Antimicrobial Resistance Profiles of Bacteria Isolated from the Animal Health Sector in Zambia (2020–2024): Opportunities to Strengthen Antimicrobial Resistance Surveillance and Stewardship Programs
Abstract
1. Introduction
2. Results
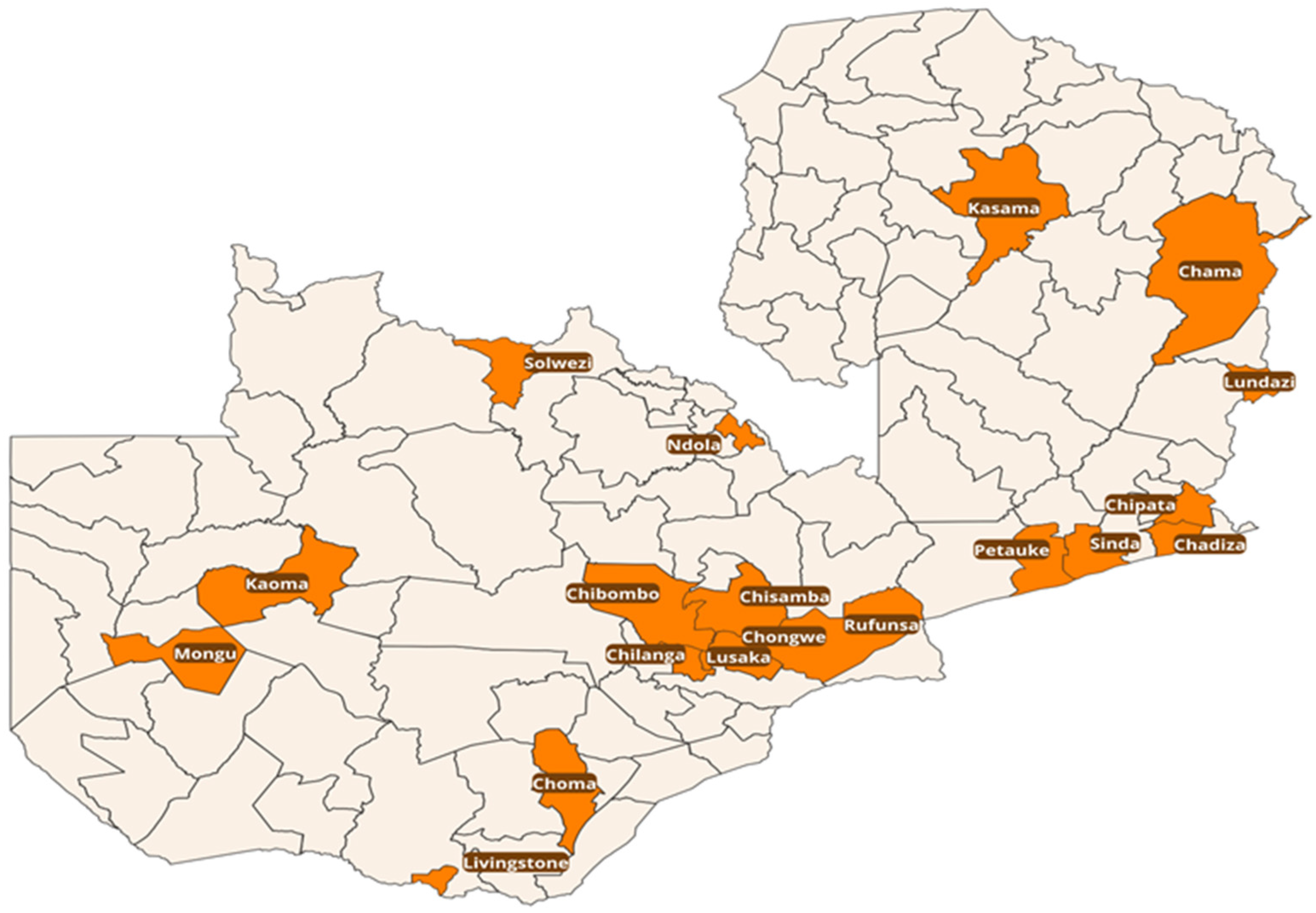
2.1. Sample Distribution and Trends over the Five Years (2020–2024)
2.2. Microbial Isolates Profile over Time (2020 to 2024)
2.3. Isolation Rate of Pathogens by Sample Type
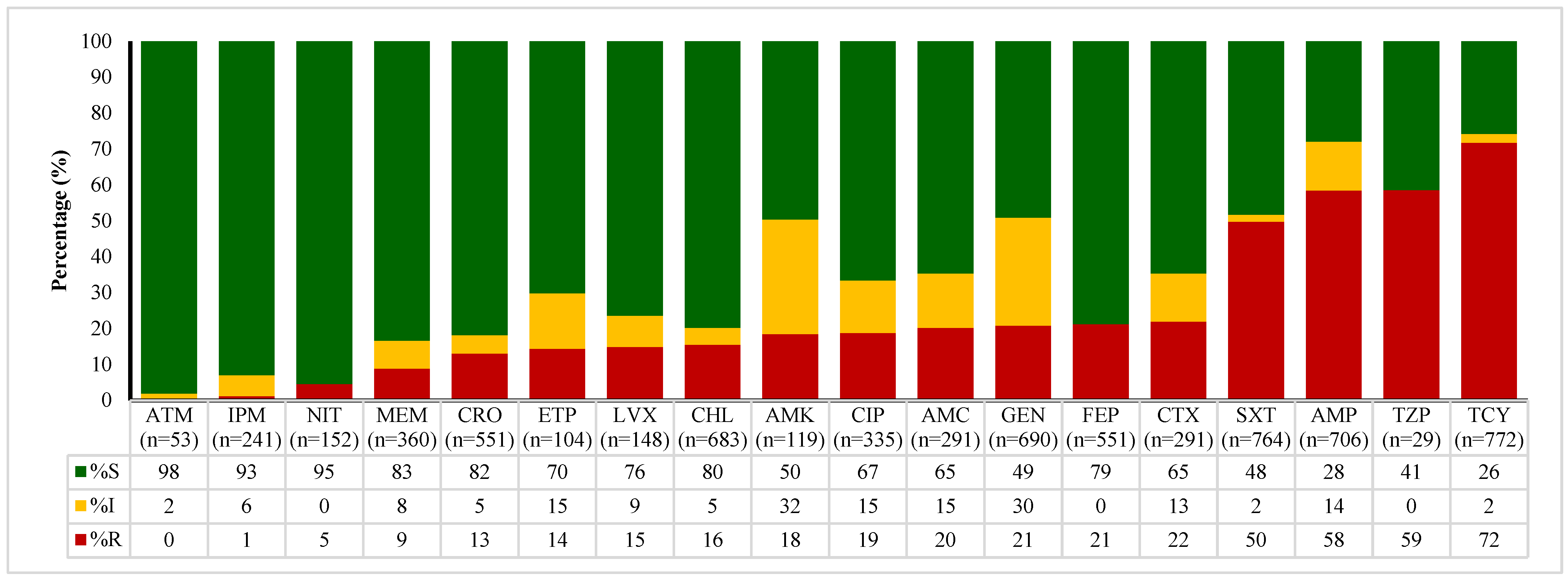
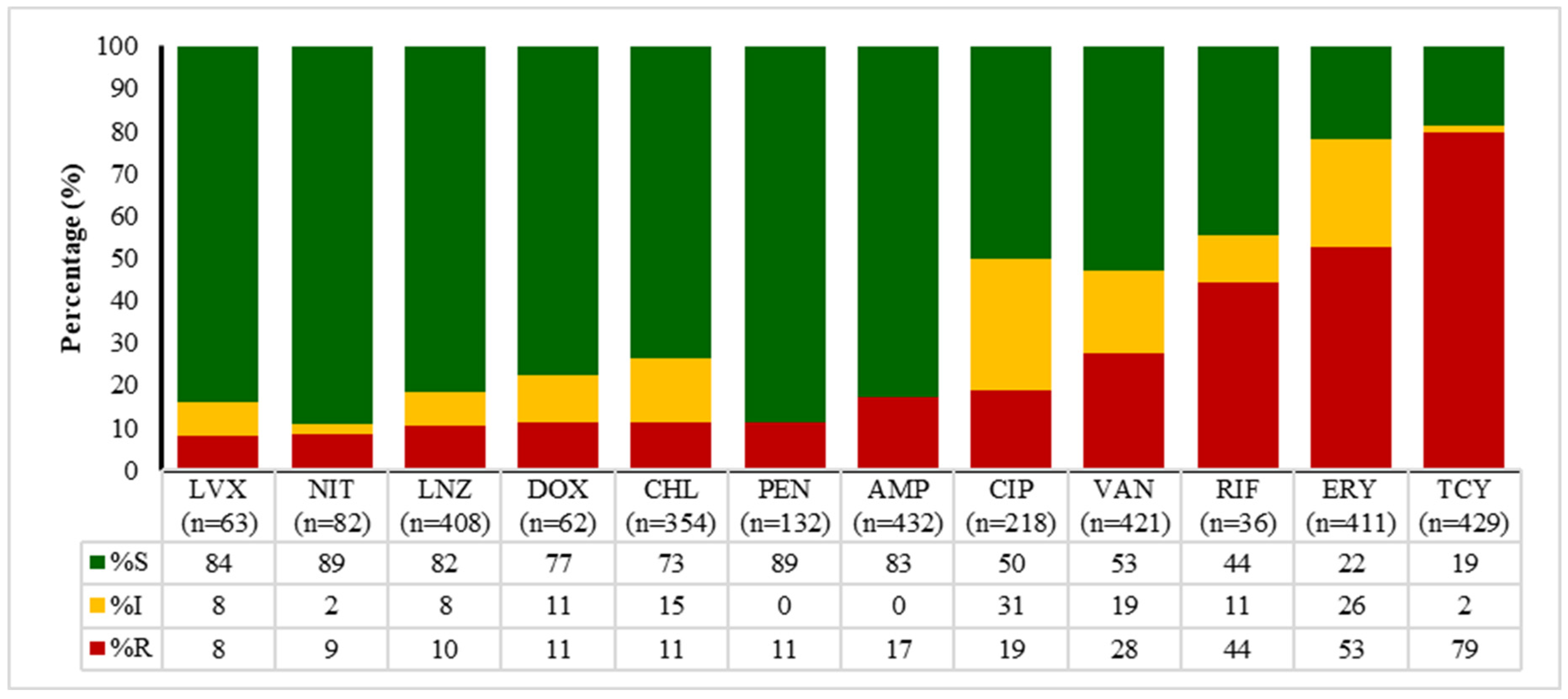
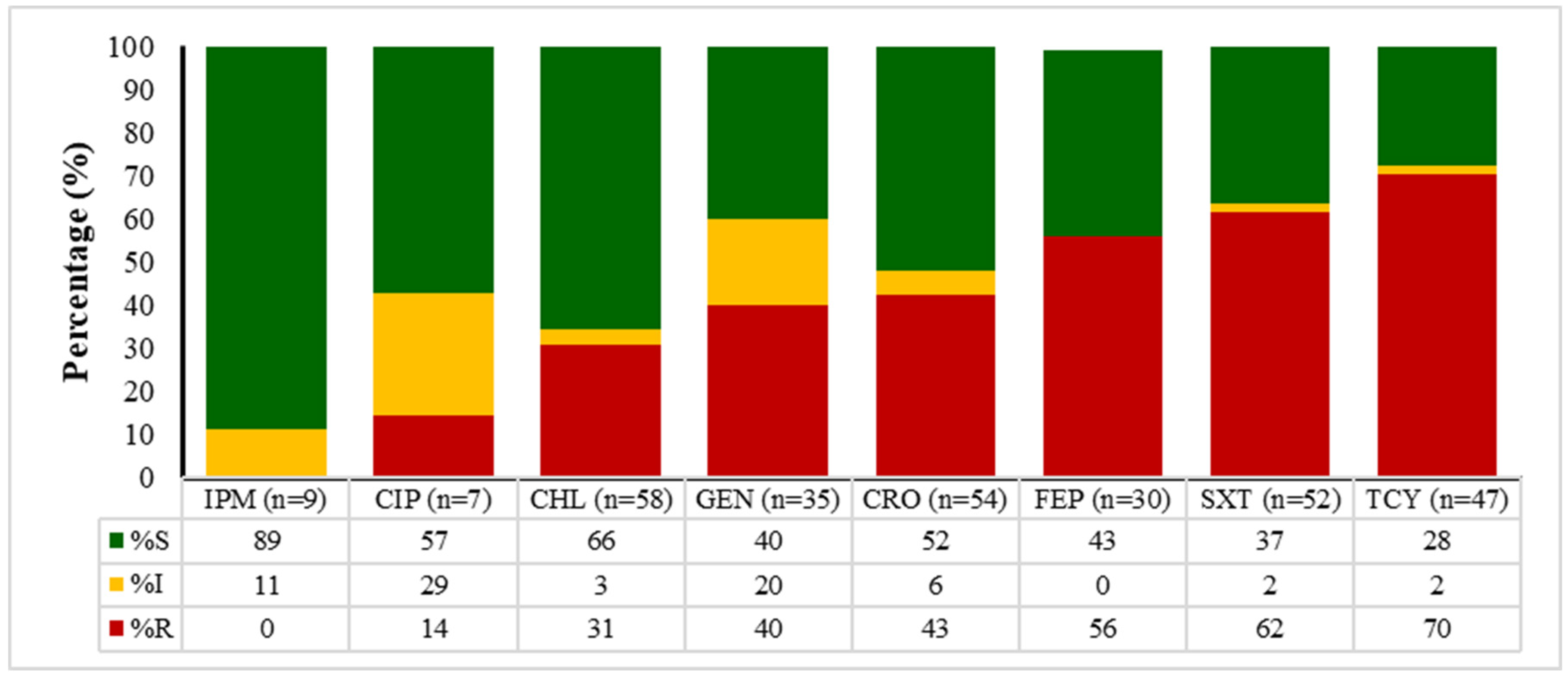
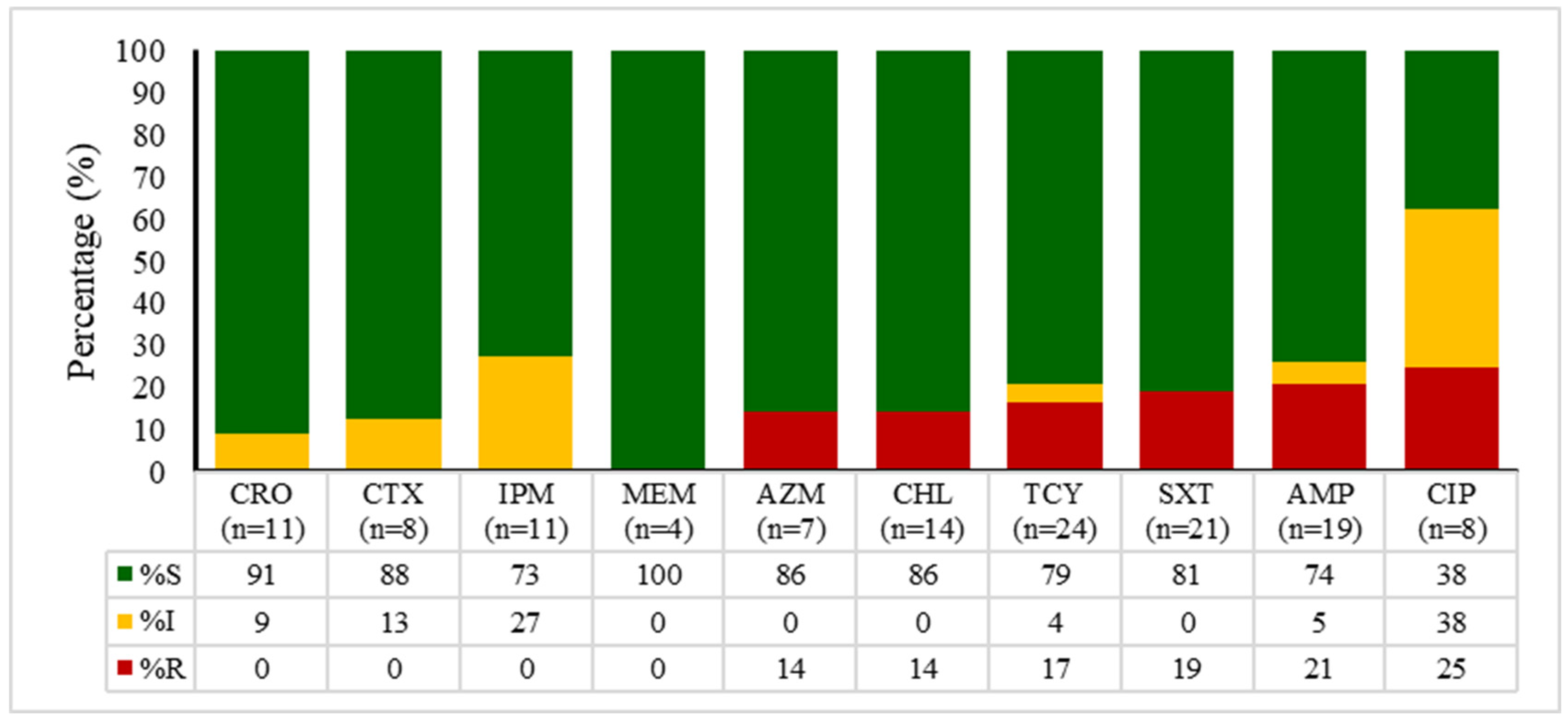
2.4. Antibiotic Susceptibility Patterns of E. coli, Enterococcus spp., Klebsiella spp., and Salmonella spp.
2.5. Trends of Antibiotic Non-Susceptibility over Time (2020–2024)
3. Discussion
4. Materials and Methods
4.1. Study Design and Setting
4.2. Study Design
4.3. Sample Type and Sample Size
4.4. Sampling Strategy
4.5. Data Collection
4.6. Antimicrobial Susceptibility Testing
4.7. Quality Control and Inter-Laboratory Comparability
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMC | Antimicrobial Consumption |
| AMR | Antimicrobial Resistance |
| AMS | Antimicrobial Stewardship |
| AMU | Antimicrobial Use |
| ANIMUSE | ANImal antiMicrobial USE global data base |
| FAO | Food and Agriculture Organization of the United Nations |
| InFARM | International FAO Antimicrobial Resistance Monitoring System |
| MDR | Multidrug Resistance |
| SADCAS | Southern African Development Community Accreditation Service |
| SPSS | Statistical Package for the Social Sciences |
| WOAH | World Organisation for Animal Health |
| ZNPHI | Zambia National Public Health Institute |
| β | Beta |
References
- Delamare-deboutteville, J.; Mohan, C.V. Antimicrobial Resistance: Preventing the Silent Pandemic in Aquatic Food Systems; WorldFish: Penang, Malaysia, 2021; Available online: https://digitalarchive.worldfishcenter.org/handle/20.500.12348/4996 (accessed on 20 December 2021).
- Mudenda, S.; Hakayuwa, C.M.; Lubanga, A.F.; Kasanga, M.; Daka, V.; Salachi, K.I.; Mwaba, M.; Chileshe, C.; Champo, M.; Kamayani, M.; et al. Global Antimicrobial Stewardship, Surveillance, and Infection Prevention and Control Programs: Leveraging One Health, Nanotechnology, and Artificial Intelligence to Combat Antimicrobial Resistance in a Climate-Impacted World. Pharmacol. Pharm. 2025, 16, 197–291. [Google Scholar] [CrossRef]
- Salam, A.; Al-Amin, Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Yoo, J.-H. Antimicrobial Resistance—The ‘Real’ Pandemic We Are Unaware Of, Yet Nearby. J. Korean Med. Sci. 2025, 40, e161. [Google Scholar] [CrossRef]
- Paneri, M.; Sevta, P. Overview of Antimicrobial Resistance: An Emerging Silent Pandemic. Glob. J. Med. Pharm. Biomed. Updat. 2023, 18, 11–13. [Google Scholar] [CrossRef]
- Wasan, H.; Singh, D.; Reeta, K.; Gupta, Y.K. Landscape of Push Funding in Antibiotic Research: Current Status and Way Forward. Biology 2023, 12, 101. [Google Scholar] [CrossRef]
- Mendelson, M.; Sharland, M.; Mpundu, M. Antibiotic resistance: Calling time on the ‘silent pandemic’. JAC-Antimicrob. Resist. 2022, 4, dlac016. [Google Scholar] [CrossRef]
- FAO. The FAO Action Plan on Antimicrobial Resistance 2021–2025; FAO: Rome, Italy, 2021; pp. 1–46. [Google Scholar]
- Ruckert, A.; Harris, F.; Aenishaenslin, C.; Aguiar, R.; Boudreau-LeBlanc, A.; Carmo, L.P.; Labonté, R.; Lambraki, I.; Parmley, E.J.; Wiktorowicz, M.E. One Health governance principles for AMR surveillance: A scoping review and conceptual framework. Res. Dir. One Health 2024, 2, e4. [Google Scholar] [CrossRef]
- Abdelfattah, E.M.; Ekong, P.S.; Okello, E.; Williams, D.R.; Karle, B.M.; Rowe, J.D.; Marshall, E.S.; Lehenbauer, T.W.; Aly, S.S. 2019 Survey of Antimicrobial Drug Use and Stewardship Practices in Adult Cows on California Dairies: Post Senate Bill 27. Microorganisms 2021, 9, 1507. [Google Scholar] [CrossRef]
- de Kraker, M.E.A.; Stewardson, A.J.; Harbarth, S. Will 10 Million People Die a Year due to Antimicrobial Resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef]
- Stanley, D.; Batacan, R.; Bajagai, Y.S. Rapid growth of antimicrobial resistance: The role of agriculture in the problem and the solutions. Appl. Microbiol. Biotechnol. 2022, 106, 6953–6962. [Google Scholar] [CrossRef]
- Cuong, N.V.; Padungtod, P.; Thwaites, G.; Carrique-Mas, J.J. Antimicrobial Usage in Animal Production: A Review of the Literature with a Focus on Low- and Middle-Income Countries. Antibiotics 2018, 7, 75. [Google Scholar] [CrossRef]
- Hedman, H.D.; Vasco, K.A.; Zhang, L. A Review of Antimicrobial Resistance in Poultry Farming within Low-Resource Settings. Animals 2020, 10, 1264. [Google Scholar] [CrossRef]
- Mohsin, M.; Van Boeckel, T.P.; Saleemi, M.K.; Umair, M.; Naseem, M.N.; He, C.; Khan, A.; Laxminarayan, R. Excessive use of medically important antimicrobials in food animals in Pakistan: A five-year surveillance survey. Glob. Health Action 2019, 12, 1697541. [Google Scholar] [CrossRef]
- Barroga, T.R.M.; Morales, R.G.; Benigno, C.C.; Castro, S.J.M.; Caniban, M.M.; Cabullo, M.F.B.; Agunos, A.; de Balogh, K.; Dorado-Garcia, A. Antimicrobials Used in Backyard and Commercial Poultry and Swine Farms in the Philippines: A Qualitative Pilot Study. Front. Veter.-Sci. 2020, 7, 329. [Google Scholar] [CrossRef]
- Mulchandani, R.; Tiseo, K.; Nandi, A.; Klein, E.; Gandra, S.; Laxminarayan, R.; Van Boeckel, T. Global trends in inappropriate use of antibiotics, 2000–2021: Scoping review and prevalence estimates. BMJ Public Health 2025, 3, e002411. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Pires, J.; Silvester, R.; Zhao, C.; Song, J.; Criscuolo, N.G.; Gilbert, M.; Bonhoeffer, S.; Laxminarayan, R. Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science 2019, 365, eaaw1944. [Google Scholar] [CrossRef]
- Booton, R.D.; Meeyai, A.; Alhusein, N.; Buller, H.; Feil, E.; Lambert, H.; Mongkolsuk, S.; Pitchforth, E.; Reyher, K.K.; Sakcamduang, W.; et al. One Health drivers of antibacterial resistance: Quantifying the relative impacts of human, animal and environmental use and transmission. One Health 2021, 12, 100220. [Google Scholar] [CrossRef]
- Kasimanickam, V.; Kasimanickam, M.; Kasimanickam, R. Antibiotics Use in Food Animal Production: Escalation of Antimicrobial Resistance: Where Are We Now in Combating AMR? Med. Sci. 2021, 9, 14. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Public Health 2024, 2. [Google Scholar] [CrossRef]
- Mudenda, S.; Bumbangi, F.N.; Yamba, K.; Munyeme, M.; Malama, S.; Mukosha, M.; Hadunka, M.A.; Daka, V.; Matafwali, S.K.; Siluchali, G.; et al. Drivers of antimicrobial resistance in layer poultry farming: Evidence from high prevalence of multidrug-resistant Escherichia coli and enterococci in Zambia. Veter.-World 2023, 16, 1803–1814. [Google Scholar] [CrossRef]
- Norris, J.M.; Zhuo, A.; Govendir, M.; Rowbotham, S.J.; Labbate, M.; Degeling, C.; Gilbert, G.L.; Dominey-Howes, D.; Ward, M.P. Factors influencing the behaviour and perceptions of Australian veterinarians towards antibiotic use and antimicrobial resistance. PLoS ONE 2019, 14, e0223534. [Google Scholar] [CrossRef]
- Khan, X.; Rymer, C.; Ray, P.; Lim, R. Quantification of antimicrobial use in Fijian livestock farms. One Health 2021, 13, 100326. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Kehrenberg, C.; Walsh, T. Use of antimicrobial agents in veterinary medicine and food animal production. Int. J. Antimicrob. Agents 2001, 17, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Samanta, I. Antimicrobial Resistance in Agri-Food Chain and Companion Animals as a Re-emerging Menace in Post-COVID Epoch: Low-and Middle-Income Countries Perspective and Mitigation Strategies. Front. Veter.-Sci. 2020, 7, 620. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.D.; Panda, A.K. Rational Use of Antimicrobials in Animal Production:A Prerequisite to Stem the Tide of Antimicrobial Resistance. Curr. Sci. 2017, 113, 1846–1857. [Google Scholar] [CrossRef]
- Sangeda, R.Z.; Baha, A.; Erick, A.; Mkumbwa, S.; Bitegeko, A.; Sillo, H.B.; Fimbo, A.M.; Chambuso, M.; Mbugi, E.V. Consumption Trends of Antibiotic for Veterinary Use in Tanzania: A Longitudinal Retrospective Survey From 2010-2017. Front. Trop. Dis. 2021, 2, 694082. [Google Scholar] [CrossRef]
- Musoke, D.; Namata, C.; Lubega, G.B.; Kitutu, F.E.; Mugisha, L.; Amir, S.; Brandish, C.; Gonza, J.; Ikhile, D.; Niyongabo, F.; et al. Access, use and disposal of antimicrobials among humans and animals in Wakiso district, Uganda: A qualitative study. J. Pharm. Policy Pr. 2021, 14, 1–12. [Google Scholar] [CrossRef]
- Nhung, N.T.; Chansiripornchai, N.; Carrique-Mas, J.J. Antimicrobial Resistance in Bacterial Poultry Pathogens: A Review. Front. Vet. Sci. 2017, 4, 126. [Google Scholar] [CrossRef] [PubMed]
- Hirwa, E.M.; Mujawamariya, G.; Shimelash, N.; Shyaka, A. Evaluation of cattle farmers’ knowledge, attitudes, and practices regarding antimicrobial use and antimicrobial resistance in Rwanda. PLoS ONE 2024, 19, e0300742. [Google Scholar] [CrossRef] [PubMed]
- Mudenda, S.; Mulenga, K.M.; Nyirongo, R.; Chabalenge, B.; Chileshe, C.; Daka, V.; M’kAndawire, E.; Jere, E.; Muma, J.B. Non-prescription sale and dispensing of antibiotics for prophylaxis in broiler chickens in Lusaka District, Zambia: Findings and implications on one health. JAC-Antimicrob. Resist. 2024, 6, dlae094. [Google Scholar] [CrossRef] [PubMed]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control. 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Howard, S.J.; Catchpole, M.; Watson, J.; Davies, S.C. Antibiotic resistance: Global response needed. Lancet Infect. Dis. 2013, 13, 1001–1003. [Google Scholar] [CrossRef]
- Tebug, S.F.; Mouiche, M.M.M.; Abia, W.A.; Teno, G.; Tiambo, C.K.; Moffo, F.; Awah-Ndukum, J. Antimicrobial use and practices by animal health professionals in 20 sub-Saharan African countries. Prev. Veter.-Med. 2021, 186, 105212. [Google Scholar] [CrossRef]
- Lambrou, A.S.; Innes, G.K.; O’sUllivan, L.; Luitel, H.; Bhattarai, R.K.; Basnet, H.B.; Heaney, C.D. Policy implications for awareness gaps in antimicrobial resistance (AMR) and antimicrobial use among commercial Nepalese poultry producers. Glob. Health Res. Policy 2021, 6, 1–9. [Google Scholar] [CrossRef]
- Alhaji, N.; Haruna, A.; Muhammad, B.; Lawan, M.; Isola, T. Antimicrobials usage assessments in commercial poultry and local birds in North-central Nigeria: Associated pathways and factors for resistance emergence and spread. Prev. Veter.-Med. 2018, 154, 139–147. [Google Scholar] [CrossRef]
- Chilawa, S.; Mudenda, S.; Daka, V.; Chileshe, M.; Matafwali, S.; Chabalenge, B.; Mpundu, P.; Mufwambi, W.; Mohamed, S.; Mfune, R.L. Knowledge, Attitudes, and Practices of Poultry Farmers on Antimicrobial Use and Resistance in Kitwe, Zambia: Implications on Antimicrobial Stewardship. Open J. Anim. Sci. 2023, 13, 60–81. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Kakarla, D.; Venkateswarlu, K.; Megharaj, M.; Yoon, Y.-E.; Lee, Y.B. Veterinary antibiotics (VAs) contamination as a global agro-ecological issue: A critical view. Agric. Ecosyst. Environ. 2018, 257, 47–59. [Google Scholar] [CrossRef]
- Sudatip, D.; Tiengrim, S.; Chasiri, K.; Kritiyakan, A.; Phanprasit, W.; Morand, S.; Thamlikitkul, V. One Health Surveillance of Antimicrobial Resistance Phenotypes in Selected Communities in Thailand. Antibiotics 2022, 11, 556. [Google Scholar] [CrossRef]
- Donado-Godoy, P.; Castellanos, R.; León, M.; Arevalo, A.; Clavijo, V.; Bernal, J.; León, D.; Tafur, M.A.; Byrne, B.A.; Smith, W.A.; et al. The Establishment of the Colombian Integrated Program for Antimicrobial Resistance Surveillance (COIPARS): A Pilot Project on Poultry Farms, Slaughterhouses and Retail Market. Zoonoses Public Health 2015, 62, 58–69. [Google Scholar] [CrossRef]
- da Costa, R.C.; Serrano, I.; Chambel, L.; Oliveira, M. The importance of “one health approach” to the AMR study and surveillance in Angola and other African countries. One Health 2024, 18, 100691. [Google Scholar] [CrossRef]
- Irfan, M.; Almotiri, A.; AlZeyadi, Z.A. Antimicrobial Resistance and Its Drivers—A Review. Antibiotics 2022, 11, 1362. [Google Scholar] [CrossRef] [PubMed]
- Nazir, A.; Nazir, A.; Zuhair, V.; Aman, S.; Sadiq, S.U.R.; Hasan, A.H.; Tariq, M.; Rehman, L.U.; Mustapha, M.J.; Bulimbe, D.B. The Global Challenge of Antimicrobial Resistance: Mechanisms, Case Studies, and Mitigation Approaches. Health Sci. Rep. 2025, 8, e71077. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.H.; Moore, L.S.P.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Belay, W.Y.; Getachew, M.; Tegegne, B.A.; Teffera, Z.H.; Dagne, A.; Zeleke, T.K.; Abebe, R.B.; Gedif, A.A.; Fenta, A.; Yirdaw, G.; et al. Mechanism of antibacterial resistance, strategies and next-generation antimicrobials to contain antimicrobial resistance: A review. Front. Pharmacol. 2024, 15, 1444781. [Google Scholar] [CrossRef]
- Matheou, A.; Abousetta, A.; Pascoe, A.P.; Papakostopoulos, D.; Charalambous, L.; Panagi, S.; Panagiotou, S.; Yiallouris, A.; Filippou, C.; Johnson, E.O. Antibiotic Use in Livestock Farming: A Driver of Multidrug Resistance? Microorganisms 2025, 13, 779. [Google Scholar] [CrossRef]
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic Resistance in Bacteria—A Review. Antibiotics 2022, 11, 1079. [Google Scholar] [CrossRef]
- Adebowale, O.; Makanjuola, M.; Bankole, N.; Olasoju, M.; Alamu, A.; Kperegbeyi, E.; Oladejo, O.; Fasanmi, O.; Adeyemo, O.; Fasina, F.O. Multi-Drug Resistant Escherichia coli, Biosecurity and Anti-Microbial Use in Live Bird Markets, Abeokuta, Nigeria. Antibiotics 2022, 11, 253. [Google Scholar] [CrossRef]
- Kahn, L.H. Antimicrobial resistance: A One Health perspective. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 255–260. [Google Scholar] [CrossRef]
- Collineau, L.; Bourély, C.; Rousset, L.; Berger-Carbonne, A.; Ploy, M.-C.; Pulcini, C.; Colomb-Cotinat, M. Towards One Health surveillance of antibiotic resistance: Characterisation and mapping of existing programmes in humans, animals, food and the environment in France, 2021. Eurosurveillance 2023, 28, 2200804. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Li, M.; Jing, Y.; Liu, K.; Wu, Y.; Peng, Z. What Are the Drivers Triggering Antimicrobial Resistance Emergence and Spread? Outlook from a One Health Perspective. Antibiotics 2025, 14, 543. [Google Scholar] [CrossRef]
- Samreen Ahmad, I.; Malak, H.A.; Abulreesh, H.H. Environmental Antimicrobial Resistance and Its Drivers: A Potential Threat to Public Health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar] [CrossRef]
- Davies, B.; Erlacher-Vindel, E.; Kuribrena, M.A.; Gochez, D.; Jeannin, M.; Magongo, M.; Valsson, O.; Yugueros-Marcos, J. Antimicrobial use in animals: A journey towards integrated surveillance. Rev. Sci. Tech. l’OIE 2023, 42, 201–209. [Google Scholar] [CrossRef]
- World Organization for Animal Health. ANIMUSE: Monitoring Antimicrobial Use in Animals; World Organization for Animal Health: Paris, France, 2023. [Google Scholar]
- Devreese, M.; Anadon, A.; Reeve-Johnson, L. The availability and use of antimicrobial agents. J. Veter.-Pharmacol. Ther. 2023, 46, 4–5. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Monitoring and Surveillance of Antimicrobial Resistance in Bacteria from Healthy Food Animals Intended for Consumption; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019; Volume 1, ISBN 9788589629768. [Google Scholar]
- Queenan, K.; Häsler, B.; Rushton, J. A One Health approach to antimicrobial resistance surveillance: Is there a business case for it? Int. J. Antimicrob. Agents 2016, 48, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, M.; Hesp, A.; Aenishaenslin, C.; Bordier, M.; Bennani, H.; Bergwerff, U.; Chantziaras, I.; De Meneghi, D.; Ellis-Iversen, J.; Filippizi, M.-E.; et al. Assessment of Evaluation Tools for Integrated Surveillance of Antimicrobial Use and Resistance Based on Selected Case Studies. Front. Veter.-Sci. 2021, 8, 663. [Google Scholar] [CrossRef] [PubMed]
- Oberin, M.; Badger, S.; Faverjon, C.; Cameron, A.; Bannister-Tyrrell, M. Electronic information systems for One Health surveillance of antimicrobial resistance: A systematic scoping review. BMJ Glob. Health 2022, 7, e007388. [Google Scholar] [CrossRef]
- Schrijver, R.; Stijntjes, M.; Rodríguez-Baño, J.; Tacconelli, E.; Rajendran, N.B.; Voss, A. Review of antimicrobial resistance surveillance programmes in livestock and meat in EU with focus on humans. Clin. Microbiol. Infect. 2018, 24, 577–590. [Google Scholar] [CrossRef]
- Agunos, A.; Gow, S.P.; Léger, D.F.; Carson, C.A.; Deckert, A.E.; Bosman, A.L.; Loest, D.; Irwin, R.J.; Reid-Smith, R.J. Antimicrobial Use and Antimicrobial Resistance Indicators—Integration of Farm-Level Surveillance Data From Broiler Chickens and Turkeys in British Columbia, Canada. Front. Veter.-Sci. 2019, 6, 131. [Google Scholar] [CrossRef]
- Robbins, S.N.; Goggs, R.; Kraus-Malett, S.; Goodman, L. Effect of institutional antimicrobial stewardship guidelines on prescription of critically important antimicrobials for dogs and cats. J. Veter.-Intern. Med. 2024, 38, 1706–1717. [Google Scholar] [CrossRef] [PubMed]
- Allerton, F.; Russell, J. Antimicrobial stewardship in veterinary medicine: A review of online resources. JAC-Antimicrob. Resist. 2023, 5, dlad058. [Google Scholar] [CrossRef]
- Lakoh, S.; Bawoh, M.; Lewis, H.; Jalloh, I.; Thomas, C.; Barlatt, S.; Jalloh, A.; Deen, G.F.; Russell, J.B.W.; Kabba, M.S.; et al. Establishing an Antimicrobial Stewardship Program in Sierra Leone: A Report of the Experience of a Low-Income Country in West Africa. Antibiotics 2023, 12, 424. [Google Scholar] [CrossRef]
- Hibbard, R.; Mendelson, M.; Page, S.W.; Ferreira, J.P.; Pulcini, C.; Paul, M.C.; Faverjon, C. Antimicrobial stewardship: A definition with a One Health perspective. Npj Antimicrob. Resist. 2024, 2, 15. [Google Scholar] [CrossRef]
- Musoke, D.; Kitutu, F.E.; Mugisha, L.; Amir, S.; Brandish, C.; Ikhile, D.; Kajumbula, H.; Kizito, I.M.; Lubega, G.B.; Niyongabo, F.; et al. A One Health Approach to Strengthening Antimicrobial Stewardship in Wakiso District, Uganda. Antibiotics 2020, 9, 764. [Google Scholar] [CrossRef]
- Mudenda, S.; Chabalenge, B.; Daka, V.; Mfune, R.L.; Salachi, K.I.; Mohamed, S.; Mufwambi, W.; Kasanga, M.; Matafwali, S.K. Global Strategies to Combat Antimicrobial Resistance: A One Health Perspective. Pharmacol. Pharm. 2023, 14, 271–328. [Google Scholar] [CrossRef]
- Zambia National Public Health Institute. Multi-Sectoral National Action Plan on Antimicrobial Resistance; Zambia National Public Health Institute: Lusaka, Zambia, 2017; Available online: https://www.afro.who.int/publications/multi-sectoral-national-action-plan-antimicrobial-resistance-2017-2027 (accessed on 20 December 2021).
- Zambia National Public Health Institute. Zambia’s Integrated Antimicrobial Resistance Surveillance Framework; Zambia National Public Health Institute: Lusaka, Zambia, 2020; Available online: https://www.afro.who.int/publications/zambias-integrated-antimicrobial-resistance-surveillance-framework (accessed on 20 December 2021).
- Sinyawa, T.; Shawa, M.; Muuka, G.M.; Goma, F.; Fandamu, P.; Chizimu, J.Y.; Khumalo, C.S.; Mulavu, M.; Ngoma, M.; Chambaro, H.M.; et al. Antimicrobial Use Survey and Detection of ESBL-Escherichia coli in Commercial and Medium-/Small-Scale Poultry Farms in Selected Districts of Zambia. Antibiotics 2024, 13, 467. [Google Scholar] [CrossRef]
- Chileshe, C.; Shawa, M.; Phiri, N.; Ndebe, J.; Khumalo, C.S.; Nakajima, C.; Kajihara, M.; Higashi, H.; Sawa, H.; Suzuki, Y.; et al. Detection of Extended-Spectrum Beta-Lactamase (ESBL)-Producing Enterobacteriaceae from Diseased Broiler Chickens in Lusaka District, Zambia. Antibiotics 2024, 13, 259. [Google Scholar] [CrossRef] [PubMed]
- Mudenda, S.; Matafwali, S.K.; Malama, S.; Munyeme, M.; Yamba, K.; Katemangwe, P.; Siluchali, G.; Mainda, G.; Mukuma, M.; Bumbangi, F.N.; et al. Prevalence and antimicrobial resistance patterns of Enterococcus species isolated from laying hens in Lusaka and Copperbelt provinces of Zambia: A call for AMR surveillance in the poultry sector. JAC-Antimicrob. Resist. 2022, 4, dlac126. [Google Scholar] [CrossRef] [PubMed]
- Chishimba, K.; Hang’oMbe, B.M.; Muzandu, K.; Mshana, S.E.; Matee, M.I.; Nakajima, C.; Suzuki, Y. Detection of Extended-Spectrum Beta-Lactamase-Producing Escherichia coli in Market-Ready Chickens in Zambia. Int. J. Microbiol. 2016, 2016, 5275724. [Google Scholar] [CrossRef] [PubMed]
- Phiri, N.; Mainda, G.; Mukuma, M.; Sinyangwe, N.N.; Banda, L.J.; Kwenda, G.; Muonga, E.M.; Flavien, B.N.; Mwansa, M.; Yamba, K.; et al. Antibiotic-resistant Salmonella species and Escherichia coli in broiler chickens from farms, abattoirs, and open markets in selected districts of Zambia. J. Epidemiol. Res. 2020, 6, 13. [Google Scholar] [CrossRef]
- Mwansa, M.; Mukuma, M.; Mulilo, E.; Kwenda, G.; Mainda, G.; Yamba, K.; Bumbangi, F.N.; Muligisa-Muonga, E.; Phiri, N.; Silwamba, I.; et al. Determination of antimicrobial resistance patterns of Escherichia coli isolates from farm workers in broiler poultry production and assessment of antibiotic resistance awareness levels among poultry farmers in Lusaka, Zambia. Front. Public Health 2023, 10, 998860. [Google Scholar] [CrossRef]
- Kabali, E.; Pandey, G.S.; Munyeme, M.; Kapila, P.; Mukubesa, A.N.; Ndebe, J.; Muma, J.B.; Mubita, C.; Muleya, W.; Muonga, E.M.; et al. Identification of Escherichia coli and Related Enterobacteriaceae and Examination of Their Phenotypic Antimicrobial Resistance Patterns: A Pilot Study at A Wildlife–Livestock Interface in Lusaka, Zambia. Antibiotics 2021, 10, 238. [Google Scholar] [CrossRef]
- Muligisa-Muonga, E.; Mainda, G.; Mukuma, M.; Kwenda, G.; Hang’oMbe, B.; Flavien, B.N.; Phiri, N.; Mwansa, M.; Munyeme, M.; Muma, J.B. Antimicrobial resistance of Escherichiacoli and Salmonella isolated from retail broiler chicken carcasses in Zambia. J. Epidemiol. Res. 2020, 6, 35. [Google Scholar] [CrossRef]
- Phiri, B.S.; Hang’OMbe, B.M.; Mulenga, E.; Mubanga, M.; Maurischat, S.; Wichmann-Schauer, H.; Schaarschmidt, S.; Fetsch, A. Prevalence and diversity of Staphylococcus aureus in the Zambian dairy value chain: A public health concern. Int. J. Food Microbiol. 2022, 375, 109737. [Google Scholar] [CrossRef]
- Samutela, M.T.; Phiri, B.S.J.; Simulundu, E.; Kwenda, G.; Moonga, L.; Bwalya, E.C.; Muleya, W.; Nyirahabimana, T.; Yamba, K.; Kainga, H.; et al. Antimicrobial Susceptibility Profiles and Molecular Characterisation of Staphylococcus aureus from Pigs and Workers at Farms and Abattoirs in Zambia. Antibiotics 2022, 11, 844. [Google Scholar] [CrossRef] [PubMed]
- Mudenda, S.; Malama, S.; Munyeme, M.; Hang’ombe, B.M.; Mainda, G.; Kapona, O.; Mukosha, M.; Yamba, K.; Bumbangi, F.N.; Mfune, R.L.; et al. Awareness of Antimicrobial Resistance and Associated Factors among Layer Poultry Farmers in Zambia: Implications for Surveillance and Antimicrobial Stewardship Programs. Antibiotics 2022, 11, 383. [Google Scholar] [CrossRef]
- Mudenda, S.; Mufwambi, W.; Mohamed, S. The Burden of Antimicrobial Resistance in Zambia, a Sub-Saharan African Country: A One Health Review of the Current Situation, Risk Factors, and Solutions. Pharmacol. Pharm. 2024, 15, 403–465. [Google Scholar] [CrossRef]
- Donkor, E.S.; Odoom, A.; Osman, A.-H.; Darkwah, S.; Kotey, F.C.N. A Systematic Review on Antimicrobial Resistance in Ghana from a One Health Perspective. Antibiotics 2024, 13, 662. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; The Review on Antimicrobial Resistance: London, UK, 2016; Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 20 December 2021).
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Food Animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022; World Health Organization: Geneva, Switzerland, 2022; ISBN 9789240005587. Available online: https://www.who.int/publications/i/item/9789240062702 (accessed on 20 December 2021).
- Mwikuma, G.; Kainga, H.; Kallu, S.A.; Nakajima, C.; Suzuki, Y.; Hang’ombe, B.M. Determination of the Prevalence and Antimicrobial Resistance of Enterococcus faecalis and Enterococcus faecium Associated with Poultry in Four Districts in Zambia. Antibiotics 2023, 12, 657. [Google Scholar] [CrossRef] [PubMed]
- Kasanga, M.; Shempela, D.M.; Daka, V.; Mwikisa, M.J.; Sikalima, J.; Chanda, D.; Mudenda, S. Antimicrobial resistance profiles of Escherichia coli isolated from clinical and environmental samples: Findings and implications. JAC-Antimicrob. Resist. 2024, 6, dlae061. [Google Scholar] [CrossRef]
- Kasanga, M.; Kwenda, G.; Wu, J.; Kasanga, M.; Mwikisa, M.J.; Chanda, R.; Mupila, Z.; Yankonde, B.; Sikazwe, M.; Mwila, E.; et al. Antimicrobial Resistance Patterns and Risk Factors Associated with ESBL-Producing and MDR Escherichia coli in Hospital and Environmental Settings in Lusaka, Zambia: Implications for One Health, Antimicrobial Stewardship and Surveillance Systems. Microorganisms 2023, 11, 1951. [Google Scholar] [CrossRef]
- Songe, M.M.; Hang’ombe, B.M.; Knight-Jones, T.J.D.; Grace, D. Antimicrobial Resistant Enteropathogenic Escherichia coli and Salmonella spp. in Houseflies Infesting Fish in Food Markets in Zambia. Int. J. Environ. Res. Public Health 2016, 14, 21. [Google Scholar] [CrossRef]
- Kaonga, N.; Hang’ombe, B.M.; Lupindu, A.M.; Hoza, A.S. Detection of CTX-M-Type Extended-Spectrum Beta-Lactamase Producing Salmonella Typhimurium in Commercial Poultry Farms in Copperbelt Province, Zambia. Ger. J. Veter.-Res. 2021, 1, 27–34. [Google Scholar] [CrossRef]
- Ribeiro, M.G.; de Morais, A.B.C.; Alves, A.C.; Bolaños, C.A.D.; de Paula, C.L.; Portilho, F.V.R.; Júnior, G.d.N.; Lara, G.H.B.; Martins, L.d.S.A.; Moraes, L.S.; et al. Klebsiella-induced infections in domestic species: A case-series study in 697 animals (1997–2019). Braz. J. Microbiol. 2022, 53, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Araújo, S.; Silva, V.; Quintelas, M.; Martins, Â.; Igrejas, G.; Poeta, P. From soil to surface water: Exploring Klebsiella ‘s clonal lineages and antibiotic resistance odyssey in environmental health. BMC Microbiol. 2025, 25, 97. [Google Scholar] [CrossRef]
- Rahman, M.; Alam, M.-U.; Luies, S.K.; Kamal, A.; Ferdous, S.; Lin, A.; Sharior, F.; Khan, R.; Rahman, Z.; Parvez, S.M.; et al. Contamination of Fresh Produce with Antibiotic-Resistant Bacteria and Associated Risks to Human Health: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 360. [Google Scholar] [CrossRef]
- Abbas, R.; Chakkour, M.; El Dine, H.Z.; Obaseki, E.F.; Obeid, S.T.; Jezzini, A.; Ghssein, G.; Ezzeddine, Z. General Overview of Klebsiella pneumonia: Epidemiology and the Role of Siderophores in Its Pathogenicity. Biology 2024, 13, 78. [Google Scholar] [CrossRef]
- Popa, G.L.; Popa, M.I. Salmonella spp. infection—A continuous threat worldwide. GERMS 2021, 11, 88–96. [Google Scholar] [CrossRef]
- Adzitey, F.; Tibile, B.A.; Addy, F.; Adu-Bonsu, G.; Amagloh, A.S.A.; Noyoro, E.J.; Tsigbey, V.E. Occurrence, antimicrobial susceptibility and genomic characterization of Salmonella enterica isolated from milk and related sources. Cogent Food Agric. 2025, 11, 2486330. [Google Scholar] [CrossRef]
- Galán-Relaño, Á.; Díaz, A.V.; Lorenzo, B.H.; Gómez-Gascón, L.; Rodríguez, M.Á.M.; Jiménez, E.C.; Rodríguez, F.P.; Márquez, R.J.A. Salmonella and Salmonellosis: An Update on Public Health Implications and Control Strategies. Animals 2023, 13, 3666. [Google Scholar] [CrossRef] [PubMed]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6, 521–547. [Google Scholar] [CrossRef] [PubMed]
- Ariyawansa, S.; Gunawardana, K.N.; Hapudeniya, M.M.; Manelgamage, N.J.; Karunarathne, C.R.; Madalagama, R.P.; Ubeyratne, K.H.; Wickramasinghe, D.; Tun, H.M.; Wu, P.; et al. One Health Surveillance of Antimicrobial Use and Resistance: Challenges and Successes of Implementing Surveillance Programs in Sri Lanka. Antibiotics 2023, 12, 446. [Google Scholar] [CrossRef] [PubMed]
- Mudenda, S.; Malama, S.; Munyeme, M.; Matafwali, S.K.; Kapila, P.; Katemangwe, P.; Mainda, G.; Mukubesa, A.N.; Hadunka, M.A.; Muma, J.B. Antimicrobial resistance profiles of Escherichia coli isolated from laying hens in Zambia: Implications and significance on one health. JAC-Antimicrob. Resist. 2023, 5, dlad060. [Google Scholar] [CrossRef]
- Madoshi, B.; Mtambo, M.; Muhairwa, A.; Lupindu, A.; Olsen, J. Isolation of vancomycin-resistant Enterococcus from apparently healthy human animal attendants, cattle and cattle wastes in Tanzania. J. Appl. Microbiol. 2018, 124, 1303–1310. [Google Scholar] [CrossRef]
- Yamba, K.; Chizimu, J.Y.; Chanda, R.; Mpundu, M.; Samutela, M.T.; Chanda, D.; Mudenda, S.; Finjika, M.; Chansa, B.N.; Siame, A.; et al. Antibiotic resistance profiles in Gram-negative bacteria causing bloodstream and urinary tract infections in paediatric and adult patients in Ndola District, Zambia, 2020–2021. Infect. Prev. Pr. 2025, 7, 100462. [Google Scholar] [CrossRef]
- Henry, M.C.; Geoffrey, K.; Mulemba, T.S.; Baron, Y.; Nawa, M.; Ruth, N.; Mildred, Z.; Sankananji, N.; Anita, K.; Amon, S.; et al. Antimicrobial resistance of clinical and environmental klebsiella pneumoniae isolates in selected areas of Lusaka, Zambia. Afr. J. Microbiol. Res. 2025, 19, 72–82. [Google Scholar] [CrossRef]
- Mudenda, S.; Mukosha, M.; Godman, B.; Fadare, J.; Malama, S.; Munyeme, M.; Hikaambo, C.N.; Kalungia, A.C.; Hamachila, A.; Kainga, H.; et al. Knowledge, Attitudes, and Practices of Community Pharmacy Professionals on Poultry Antibiotic Dispensing, Use, and Bacterial Antimicrobial Resistance in Zambia: Implications on Antibiotic Stewardship and WHO AWaRe Classification of Antibiotics. Antibiotics 2022, 11, 1210. [Google Scholar] [CrossRef]
- Lagana, D.M.; Taylor, D.D.; Walter, E.J.S. Advancing antimicrobial stewardship in companion animal veterinary medicine: A qualitative study on perceptions and solutions to a One Health problem. J. Am. Veter.-Med. Assoc. 2023, 261, 1200–1207. [Google Scholar] [CrossRef]
- Azabo, R.R.; Mshana, S.E.; Matee, M.I.; Kimera, S.I. Antimicrobial Resistance Pattern of Escherichia coli Isolates from Small Scale Dairy Cattle in Dar es Salaam, Tanzania. Animals 2022, 12, 1853. [Google Scholar] [CrossRef] [PubMed]
- Munengwa, A.; Nation, C.; Alban, M.; Lenin, D. Susceptibility profile of Zimbabwean livestock fecal Escherichia coli isolates to veterinary antibiotics: Implications for standardization of antimicrobial resistance surveillance in livestock production. Aceh J. Anim. Sci. 2022, 7, 34–40. [Google Scholar] [CrossRef]
- Azabo, R.; Dulle, F.; Mshana, S.E. Antimicrobial Use in Cattle and Poultry Production on Occurrence of Multidrug Resistant Escherichia Coli. A Systematic Review with Focus on Sub-Saharan Africa. Front. Vet. Sci. 2022, 9, 1000457. [Google Scholar] [CrossRef]
- Founou, L.L.; Amoako, D.G.; Founou, R.C.; Essack, S.Y. Antibiotic Resistance in Food Animals in Africa: A Systematic Review and Meta-Analysis. Microb. Drug Resist. 2018, 24, 648–665. [Google Scholar] [CrossRef] [PubMed]
- Rüegg, S.R.; Antoine-Moussiaux, N.; Aenishaenslin, C.; Alban, L.; Bordier, M.; Bennani, H.; Schauer, B.; Arnold, J.-C.; Gabain, I.; Sauter-Louis, C.; et al. Guidance for evaluating integrated surveillance of antimicrobial use and resistance. CABI One Health 2022, 2022, ohcs20220007. [Google Scholar] [CrossRef]
- Aenishaenslin, C.; Häsler, B.; Ravel, A.; Parmley, E.J.; Mediouni, S.; Bennani, H.; Stärk, K.D.C.; Buckeridge, D.L. Evaluating the Integration of One Health in Surveillance Systems for Antimicrobial Use and Resistance: A Conceptual Framework. Front. Veter.-Sci. 2021, 8, 611931. [Google Scholar] [CrossRef]
- World Health Organization. GLASS Methodology for Surveillance of National Antimicrobial Consumption; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/9789240012639 (accessed on 20 December 2021).
- World Health Organization. WHO Bacterial Priority Pathogens List 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024; Available online: https://iris.who.int/bitstream/handle/10665/376776/9789240093461-eng.pdf?sequence=1 (accessed on 20 December 2021).
- Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing, Thirtieth Edition: M100. Available online: https://unitedvrg.com/2021/05/20/m100-performance-standards-for-antimicrobial-susceptibility-testing-30th-edition-2020-pdf/ (accessed on 26 August 2021).

| Sample Type | Total | 2020 | 2021 | 2022 | 2023 | 2024 | p Value * |
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Faecal | 1478 (87.6%) | 524 (98.5%) | 336 (92.0%) | 219 (92.0%) | 255 (85.6%) | 144 (56.5%) | 0.086 |
| Animal environmental | 169 (10.0%) | 0 (0%) | 5 (1.4%) | 18 (7.6%) | 41 (13.8%) | 105 (41.2%) | 0.027 |
| Meat | 26 (1.5%) | 0 (0%) | 24 (6.6%) | 0 (0%) | 2 (0.6%) | 0 (0%) | 1.000 |
| Food | 15 (0.9%) | 8 (1.5%) | 0 (0%) | 1 (0.4%) | 0 (0%) | 6 (2.3%) | 1.000 |
| Total | 1688 | 532 | 365 | 238 | 298 | 255 | 0.086 |
| Organism | Faecal (n = 1478) | Animal Environmental (n = 169) | Meat (n = 26) | Food (n = 15) | Total (n = 1688) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Escherichia coli | 754 | 51.0% | 77 | 45.6% | 8 | 30.8% | 11 | 73.3% | 850 | 50.4% |
| Enterococcus spp. | 498 | 33.7% | 6 | 3.6% | 3 | 11.5% | 0 | 0.0% | 507 | 30.0% |
| Klebsiella spp. | 13 | 0.9% | 46 | 27.2% | 0 | 0.0% | 0 | 0.0% | 59 | 3.5% |
| Other GNRs | 23 | 1.6% | 21 | 12.4% | 2 | 7.7% | 1 | 6.7% | 47 | 2.8% |
| Salmonella spp. | 19 | 1.3% | 7 | 4.1% | 0 | 0.0% | 1 | 6.7% | 27 | 1.6% |
| Non-enterococcal Strep Group D | 6 | 0.4% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 6 | 0.4% |
| Staphylococcus aureus | 1 | 0.1% | 0 | 0.0% | 2 | 7.7% | 2 | 13.3% | 5 | 0.3% |
| CONS | 0 | 0.0% | 0 | 0.0% | 2 | 7.7% | 0 | 0.0% | 2 | 0.1% |
| Antibiotics | Period: 2020–2024 | Mann–Kendall’s Tau Test | ||||||
|---|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2022 | 2023 | 2024 | ||||
| %NS | %NS | %NS | %NS | %NS | Kendall’s Tau | p-Value | Sen’s Slope | |
| AMC %NS | 22.2 | 26.8 | 36.9 | 48.8 | 81.8 | 1.000 | 0.027 | 11.450 |
| AMP %NS | 51.1 | 51.2 | 49 | 44.7 | 71.9 | 0.000 | 1.000 | −0.475 |
| CHL %NS | 23.3 | 17.9 | 21.2 | 27.7 | 25.5 | 0.400 | 0.462 | 1.808 |
| CIP %NS | 35.7 | 25.7 | 57.1 | 40.5 | 52.4 | 0.400 | 0.462 | 5.788 |
| FEP %NS | 0.6 | 5.2 | 14.3 | 7.7 | 28.3 | 0.800 | 0.086 | 6.888 |
| CTX %NS | 31.3 | 20.9 | 44.4 | 87.5 | 35.5 | 0.400 | 0.462 | 5.708 |
| CRO %NS | 7.5 | 8.3 | 25.5 | 14 | 48.8 | 0.800 | 0.086 | 9.663 |
| CAZ %NS | 5.5 | 2.6 | 22.7 | 29.4 | 44.1 | 0.800 | 0.086 | 10.175 |
| ERY %NS | 86.5 | 75.2 | 86.6 | 68.3 | 76.7 | −0.200 | 0.806 | −2.950 |
| GEN %NS | 44 | 49 | 58 | 40.5 | 42.5 | −0.200 | 0.806 | −0.771 |
| IPM %NS | 4.3 | 17.9 | 9.1 | 9.2 | 10.5 | 0.400 | 0.462 | 1.000 |
| LNZ %NS | 28.1 | 20.5 | 35.4 | 23.2 | 20.8 | −0.200 | 0.806 | −1.729 |
| MEM %NS | 0 | 10.1 | 33.3 | 20.3 | 39.1 | 0.800 | 0.086 | 9.721 |
| TCY %NS | 80.9 | 72.1 | 79.5 | 59.7 | 79.9 | −0.200 | 0.806 | −0.475 |
| SXT %NS | 50 | 52.7 | 45.2 | 48.1 | 56.2 | 0.200 | 0.806 | 1.358 |
| VAN %NS | 45.1 | 35.3 | 83.5 | 38.6 | 81.8 | 0.200 | 0.806 | 5.413 |
| LVX %NS | 0 | 15.7 | 22.1 | 76.9 | 10 | 0.400 | 0.462 | 8.725 |
| AMK %NS | 30.8 | 0 | 100 | 73.8 | 60 | 0.200 | 0.806 | 10.817 |
| Organism | Number of Isolates | MDR | Possible XDR | Possible PDR |
|---|---|---|---|---|
| E. coli | 850 | 411 (48.4%) | 313 (36.8%) | 110 (12.9%) |
| Enterococcus spp. | 507 | 60 (11.8%) | 54 (10.7%) | 21 (4.1%) |
| Klebsiella spp. | 59 | 11 (18.6%) | 11 (18.6%) | 10 (16.9%) |
| Total | 1416 | 482 | 378 | 141 |
| Key Finding | Policy Implication | Proposed Action | Responsible Stakeholders |
|---|---|---|---|
| High proportion of faecal samples (87.6%) and limited animal environmental, food, and meat sampling | Surveillance scope is narrow, potentially missing AMR sources in the animal environment and food chain | Expand sentinel site protocols to include routine animal environmental, meat, and food sample collection to support One Health surveillance | Ministry of Fisheries and Livestock (MFL); Zambia National Public Health Institute (ZNPHI); Ministry of Health (MoH); FAO |
| Significant increase in animal environmental samples (p = 0.027) | Growing recognition of animal environmental AMR risks | Institutionalise animal environmental sampling in AMR surveillance guidelines and integrate with environmental health monitoring | MFL; ZNPHI; Zambia Environmental Management Agency (ZEMA) |
| E. coli and Enterococcus spp. dominate isolates | These pathogens are priority AMR indicators and can spread resistance genes | Maintain focus on these species while adding other relevant pathogens for comprehensive risk profiling | MFL; MoH; FAO; WOAH |
| Klebsiella spp. and Salmonella spp. are more prevalent in environmental samples | Environmental contamination may be a significant reservoir for resistant pathogens | Strengthen farm-level and slaughterhouse biosecurity measures; enforce waste management standards | MFL; Local Authorities; ZEMA; Food Safety Agencies |
| Low susceptibility of E. coli to tetracycline (26%) and ampicillin (28%) | Overuse of common antimicrobials in veterinary practice is likely contributing to resistance | Regulate veterinary antimicrobial sales; implement restrictions on growth-promoter use; encourage alternatives to antibiotics | Veterinary Council of Zambia (VCZ); MFL; Zambia Medicines Regulatory Authority (ZAMRA) |
| Borderline resistance in Enterococcus spp. to vancomycin (53%) and linezolid (50%) | Potential emergence of resistance to critically important human medicines | Enforce strict controls on veterinary use of critical antimicrobials; introduce a national “protected list” of antibiotics | MoH; MFL; ZAMRA; VCZ |
| Increasing resistance to amoxicillin/clavulanic acid (22.2% → 81.8%, p = 0.027) | Rapid resistance escalation to a key broad-spectrum antibiotic | Review and restrict empirical veterinary use of amoxicillin/clavulanic acid; promote targeted therapy based on susceptibility testing | MFL; VCZ; ZAMRA |
| Preserved susceptibility to carbapenems (imipenem, meropenem) | Critical antimicrobials remain effective | Maintain carbapenems as “last-resort” drugs; ban routine veterinary use to prevent resistance development | MoH; MFL; ZAMRA |
| Fluctuating ciprofloxacin resistance (peaking > 50%) | Risk to human medicine, as fluoroquinolones are important in both sectors | Introduce stewardship protocols for fluoroquinolone use in livestock; require culture and sensitivity testing before administration | MoH; MFL; VCZ |
| Limited molecular epidemiology data | Lack of genetic AMR surveillance limits understanding of resistance spread | Invest in laboratory capacity for molecular typing and AMR gene detection; integrate data with global platforms (FAO InFARM, WOAH ANIMUSE) | MFL; ZNPHI; MoH; FAO; WOAH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinyawa, T.; Goma, F.; Chileshe, C.; Mudenda, N.B.; Mudenda, S.; Siame, A.; Simwinji, F.M.; Hadunka, M.A.; Chibwe, B.; Kaunda, K.; et al. Antimicrobial Resistance Profiles of Bacteria Isolated from the Animal Health Sector in Zambia (2020–2024): Opportunities to Strengthen Antimicrobial Resistance Surveillance and Stewardship Programs. Antibiotics 2025, 14, 1102. https://doi.org/10.3390/antibiotics14111102
Sinyawa T, Goma F, Chileshe C, Mudenda NB, Mudenda S, Siame A, Simwinji FM, Hadunka MA, Chibwe B, Kaunda K, et al. Antimicrobial Resistance Profiles of Bacteria Isolated from the Animal Health Sector in Zambia (2020–2024): Opportunities to Strengthen Antimicrobial Resistance Surveillance and Stewardship Programs. Antibiotics. 2025; 14(11):1102. https://doi.org/10.3390/antibiotics14111102
Chicago/Turabian StyleSinyawa, Taona, Fusya Goma, Chikwanda Chileshe, Ntombi B. Mudenda, Steward Mudenda, Amon Siame, Fred Mulako Simwinji, Mwendalubi Albert Hadunka, Bertha Chibwe, Kaunda Kaunda, and et al. 2025. "Antimicrobial Resistance Profiles of Bacteria Isolated from the Animal Health Sector in Zambia (2020–2024): Opportunities to Strengthen Antimicrobial Resistance Surveillance and Stewardship Programs" Antibiotics 14, no. 11: 1102. https://doi.org/10.3390/antibiotics14111102
APA StyleSinyawa, T., Goma, F., Chileshe, C., Mudenda, N. B., Mudenda, S., Siame, A., Simwinji, F. M., Hadunka, M. A., Chibwe, B., Kaunda, K., Mainda, G., Phiri, B. S. J., Kasanga, M., Mufwambi, W., Mukale, S., Bambala, A., Hangoma, J., Mabuku, N., Bowa, B., ... Chilengi, R. (2025). Antimicrobial Resistance Profiles of Bacteria Isolated from the Animal Health Sector in Zambia (2020–2024): Opportunities to Strengthen Antimicrobial Resistance Surveillance and Stewardship Programs. Antibiotics, 14(11), 1102. https://doi.org/10.3390/antibiotics14111102








