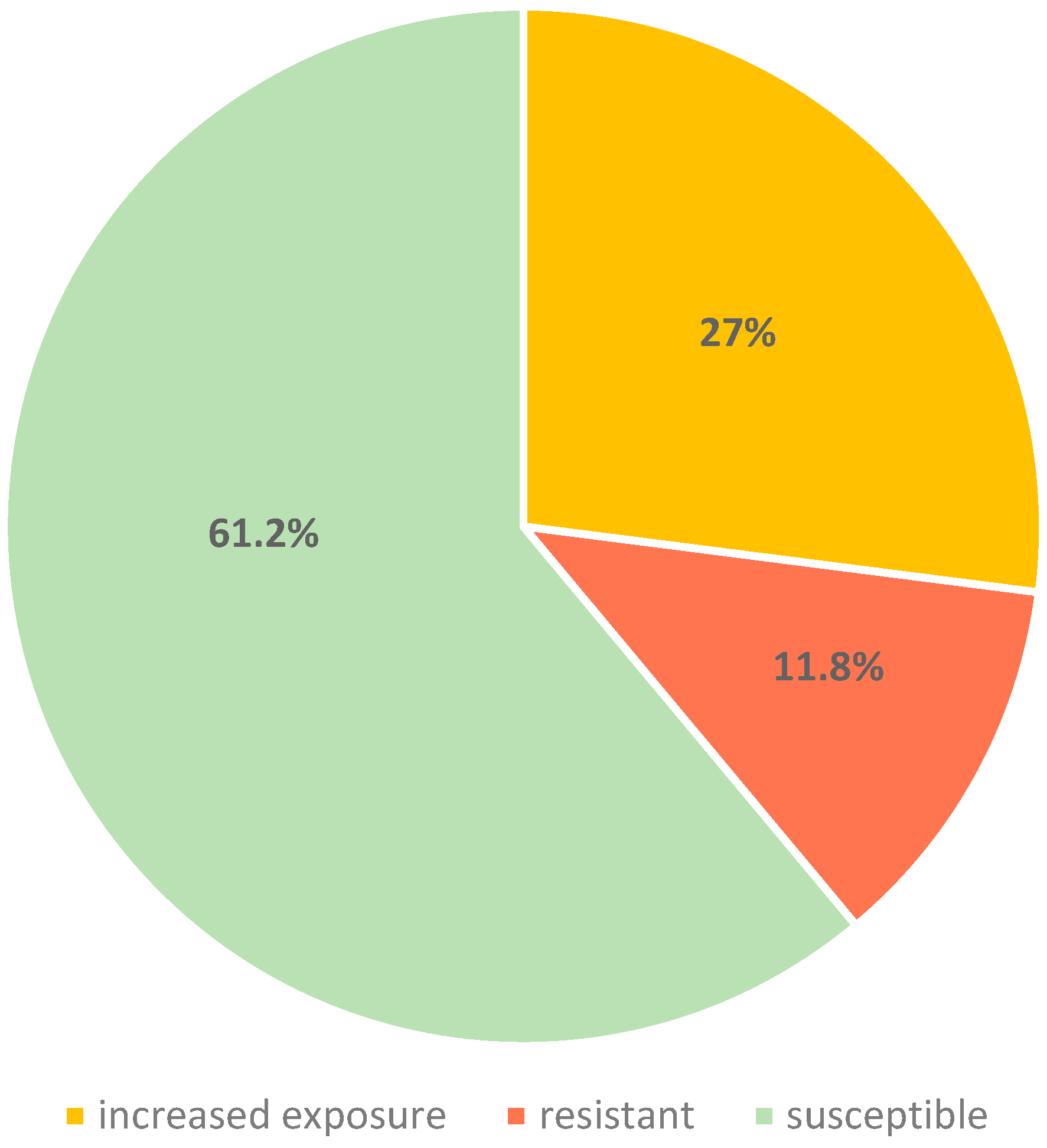
2.2. Antimicrobial Susceptibility Testing
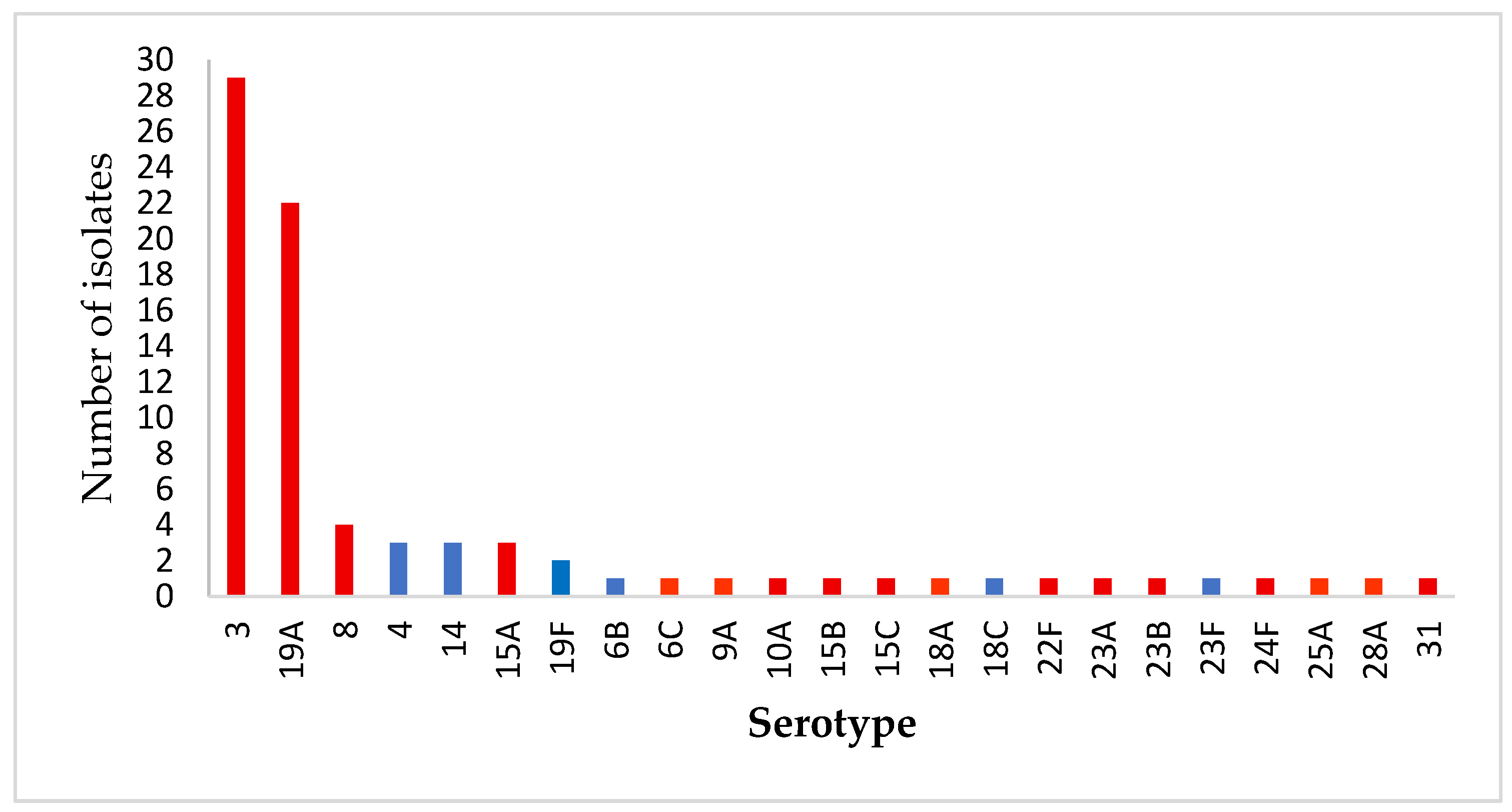
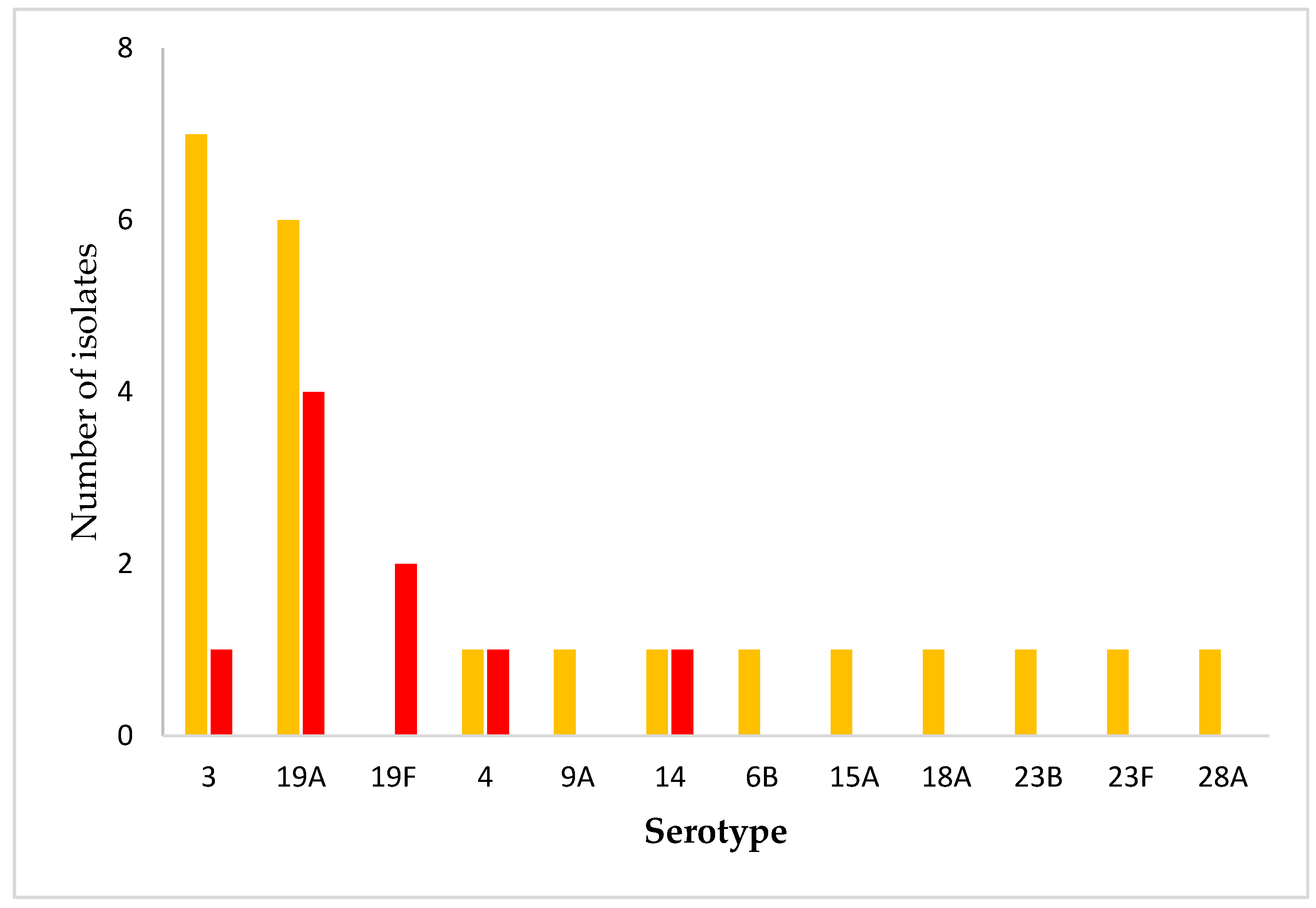
2.3. Serotype Distribution
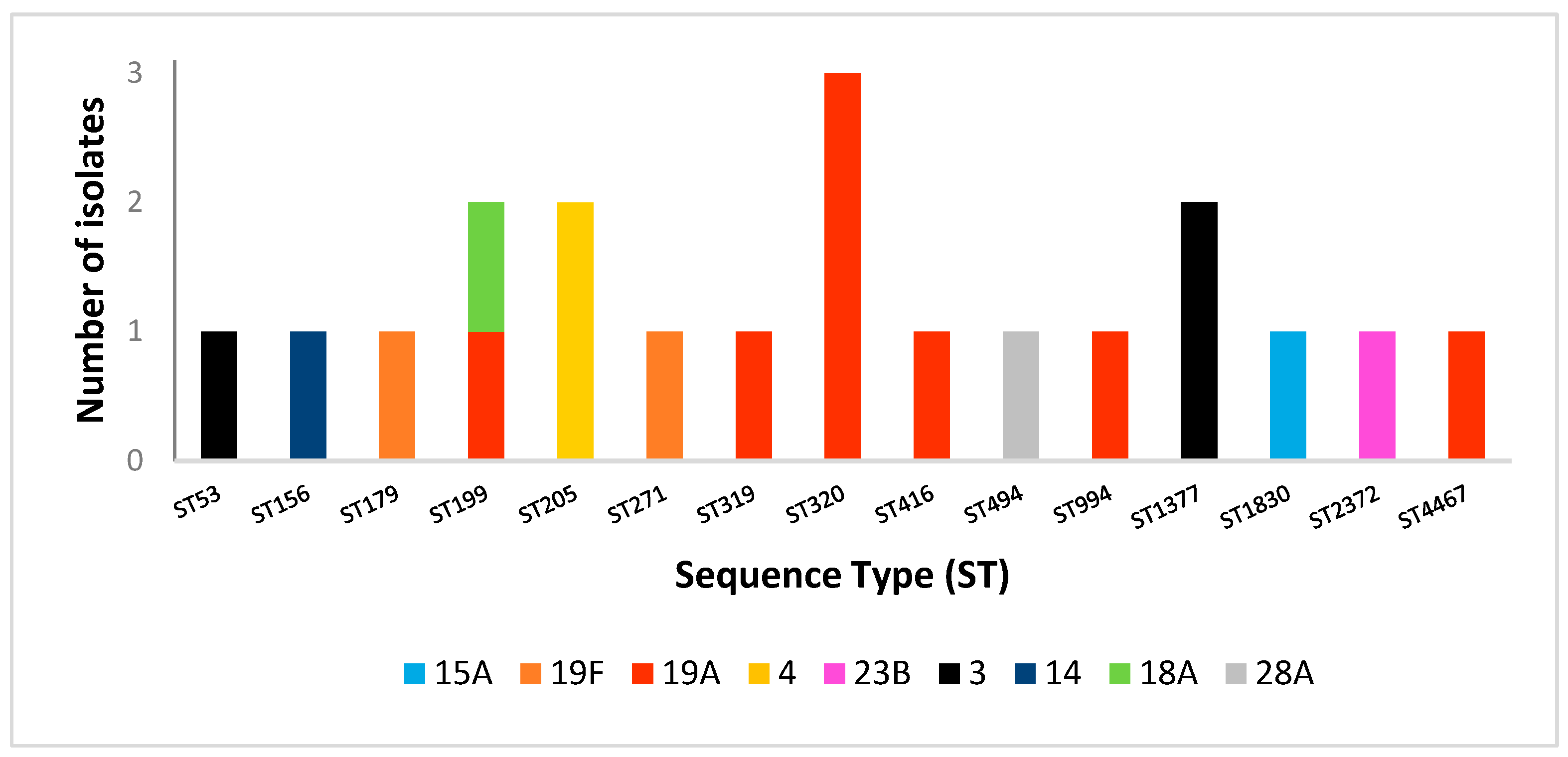
2.4. Multilocus Sequence Typing Analysis
3. Discussion
3.1. Clinical Characteristics and Outcomes
3.2. β-Lactam Susceptibility
3.3. Molecular Epidemiology and Serotype Distribution
3.4. Non-β-Lactam Susceptibility
3.5. Vaccination and Public Health Implications
3.6. Study Limitations
4. Materials and Methods
4.1. Study Setting
4.2. Antibiotic Susceptibility Testing and Serotyping
4.3. DNA Extraction and MLST
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CLI | Clindamycin |
| CSF | Cerebrospinal fluid |
| ERY | Erythromycin |
| ECDC | European Centre for Disease Prevention and Control |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| ICU | Intensive Care Unit |
| IPD | Invasive pneumococcal disease |
| MDR | Multidrug-resistant |
| MIC | Minimum inhibitory concentration |
| MLST | Multilocus sequence typing |
| MOX | Moxifloxacin |
| PCV | Pneumococcal conjugate vaccine |
| PPV | Pneumococcal polysaccharide vaccine |
| ST | Sequence type |
| SXT | Trimethoprim-sulfamethoxazole |
| TE | Tetracycline |
References
- Centers for Disease Control and Prevention, CDC. Pneumococcal Disease. Pneumococcal Vaccination. Available online: https://www.cdc.gov/pneumococcal/vaccines/index.html (accessed on 30 July 2025).
- Hrvatski Zavod za Javno Zdravstvo. Preporuke za Cijepljenje Odraslih Osoba Protiv Pneumokoka. Available online: https://www.hzjz.hr/sluzba-epidemiologija-zarazne-bolesti/preporuke-za-cijepljenje-odraslih-osoba-protiv-pneumokoka (accessed on 30 July 2025).
- Ahmed, S.S.; Pondo, T.; Xing, W.; McGee, L.; Farley, M.; Schaffner, W.; Thomas, A.; Reingold, A.; Harrison, L.H.; Lynfield, R.; et al. Early Impact of 13-Valent Pneumococcal Conjugate Vaccine Use on Invasive Pneumococcal Disease Among Adults with and Without Underlying Medical Conditions—United States. Clin. Infect. Dis. 2020, 70, 2484–2492. [Google Scholar] [CrossRef] [PubMed]
- Hanquet, G.; Krizova, P.; Valentiner-Branth, P.; Ladhani, S.N.; Nuorti, J.P.; Lepoutre, A.; Mereckiene, J.; Knol, M.; Winje, B.A.; Ciruela, P.; et al. Effect of childhood pneumococcal conjugate vaccination on invasive disease in older adults of 10 European countries: Implications for adult vaccination. Thorax 2019, 74, 473–482. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Invasive Pneumococcal Disease. Annual Epidemiological Report for 2022. Available online: https://www.ecdc.europa.eu/en/publications-data/invasive-pneumococcal-disease-annual-epidemiological-report-2022 (accessed on 30 July 2025).
- Gergova, R.; Boyanov, V.; Muhtarova, A.; Alexandrova, A. A Review of the Impact of Streptococcal Infections and Antimicrobial Resistance on Human Health. Antibiotics 2024, 13, 360. [Google Scholar] [CrossRef]
- de Miguel, S.; Pérez-Abeledo, M.; Ramos, B.; García, L.; Arce, A.; Martínez-Arce, R.; Yuste, J.; Sanz, J.C. Distribution of Multidrug-Resistant Invasive Serotypes of Streptococcus pneumoniae during the Period 2007–2021 in Madrid, Spain. Antibiotics 2023, 12, 342. [Google Scholar] [CrossRef]
- Maraki, S.; Mavromanolaki, V.E.; Stafylaki, D.; Iliaki-Giannakoudaki, E.; Kasimati, A.; Hamilos, G. Antimicrobial Resistance of Streptococcus pneumoniae Clinical Serotypes between 2017 and 2022 in Crete, Greece. Infect. Chemother. 2024, 56, 73–82. [Google Scholar] [CrossRef]
- Public Databases for Molecular Typing and Microbial Genome Diversity, PubMLST. Available online: https://pubmlst.org/bigsdb?db=pubmlst_spneumoniae_isolates&page=query (accessed on 17 September 2025).
- Državni Zavod za Statistiku Republika Hrvatska. Natural Change in Population in the Republic of Croatia. 2024. Available online: https://podaci.dzs.hr/media/pcndmrk2/stan-2025-1-1-natural-change-in-population-in-the-republic-of-croatia-2024.pdf (accessed on 30 August 2025).
- Bailey, M.D.; Farge, G.; Mohanty, S.; Breau-Brunel, M.; Roy, G.; de Pouvourville, G.; de Wazieres, B.; Janssen, C.; Tauty, S.; Bugnard, F.; et al. Clinical burden of pneumococcal disease among adults in France: A retrospective cohort study. Hum. Vaccines Immunother. 2025, 21, 2515760. [Google Scholar] [CrossRef]
- Čivljak, R.; Draženović, K.; Butić, I.; Kljaković Gašpić Batinjan, M.; Huljev, E.; Vicković, N.; Lizatović, I.K.; Grgić, B.; Budimir, A.; Janeš, A.; et al. Invasive pneumococcal disease in adults after the introduction of pneumococcal vaccination: A retrospective study in the metropolitan area of Zagreb, Croatia (2010–2022). Front. Public Health 2024, 12, 1480348. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Onizuka, H.; Nishimura, N.; Kiyohara, K. Risk factors for pneumococcal disease in persons with chronic medical conditions: Results from the LIFE Study. Int. J. Infect. Dis. 2022, 116, 216–222. [Google Scholar] [CrossRef]
- Ditzel, K.; Giardina, F.; ten Oever, J.; Cremers, A.J.H. Risk factors for invasive pneumococcal disease in adults: A systematic review and meta-analysis. medRxiv 2025. medRxiv:03.13.25323815. [Google Scholar] [CrossRef]
- Gil-Prieto, R.; Allouch, N.; Jimeno, I.; Hernández-Barrera, V.; Arguedas-Sanz, R.; Gil-de-Miguel, Á. Burden of Hospitalizations Related to Pneumococcal Infection in Spain (2016–2020). Antibiotics 2023, 12, 172. [Google Scholar] [CrossRef] [PubMed]
- Butić, I.; Gužvinec, M.; Jelić, M.; Groš, I.; Lucić, S.; Bošnjak, M.; Andrašević, A.T. Working Group for Invasive Isolates of the Croatian Committee for Antibiotic Resistance Surveillance. Serotype distribution and antimicrobial resistance of invasive Streptococcus pneumoniae isolates among Croatian adults during a fifteen-year period (2005–2019). Croat. Med. J. 2022, 63, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Hanquet, G.; Krizova, P.; Dalby, T.; Ladhani, S.N.; Nuorti, J.; Danis, K.; Mereckiene, J.; Knol, M.J.; Winje, B.A.; Ciruela, P.; et al. Serotype Replacement after Introduction of 10-Valent and 13-Valent Pneumococcal Conjugate Vaccines in 10 Countries, Europe. Emerg. Infect. Dis. 2022, 28, 137–138. [Google Scholar] [CrossRef]
- Shi, X.; Patil, S.; Wang, Q.; Liu, Z.; Zhu, C.; Wang, H.; Chen, Y.; Li, L.; Yang, L.; Zheng, Y.; et al. Prevalence and resistance characteristics of multidrug-resistant Streptococcus pneumoniae isolated from the respiratory tracts of hospitalized children in Shenzhen, China. Front. Cell. Infect. Microbiol. 2024, 13, 1332472. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Surveillance Atlas of Infectious Diseases. Available online: https://www.ecdc.europa.eu/en/surveillance-atlas-infectious-diseases (accessed on 30 August 2025).
- Varghese, R.; Basu, S.; Neeravi, A.; Pragasam, A.; Aravind, V.; Gupta, R.; Miraclin, A.; Ramaiah, S.; Anbarasu, A.; Veeraraghavan, B. Emergence of Meropenem Resistance Among Cefotaxime Non-Susceptible Streptococcus pneumoniae: Evidence and Challenges. Front. Microbiol. 2022, 12, 810414. [Google Scholar] [CrossRef]
- Petrutienė, A.; Sinotova, J.; Pupienienė, N.; Marcinonytė, R.; Padvilikytė, I.; Razmuk, J.; Muralytė, S.; Bulavaitė, A.; Plečkaitytė, M. A decade of 10-valent pneumococcal conjugate vaccine use in Lithuania: Trends in invasive pneumococcal serotype dynamics. Front. Public Health. 2025, 13, 1633396. [Google Scholar] [CrossRef]
- Opavski, N.; Jovićević, M.; Kabić, J.; Kekić, D.; Gajić, I. Study Group for Laboratory Surveillance of Invasive Pneumococcal Diseases. Effect of Childhood Pneumococcal Conjugate Vaccination on Invasive Disease Serotypes in Serbia. Vaccines 2024, 12, 940. [Google Scholar] [CrossRef]
- Krajcar, N.; Trkulja, V.; Butić, I.; Tešović, G. Pneumococcal CROcarriage Study Group. Impact of the 10-valent pneumococcal conjugate vaccine (PCV10) on pneumococcal carriage in healthy children and children with acute otitis media and pneumonia: Emergence of serotypes 3, 6C and 19A in Croatia. Vaccine 2025, 50, 126848. [Google Scholar] [CrossRef]
- Kim, S.H.; Song, J.H.; Chung, D.R.; Thamlikitkul, V.; Yang, Y.; Wang, H.; Lu, M.; So, T.M.-K.; Hsueh, P.-R.; Yasin, R.M.; et al. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: An Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob. Agents Chemother. 2012, 56, 1418–1426. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.R.; Gertz, R.E., Jr.; Woodbury, R.L.; Barkocy-Gallagher, G.A.; Schaffner, W.; Lexau, C.; Gershman, K.; Reingold, A.; Farley, M.; Harrison, L.H.; et al. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J. Infect. Dis. 2008, 197, 1016–1027. [Google Scholar] [CrossRef]
- van der Linden, M.; Imöhl, M.; Perniciaro, S. Limited indirect effects of an infant pneumococcal vaccination program in an aging population. PLoS ONE 2019, 14, e0220453. [Google Scholar] [CrossRef] [PubMed]
- Wyres, K.L.; Lambertsen, L.M.; Croucher, N.J.; McGee, L.; von Gottberg, A.; Liñares, J.; Jacobs, M.R.; Kristinsson, K.G.; Beall, B.W.; Klugman, K.P.; et al. Pneumococcal Capsular Switching: A Historical Perspective. J. Infect. Dis. 2013, 207, 439–449. [Google Scholar] [CrossRef]
- Schellenberg, J.J.; Adam, H.J.; Baxter, M.R.; Karlowsky, J.A.; Golden, A.R.; Martin, I.; Zhanel, G.G. Phenotypic and molecular characterization of Streptococcus pneumoniae serotype 3 isolates from blood and respiratory samples in Canada: CANWARD 2007-21. J. Antimicrob. Chemother. 2024, 79, 2653–2661. [Google Scholar] [CrossRef]
- Aydin, M.A.; Janapatla, R.P.; Chen, C.L.; Li, H.C.; Su, L.H.; Chiu, C.H. Microbiological and clinical characteristics of Streptococcus pneumoniae serotype 3 infection and risk factors for severe outcome: A multicenter observational study. J. Microbiol. Immunol. Infect. 2023, 56, 598–604. [Google Scholar] [CrossRef]
- Calvo-Silveria, S.; González-Díaz, A.; Grau, I.; Marimón, J.M.; Cercenado, E.; Quesada, M.D.; Casabella, A.; Larrosa, N.; Yuste, J.; Berbel, D.; et al. Evolution of invasive pneumococcal disease by serotype 3 in adults: A Spanish three-decade retrospective study. Lancet Reg. Health Eur. 2024, 41, 100913. [Google Scholar] [CrossRef] [PubMed]
- Stanley, S.; Silva-Costa, C.; Gomes-Silva, J.; Melo-Cristino, J.; Malley, R.; Ramirez, M. CC180 clade dynamics do not universally explain Streptococcus pneumoniae serotype 3 persistence post-vaccine: A global comparative population genomics study. medRxiv 2024. medRxiv:2024.08.29.24312665. [Google Scholar] [CrossRef] [PubMed]
- Opavski, N.; Jovicevic, M.; Kabic, J.; Kekic, D.; Vasiljevic, Z.; Tosic, T.; Medic, D.; Laban, S.; Ranin, L.; Gajic, I. Serotype distribution, antimicrobial susceptibility and molecular epidemiology of invasive Streptococcus pneumoniae in the nine-year period in Serbia. Front. Microbiol. 2023, 14, 1244366. [Google Scholar] [CrossRef]
- Müller Premru, M.; Beović, B.; Pokorn, M.; Špik, V.C. Serotypes and genotypes of invasive pneumococci in the central part of Slovenia. Wien. Klin. Wochenschr. 2015, 127, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Garcia Quesada, M.; Peterson, M.E.; Bennett, J.C.; Hayford, K.; Zeger, S.L.; Yang, Y.; Hetrich, M.K.; Feikin, D.R.; Cohen, A.L.; von Gottberg, A.; et al. Serotype distribution of remaining invasive pneumococcal disease after extensive use of ten-valent and 13-valent pneumococcal conjugate vaccines (the PSERENADE project): A global surveillance analysis. Lancet Infect. Dis. 2025, 25, 445–456. [Google Scholar] [CrossRef]
- Cillóniz, C.; Garcia-Vidal, C.; Ceccato, A.; Torres, A. Antimicrobial Resistance Among Streptococcus pneumoniae. In Antimicrobial Resistance in the 21st Century; Fong, I., Shlaes, D., Drlica, K., Eds.; Springer: Cham, Switzerland, 2018; pp. 13–38. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2023. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-eueea-ears-net-annual-epidemiological-report-2023 (accessed on 20 May 2025).
- EUCAST, European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 15.0; EUCAST: Växjö, Sweden, 2025; Available online: https://www.eucast.org./clinical_breakpoints (accessed on 20 May 2025).
- Enright, M.C.; Spratt, B.G. A multilocus sequence typing scheme for Streptococcus pneumoniae: Identification of clones associated with serious invasive disease. Microbiology 1998, 144 Pt 11, 3049–3060. [Google Scholar] [CrossRef]
- CodonCode Aligner. Available online: https://www.codoncode.com/aligner/download.htm (accessed on 20 May 2025).
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef]
| Age Group | n (%) |
|---|---|
| 18–35 | 2 (2.4) |
| 36–49 | 7 (8.2) |
| 50–64 | 25 (29.4) |
| ≥65 | 51 (60) |
| All Patients (n = 85) | p | |
|---|---|---|
| a Age, mean ± SD | 68.4 ± 15.7 | |
| b Gender | 0.173 | |
| Female, n (%) | 39 (45.9) | |
| Men, n (%) | 46 (54.1) | |
| c Underlying conditions | <0.001 * | |
| No comorbidities, n (%) | 9 (10.6) | |
| 1 | 21 (24.7) | |
| ≥2 | 55 (64.7) | |
| ICU admission, n (%) | 33 (38.8) | |
| CCI median (IQR) | 4 (3–6) | |
| b Mortality, R n (%) | 25 (29.4) | <0.001 * |
| c Culture Site | <0.001 * | |
| Blood, n (%) | 74 (87.1) | |
| CSF, n (%) | 6 (7.1) | |
| Other, n (%) | 5 (5.8) |
| Survived (n = 60) | Deceased (n = 25) | p | |
|---|---|---|---|
| a Age, mean ± SD | 66.1 ± 16 | 74.2 ± 13.5 | 0.029 * |
| b Gender | 0.591 | ||
| Female, n (%) | 29 (74.4) | 10 (25.6) | |
| Men, n (%) | 30 (66.7) | 15 (33.3) | |
| b Underlying conditions | 0.07 | ||
| No comorbidities, n (%) | 9 (15) | 0 (0) | |
| 1, n (%) | 17 (28.3) | 4 (16) | |
| ≥2, n (%) | 34 (56.7) | 21 (84) | |
| ICU admission | <0.001 * | ||
| Yes, n (%) | 16 (26.7) | 17 (68) | |
| No, n (%) | 44 (73.3) | 8 (32) | |
| c CCI median (IQR) | 4 (2–5) | 5 (3–7) | 0.010 * |
| Antibiotic | MIC90 mg/L | I n (%) | R n (%) |
|---|---|---|---|
| Penicillin | 0.75 | 23 (27) | 10 (11.8) |
| Ceftriaxone | 0.5 | 4 (4.7) | 4 (4.7) |
| Meropenem | 0.094 | 0 (0) | 1 (1.2) |
| Antibiotic | R (%) |
|---|---|
| Erythromycin | 15 (17.7) |
| Moxifloxacin | 5 (5.9) |
| Tetracycline | 13 (15.2) |
| Trimethoprim-sulfamethoxazole | 17 (20) |
| Vancomycin | 0 |
| Isolates Number | Serotype | Antimicrobial Susceptibility | MDR | Sequence Type | ||||
|---|---|---|---|---|---|---|---|---|
| ERY | CLI | MOX | SXT | TE | ||||
| 1 | 15A | R | R | R | S | R | yes | 1830 |
| 2 | 19F | R | R | R | I | R | yes | 179 |
| 3 | 19A | R | R | R | R | R | yes | 320 |
| 4 | 19A | R | R | S | R | R | yes | 320 |
| 5 | 19A | R | R | S | R | R | yes | 320 |
| 6 | 4 | S | S | S | S | S | no | 205 |
| 7 | 19A | S | S | S | S | S | no | 199 |
| 8 | 19F | R | R | S | R | R | yes | 271 |
| 9 | 23B | S | S | S | R | S | no | 2372 |
| 10 | 3 | S | S | S | S | S | no | 1377 |
| 11 | 4 | R | R | S | S | S | no | 205 |
| 12 | 19A | S | S | S | S | S | no | 994 |
| 13 | 14 | S | S | S | R | S | no | 156 |
| 14 | 3 | S | S | S | S | S | no | 53 |
| 15 | 18A | S | S | S | S | S | no | 199 |
| 16 | 28A | S | S | S | S | S | no | 494 |
| 17 | 3 | S | S | S | S | S | no | 1377 |
| 18 | 19A | R | R | S | R | R | yes | 319 |
| 19 | 19A | R | R | S | S | R | yes | 416 |
| 20 | 19A | R | R | S | R | R | yes | 4467 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franjić Amančić, K.; Mohar-Vitezić, B.; Cekinović Grbeša, Đ.; Grubić Kezele, T.; Abram, M.; Bubonja-Šonje, M. Molecular Epidemiology, Antimicrobial Resistance, and Clinical Characteristics of Streptococcus pneumoniae Isolated from Adult Patients with Invasive Pneumococcal Disease. Antibiotics 2025, 14, 1158. https://doi.org/10.3390/antibiotics14111158
Franjić Amančić K, Mohar-Vitezić B, Cekinović Grbeša Đ, Grubić Kezele T, Abram M, Bubonja-Šonje M. Molecular Epidemiology, Antimicrobial Resistance, and Clinical Characteristics of Streptococcus pneumoniae Isolated from Adult Patients with Invasive Pneumococcal Disease. Antibiotics. 2025; 14(11):1158. https://doi.org/10.3390/antibiotics14111158
Chicago/Turabian StyleFranjić Amančić, Kristina, Bojana Mohar-Vitezić, Đurđica Cekinović Grbeša, Tanja Grubić Kezele, Maja Abram, and Marina Bubonja-Šonje. 2025. "Molecular Epidemiology, Antimicrobial Resistance, and Clinical Characteristics of Streptococcus pneumoniae Isolated from Adult Patients with Invasive Pneumococcal Disease" Antibiotics 14, no. 11: 1158. https://doi.org/10.3390/antibiotics14111158
APA StyleFranjić Amančić, K., Mohar-Vitezić, B., Cekinović Grbeša, Đ., Grubić Kezele, T., Abram, M., & Bubonja-Šonje, M. (2025). Molecular Epidemiology, Antimicrobial Resistance, and Clinical Characteristics of Streptococcus pneumoniae Isolated from Adult Patients with Invasive Pneumococcal Disease. Antibiotics, 14(11), 1158. https://doi.org/10.3390/antibiotics14111158







