Beyond the Resistome: Molecular Insights, Emerging Therapies, and Environmental Drivers of Antibiotic Resistance
Abstract
1. Introduction
2. The Molecular Resistome: Mechanisms and Evolutionary Fitness
2.1. Chromosomal Mutations and Stress-Induced Variability
2.2. Plasmids and Mobile Genetic Elements
2.3. Efflux Pumps and Regulatory Networks
2.4. Integrons and Gene Cassettes
2.5. CRISPR-Cas Systems: Dual Roles in Resistance
2.6. Fitness Costs and Compensatory Evolution
3. The Resistome as a Reservoir for Future Resistance
4. Emerging Therapies and Molecularly Informed Interventions
4.1. Electrochemical and Catalytic Strategies
4.2. Nanomaterials and the Plastisphere
4.3. Bio-Inspired Natural Compounds
4.4. Molecular Surveillance and CRISPR-Based Tools
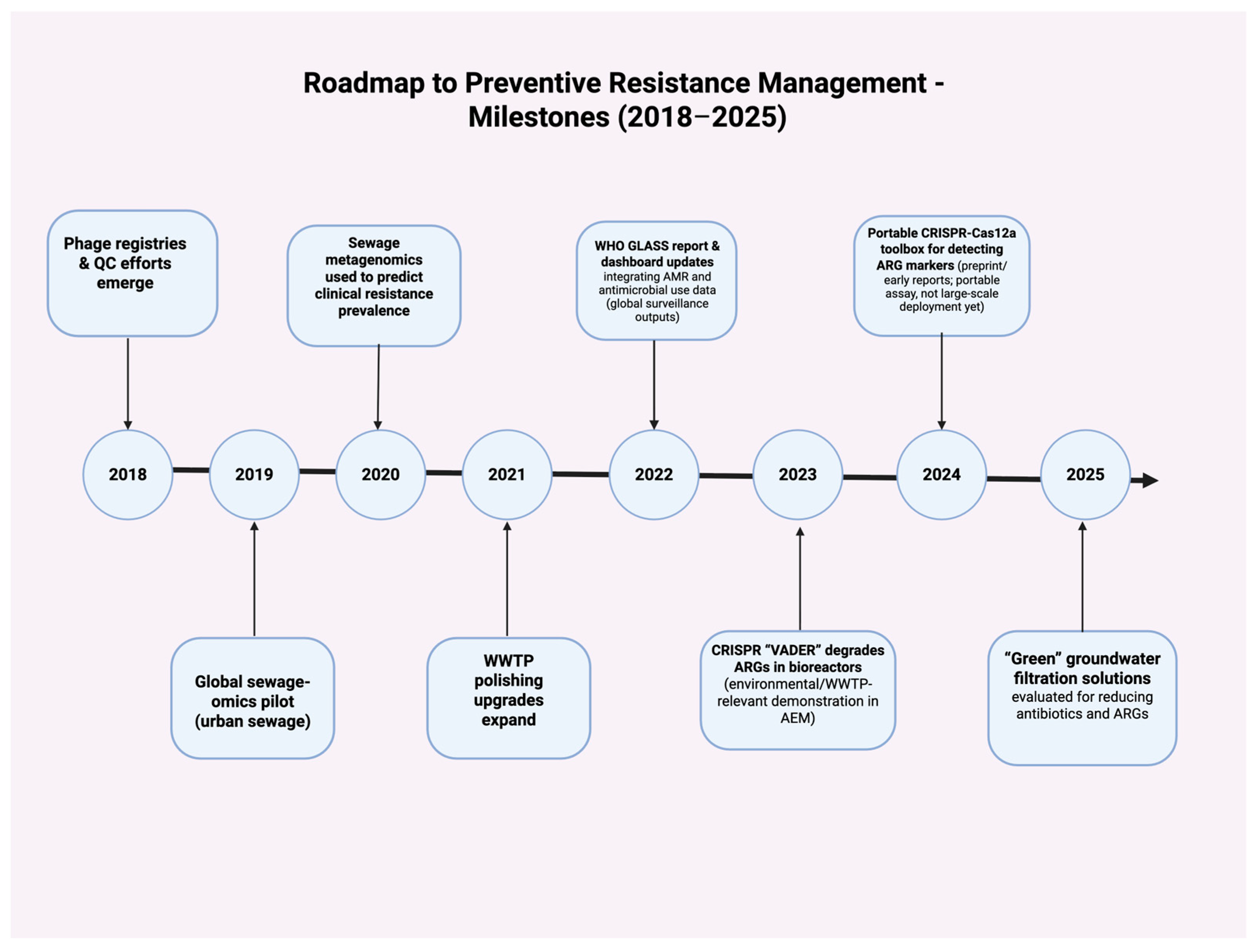
4.5. Integrative Therapeutic Roadmap
- ○
- At the environmental interface, advanced oxidation, nanomaterials, and green remediation must ensure the complete removal of antibiotics and ARGs before ecological release.
- ○
- At the molecular interface, natural products (such as quercetin and macolacin) and engineered antimicrobials (including CRISPR and phages) provide new scaffolds to bypass resistance mechanisms.
- ○
- At the surveillance interface: Portable CRISPR-Cas detection and metagenomic pipelines enable real-time ARG tracking across soil, water, and clinical isolates.
5. Surveillance and One Health Approaches
5.1. Environmental Surveillance of ARGs
5.2. Clinical and Zoonotic Monitoring
5.3. Mobile Genetic Elements as Surveillance Targets
5.4. Phage and Resistome Surveillance
5.5. Artificial Intelligence in ARG Prediction
5.6. One Health Integration
5.7. Policy and Governance
6. Prospects and Future Directions
6.1. Next-Generation Antibiotics and Adjuvants
6.2. Phage Therapy and Engineered Enzybiotics
6.3. CRISPR-Based Therapeutics and Diagnostics
6.4. Nanotechnology and Advanced Materials
6.5. Artificial Intelligence and Predictive Surveillance
6.6. One Health Policy and Global Cooperation
7. Vision: Preventive Resistance Management
- Molecular precision tools: Approaches such as CRISPR-based antimicrobials and AI-designed therapeutics offer the capacity to preemptively block the mobilization and spread of resistance genes.
- Environmental containment: Nanotechnology, biochar amendments, and green filtration strategies reduce the recycling of ARGs in wastewater, soils, and aquatic ecosystems.
- Surveillance integration: Global resistome monitoring—linking clinical, veterinary, and environmental data through metagenomics and portable CRISPR diagnostics—enables early detection and targeted interventions.
- Policy enforcement: Coordinated international frameworks are essential to align antibiotic stewardship with sustainability and One Health principles, ensuring that scientific progress is effectively implemented in practice.
8. Conclusions
- ○
- Global surveillance integration, linking clinical, agricultural, and environmental ARG data.
- ○
- Sustainable therapeutic innovation, balancing new antibiotics with phages, CRISPR-based tools, and natural adjuvants.
- ○
- Environmental remediation, ensuring wastewater treatment, soil bioremediation, and plastic pollution control, reduces ARG dissemination.
- ○
- Policy enforcement, limiting unnecessary antibiotic use, and incentivizing stewardship across sectors.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AEM | Applied and Environmental Microbiology |
| AI/ML | Artificial intelligence/machine learning |
| AMPs | Antimicrobial peptides |
| AMR | Antimicrobial resistance |
| ARG | Antibiotic resistance gene |
| AST | Antimicrobial susceptibility testing |
| BGC | Biosynthetic gene cluster |
| BL/BLI | β-lactam/β-lactamase inhibitor (combination therapy) |
| CAP | Chloramphenicol |
| Cas12a | CRISPR-associated endonuclease 12a |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| DBO | Diazabicyclooctane (β-lactamase inhibitor class) |
| Dx | Diagnostics |
| EAOPs | Electrochemical advanced oxidation processes |
| eDNA | Extracellular DNA |
| ESBL | Extended-spectrum β-lactamase |
| GLASS | Global Antimicrobial Resistance and Use Surveillance System (WHO) |
| HGT | Horizontal gene transfer |
| ICE | Integrative and conjugative element |
| KPC | Klebsiella pneumoniae carbapenemase |
| LPS | Lipopolysaccharide |
| mcr | mobilized colistin resistance genes (e.g., mcr-1–mcr-10) |
| MBL | Metallo-β-lactamase |
| MFS | Major facilitator superfamily (efflux pumps) |
| MGE | Mobile genetic element |
| MNPs | Micro- and nanoplastics |
| MOFs | Metal–organic frameworks |
| NDM | New Delhi metallo-β-lactamase |
| NF | Nanofiltration |
| OM | Outer membrane |
| OXA | Class D oxacillinase-type β-lactamase |
| PBPs | Penicillin-binding proteins |
| PEtN | Phosphoethanolamine |
| PHB | Polyhydroxybutyrate (bioplastic) |
| PLA | Polylactic acid (bioplastic) |
| QC | Quality control |
| QRDR | Quinolone resistance–determining region |
| QS | Quorum sensing |
| RND | Resistance–nodulation–division (efflux pumps) |
| ROS | Reactive oxygen species |
| TOC | Total organic carbon |
| UF | Ultrafiltration |
| WBE | Wastewater-based epidemiology |
| WGS | Whole-genome sequencing |
| WWTP | Wastewater treatment plant |
References
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Review on Antimicrobial Resistance. 2016. Available online: https://amr-review.org/ (accessed on 28 August 2025).
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Defining and combating antibiotic resistance from One Health and Global Health perspectives. Nat. Microbiol. 2019, 4, 1432–1442. [Google Scholar] [CrossRef]
- Skandalis, N.; Maeusli, M.; Papafotis, D.; Miller, S.; Lee, B.; Theologidis, I.; Luna, B. Environmental spread of antibiotic resistance. Antibiotics 2021, 10, 640. [Google Scholar] [CrossRef]
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.L.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Debruyne, R.; et al. Antibiotic resistance is ancient. Nature 2011, 477, 457–461. [Google Scholar] [CrossRef]
- Perry, J.; Waglechner, N.; Wright, G. The prehistory of antibiotic resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025197. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Pires, J.; Silvester, R.; Zhao, C.; Song, J.; Criscuolo, N.G.; Gilbert, M.; Bonhoeffer, S.; Laxminarayan, R. Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science 2019, 365, eaaw1944. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.I.; Hughes, D. Selection and Transmission of Antibiotic-Resistant Bacteria. Microbiol. Spectr. 2017, 5, mtbp-0013. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Hu, H.; Xu, C.; Zhou, M.; Li, Y.; Li, Y.; Wu, S.; Dong, N. Characterization of NDM-5-Producing Escherichia coli Strains Isolated from Pediatric Patients with Bloodstream Infections in a Chinese Hospital. Genes 2023, 14, 520. [Google Scholar] [CrossRef]
- Palzkill, T. Structural and mechanistic basis for extended-spectrum drug-resistance mutations in altering the specificity of TEM, CTX-M, and KPC β-lactamases. Front. Mol. Biosci. 2018, 5, 16. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, C.; Ji, Y.; Song, J.; Liu, Y.; Guo, Y.; Zhou, K. Identification of mcr-10 carried by self-transmissible plasmids and chromosome in Enterobacter roggenkampii strains isolated from hospital sewage water. Environ. Pollut. 2021, 268, 115706. [Google Scholar] [CrossRef]
- Blázquez, J.; Couce, A.; Rodríguez-Beltrán, J.; Rodríguez-Rojas, A. Antimicrobials as promoters of genetic variation. Curr. Opin. Microbiol. 2012, 15, 561–569. [Google Scholar] [CrossRef]
- Sanchez-Cid, C.; Guironnet, A.; Keuschnig, C.; Wiest, L.; Vulliet, E.; Vogel, T.M. Sub-inhibitory gentamicin selects for antibiotic resistance in environmental microbiomes. ISME Commun. 2022, 2, 29. [Google Scholar] [CrossRef]
- Vandecraen, J.; Chandler, M.; Aertsen, A.; Van Houdt, R. The impact of insertion sequences on bacterial genome plasticity and adaptability. Crit. Rev. Microbiol. 2017, 43, 709–730. [Google Scholar] [CrossRef]
- Rolbiecki, D.; Harnisz, M.; Korzeniewska, E.; Buta, M.; Hubeny, J.; Zieliński, W. Detection of carbapenemase-producing, hypervirulent Klebsiella spp. in wastewater and their potential transmission to river water and WWTP employees. Int. J. Hyg. Environ. Health 2021, 237, 113831. [Google Scholar] [CrossRef]
- Smith, W.P.J.; Wucher, B.R.; Nadell, C.D.; Foster, K.R. Bacterial defences: Mechanisms, evolution and antimicrobial resistance. Nat. Rev. Microbiol. 2023, 21, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Yosef, I.; Manor, M.; Kiro, R.; Qimron, U. Temperate and lytic bacteriophages programmed to sensitize and kill antibiotic-resistant bacteria. Proc. Natl. Acad. Sci. USA 2015, 112, 7267–7272. [Google Scholar] [CrossRef]
- Hall, J.P.; Wright, R.C.; Harrison, E.; Muddiman, K.J.; Wood, A.J.; Paterson, S.; Brockhurst, M.A. Plasmid fitness costs are caused by specific genetic conflicts enabling resolution by compensatory mutation. PLoS Biol. 2021, 19, e3001225. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Lawson, C.D.; Adam, H.J.; Schweizer, F.; Zelenitsky, S.; Lagacé-Wiens, P.R.; Denisuik, A.; Rubinstein, E.; Gin, A.S.; Hoban, D.J.; et al. New β-lactam–β-lactamase inhibitor combinations: 2023 update. Drugs 2023, 83, 55–91. [Google Scholar]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage therapy: A renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Björn, C.; Ekblom, J. Antimicrobial peptides as therapeutic agents: Opportunities and challenges. Crit. Rev. Biotechnol. 2020, 40, 978–992. [Google Scholar] [CrossRef]
- Stokes, J.M.; Yang, K.; Swanson, K.; Jin, W.; Cubillos-Ruiz, A.; Donghia, N.M.; MacNair, C.R.; French, S.; Carfrae, L.A.; Bloom-Ackermann, Z.; et al. A deep learning approach to antibiotic discovery. Cell 2020, 180, 688–702.e13. [Google Scholar] [CrossRef]
- Hendriksen, R.S.; Munk, P.; Njage, P.; Van Bunnik, B.; McNally, L.; Lukjancenko, O.; Röder, T.; Nieuwenhuijse, D.; Pedersen, S.K.; Kjeldgaard, J.; et al. Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage. Nat. Commun. 2019, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Karkman, A.; Berglund, F.; Flach, C.-F.; Kristiansson, E.; Larsson, D.G.J. Predicting clinical resistance prevalence using sewage metagenomic data. Commun. Biol. 2020, 3, 29. [Google Scholar] [CrossRef]
- Wright, G.D. The antibiotic resistome: The nexus of chemical and genetic diversity. Nat. Rev. Microbiol. 2007, 5, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Gao, H. Antibiotic Resistance Genes in Integrated Surface Ice, Cryoconite, and Glacier-Fed Stream in a Mountain Glacier in Central Asia. Environ. Int. 2024, 184, 108482. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Huang, J.; Zhang, Y.; Chen, X.; Liu, H.; Zhao, L.; Wang, Q.; Li, M.; Sun, Y.; Zhou, P.; et al. Profiles and risk assessment of antibiotic resistome between Qinghai-Xizang Plateau and Polar Regions. GeoSus 2025, 6, 100342. [Google Scholar] [CrossRef]
- Hooper, D.C.; Jacoby, G.A. Mechanisms of drug resistance: Quinolone resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 12–31. [Google Scholar] [CrossRef]
- Wilson, D.N. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat. Rev. Microbiol. 2014, 12, 35–48. [Google Scholar] [CrossRef]
- Gullberg, E.; Cao, S.; Berg, O.G.; Ilbäck, C.; Sandegren, L.; Hughes, D.; Andersson, D.I. Selection of Resistant Bacteria at Very Low Antibiotic Concentrations. PLoS Pathog. 2011, 7, e1002158. [Google Scholar] [CrossRef]
- Kohanski, M.A.; DePristo, M.A.; Collins, J.J. Sublethal Antibiotic Treatment Leads to Multidrug Resistance via Radical-Induced Mutagenesis. Mol. Cell 2010, 37, 311–320. [Google Scholar] [CrossRef]
- Klümper, U.; Recker, M.; Zhang, L.; Yin, X.; Zhang, T.; Buckling, A. Selection for antimicrobial resistance is reduced when embedded in a natural microbial community. ISME Commun. 2019, 3, 52. [Google Scholar] [CrossRef]
- Rozwandowicz, M.; Brouwer, M.S.M.; Fischer, J.; Wagenaar, J.A.; Gonzalez-Zorn, B.; Guerra, B.; Mevius, D.J.; Hordijk, J. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 1121–1137. [Google Scholar] [CrossRef]
- Harrison, E.; Guymer, D.; Spiers, A.J.; Paterson, S.; Brockhurst, M.A. Parallel compensatory evolution stabilizes plasmids across the parasitism–mutualism continuum. Curr. Biol. 2015, 25, 2034–2039. [Google Scholar] [CrossRef]
- Vogwill, T.; MacLean, R.C. The genetic basis of the fitness costs of antimicrobial resistance: A meta-analysis approach. Evol. Appl. 2015, 8, 284–295. [Google Scholar] [CrossRef]
- Sourenian, T.; Palkovicova, J.; Papagiannitsis, C.C.; Dolejska, M.; Hrabak, J.; Bitar, I. A novel F-type plasmid encoding mcr-10 in a clinical Enterobacter ludwigii strain from a tertiary hospital in the Czech Republic. J. Glob. Antimicrob. Resist. 2024, 37, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Wyres, K.L.; Holt, K.E. Klebsiella pneumoniae as a key trafficker of drug resistance genes from environmental to clinically important bacteria. Nat. Rev. Microbiol. 2018, 16, 688–699. [Google Scholar] [CrossRef]
- Kanai, Y.; Tsuru, S.; Furusawa, C. Insertion Sequences: Simple Mobile Elements with Rich Ecological and Evolutionary Structures. Curr. Opin. Syst. Biol. 2023, 36, 100481. [Google Scholar] [CrossRef]
- Mayorga-Ramos, A.; Zúñiga-Miranda, J.; Carrera-Pacheco, S.E.; Barba-Ostria, C.; Guamán, L.P. CRISPR-Cas-Based Antimicrobials: Design, Challenges, and Bacterial Mechanisms of Resistance. ACS Infect. Dis. 2023, 9, 1283–1302. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Plésiat, P.; Nikaido, H. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin. Microbiol. Rev. 2015, 28, 337–418. [Google Scholar] [CrossRef]
- Poole, K. Efflux-mediated antimicrobial resistance. J. Antimicrob. Chemother. 2005, 56, 20–51. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Papenfort, K.; Vogel, J. Regulatory RNA in bacterial pathogens. Cell Host Microbe 2010, 8, 116–127. [Google Scholar] [CrossRef]
- Gillings, M.R. Integrons: Past, present, and future. Microbiol. Mol. Biol. Rev. 2014, 78, 257–277. [Google Scholar] [CrossRef]
- Botelho, J.; Schulenburg, H. The Role of Integrative and Conjugative Elements in Antibiotic Resistance Evolution. Trends Microbiol. 2021, 29, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, A.H.; Wong, A.; Kassen, R. The fitness costs of antibiotic resistance mutations: A review. Evol. Appl. 2015, 8, 273–283. [Google Scholar] [CrossRef] [PubMed]
- San Millan, A.; MacLean, R.C. Fitness costs of plasmids: A limit to plasmid transmission. Microbiol. Spectr. 2017, 5, mtbp-0016. [Google Scholar] [CrossRef] [PubMed]
- Kloos, J.; Gama, J.A.; Hegstad, J.; Samuelsen, Ø.; Johnsen, P.J. Piggybacking on niche adaptation improves the maintenance of multidrug-resistance plasmids. Mol. Biol. Evol. 2021, 38, 3188–3201. [Google Scholar] [CrossRef]
- Bottery, M.J.; Wood, A.J.; Brockhurst, M.A. Adaptive modulation of antibiotic resistance through intragenomic coevolution. Nat. Ecol. Evol. 2017, 1, 1364–1369. [Google Scholar] [CrossRef]
- Okesanya, O.J.; Ahmed, M.M.; Ogaya, J.B.; Amisu, B.O.; Ukoaka, B.M.; Adigun, O.A.; Manirambona, E.; Adebusuyi, O.; Othman, Z.K.; Oluwakemi, O.G.; et al. Reinvigorating AMR Resilience: Leveraging CRISPR-Cas Technology Potentials to Combat the 2024 WHO Bacterial Priority Pathogens for Enhanced Global Health Security—A Systematic Review. Trop. Med. Health 2025, 53, 43. [Google Scholar] [CrossRef]
- Faure, G.; Shmakov, S.A.; Yan, W.X.; Cheng, D.R.; Scott, D.A.; Peters, J.E.; Makarova, K.S.; Koonin, E.V. CRISPR-Cas in mobile genetic elements: Counter-defence and beyond. Nat. Rev. Microbiol. 2019, 17, 513–525. [Google Scholar] [CrossRef]
- Rodrigues, M.; McBride, S.W.; Hullahalli, K.; Palmer, K.L.; Duerkop, B.A. Conjugative delivery of CRISPR-Cas9 for the selective depletion of antibiotic-resistant enterococci. Antimicrob. Agents Chemother. 2019, 63, e01454-19. [Google Scholar] [CrossRef]
- Collignon, P.; Beggs, J.J.; Walsh, T.R.; Gandra, S.; Laxminarayan, R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: A univariate and multivariable analysis. Lancet Planet. Health 2018, 2, e398–e405. [Google Scholar] [CrossRef]
- Inda-Díaz, J.S.; Wang, M.; Su, J.-Q.; Zhu, Y.-G.; Sánchez, L.; Hernández, M.; Brown, C.; Zhang, T.; Li, D.; Zhao, Y.; et al. Latent antibiotic resistance genes are abundant, diverse and ubiquitously present across ecosystems. Microbiome 2023, 11, 129. [Google Scholar] [CrossRef]
- Berglund, F.; Marathe, N.P.; Österlund, T.; Bengtsson-Palme, J.; Kotsakis, S.; Flach, C.-F.; Larsson, D.G.J.; Kristiansson, E. Identification of 76 novel metallo-β-lactamases through large-scale screening of metagenomic data. Microbiome 2017, 5, 134. [Google Scholar] [CrossRef] [PubMed]
- Mahfouz, N.; Ferreira, I.; Beisken, S.; von Haeseler, A.; Posch, A.E. Large-scale assessment of antimicrobial resistance mobilization using machine learning. BMC Bioinform. 2020, 21, 307. [Google Scholar]
- Liu, H.; Zhai, L.; Wang, P.; Li, Y.; Gu, Y. Ti/PbO2 Electrode Efficiency in Catalytic Chloramphenicol Degradation and Its Effect on Antibiotic Resistance Genes. Int. J. Environ. Res. Public Health 2022, 19, 15632. [Google Scholar] [CrossRef] [PubMed]
- Mandal, T.K. Nanomaterial-Enhanced Hybrid Disinfection: A Solution to Combat Multidrug-Resistant Bacteria and Antibiotic Resistance Genes in Wastewater. Nanomaterials 2024, 14, 1847. [Google Scholar] [CrossRef]
- Joo, S.H.; Knauer, K.; Su, C.; Toborek, M. Antibiotic Resistance in Plastisphere. J. Environ. Chem. Eng. 2025, 13, 115217. [Google Scholar] [CrossRef] [PubMed]
- Subirats, J.; Pastor-López, E.J.; Pascó, J.; Mendoza, M.; Guivernau, M.; Fernández, B.; Robajo, R.; Viñas, M.; Biel, C.; Sánchez, D.; et al. Green Solutions for Treating Groundwater Polluted with Nitrates, Pesticides, Antibiotics, and Antibiotic Resistance Genes for Drinking Water Production. J. Environ. Manag. 2025, 375, 124263. [Google Scholar] [CrossRef]
- Gentile, A.; Di Stasio, L.; Oliva, G.; Vigliotta, G.; Cicatelli, A.; Guarino, F.; Nissim, W.G.; Labra, M.; Castiglione, S. Antibiotic Resistance in Urban Soils: Dynamics and Mitigation Strategies. Environ. Res. 2024, 263, 120120. [Google Scholar] [CrossRef]
- Judan Cruz, K.G.; Okamoto, T.; Bongulto, K.A.; Gandalera, E.E.; Kagia, N.; Watanabe, K. Natural compound-induced downregulation of antimicrobial resistance and biofilm-linked genes in wastewater Aeromonas species. Front. Cell. Infect. Microbiol. 2024, 14, 1456700. [Google Scholar] [CrossRef]
- Imai, Y.; Meyer, K.J.; Iinishi, A.; Favre-Godal, Q.; Green, R.; Manuse, S.; Caboni, M.; Mori, M.; Niles, S.; Ghiglieri, M.; et al. A new antibiotic selectively kills Gram-negative pathogens. Nature 2019, 576, 459–464. [Google Scholar] [CrossRef]
- Sabri, N.A.; van Holst, S.; Schmitt, H.; van der Zaan, B.M.; Gerritsen, H.W.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Fate of antibiotics and antibiotic resistance genes during conventional and additional treatment technologies in wastewater treatment plants. Sci. Total Environ. 2020, 741, 140199. [Google Scholar] [CrossRef]
- Citorik, R.J.; Mimee, M.; Lu, T.K. Sequence-specific antimicrobials using efficiently delivered RNA-guided nucleases. Nat. Biotechnol. 2014, 32, 1141–1145. [Google Scholar] [CrossRef]
- Yang, J.W.; Kim, H.; Hyeon, L.-S.; Yoo, J.S.; Kang, S. Development of a Recombinase Polymerase Amplification-Coupled CRISPR/Cas12a Platform for Rapid Detection of Antimicrobial-Resistant Genes in Carbapenem-Resistant Enterobacterales. Biosensors 2024, 14, 536. [Google Scholar] [CrossRef]
- Vargas-Reyes, M.; Alcántara, R.; Alfonsi, S.; Peñaranda, K.; Petrelli, D.; Spurio, R.; Pajuelo, M.J.; Milon, P. Versatile and Portable Cas12a-Mediated Detection of Antibiotic Resistance Markers. bioRxiv 2024. [Google Scholar] [CrossRef]
- Tao, S.; Chen, H.; Li, N.; Wang, T.; Liang, W. The Spread of Antibiotic Resistance Genes In Vivo Model. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 3348695. [Google Scholar] [CrossRef] [PubMed]
- Brown-Jaque, M.; Calero-Cáceres, W.; Muniesa, M. Transfer of antibiotic-resistance genes via phage-related mobile elements. Plasmid 2015, 79, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Munk, P.; Brinch, C.; Møller, F.D.; Petersen, T.N.; Hendriksen, R.S.; Seyfarth, A.M.; Kjeldgaard, J.S.; Svendsen, C.A.; van Bunnik, B.; Berglund, F.; et al. Genomic Analysis of Sewage from 101 Countries Reveals Global Landscape of Antimicrobial Resistance. Nat. Commun. 2022, 13, 7251. [Google Scholar] [CrossRef]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global Increase and Geographic Convergence in Antibiotic Consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef]
- Miller, R.D.; Iinishi, A.; Modaresi, S.M.; Yoo, B.-K.; Curtis, T.D.; Lariviere, P.J.; Liang, L.; Son, S.; Nicolau, S.; Bargabos, R.; et al. Computational identification of a systemic antibiotic for Gram-negative bacteria (dynobactin). Nat. Microbiol. 2022, 7, 1661–1672. [Google Scholar] [CrossRef]
- Wu, K.J.Y.; Tresco, B.I.C.; Ramkissoon, A.; Aleksandrova, E.A.; Syroegin, E.A.; See, D.N.Y.; Liow, P.; Dittemore, G.A.; Yu, M.; Testolin, G.; et al. An antibiotic preorganized for ribosomal binding overcomes antimicrobial resistance (cresomycin). Science 2024, 383, 721–726. [Google Scholar] [CrossRef]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.A.; Takebayashi, Y.; Spencer, J. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J. Mol. Biol. 2019, 431, 3472–3500. [Google Scholar] [CrossRef]
- Mishra, R.; Panda, A.K.; De Mandal, S.; Shakeel, M.; Bisht, S.S.; Khan, J. Natural Anti-Biofilm Agents: Strategies to Control Biofilm-Forming Pathogens. Front. Microbiol. 2020, 11, 566325. [Google Scholar] [CrossRef]
- Cui, L.; Watanabe, S.; Miyanaga, K.; Kiga, K.; Sasahara, T.; Aiba, Y.; Tan, X.-E.; Veeranarayanan, S.; Thitiananpakorn, K.; Nguyen, H.M.; et al. A Comprehensive Review on Phage Therapy and Phage-Based Drug Development. Antibiotics 2024, 13, 870. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019, 25, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Sawa, T.; Moriyama, K.; Kinoshita, M. Current Status of Bacteriophage Therapy for Severe Bacterial Infections. J. Intensive Care 2024, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.K.; Browne, P.D.; Hansen, L.H. Antibiotic Resistance Genes Are Differentially Mobilized According to Resistance Mechanism. GigaScience 2022, 11, giac072. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bao, N.; Yan, Z.; Yuan, X.-Z.; Wang, S.-G.; Xia, P.-F. Degradation of Antibiotic Resistance Genes by VADER with CRISPR-Cas Immunity. Appl. Environ. Microbiol. 2023, 89, e00053-23. [Google Scholar] [CrossRef]
- Shin, J.; Kim, S.R.; Xie, Z.; Jin, Y.-S.; Wang, Y.-C. A CRISPR/Cas12a-Based System for Sensitive Detection of Antimicrobial-Resistant Genes in Carbapenem-Resistant Enterobacterales. Biosensors 2024, 14, 194. [Google Scholar] [CrossRef]
- Zhou, J.-C.; Li, Z.-H.; Zhang, X.; Leung, K.M.Y.; Yuan, L.; Sheng, G.-P. Facet-Specific Photocatalytic Degradation of Extracellular Antibiotic Resistance Genes by Hematite Nanoparticles in Aquatic Environments. Environ. Sci. Technol. 2023, 57, 17259–17269. [Google Scholar] [CrossRef]
- Şahin, S.; Akpinar, I.; Sivri, N. An alternative material for an effective treatment technique proposal in the light of bibliometric profile of global scientific research on antibiotic resistance and Escherichia coli. Environ. Monit. Assess. 2020, 192, 714. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Zhang, D.; Yu, P.; Sun, R.; Javed, H.; Wu, G.; Alvarez, P.J.J. Selective Adsorption and Photocatalytic Degradation of Extracellular Antibiotic Resistance Genes by Molecularly-Imprinted Graphitic Carbon Nitride. Environ. Sci. Technol. 2020, 54, 4621–4630. [Google Scholar] [CrossRef] [PubMed]
- Becsei, Á.; Fuschi, A.; Otani, S.; Kant, R.; Weinstein, I.; Alba, P.; Stéger, J.; Visontai, D.; Brinch, C.; de Graaf, M.; et al. Time-series sewage metagenomics distinguishes seasonal, human-derived and environmental microbial communities potentially allowing source-attributed surveillance. Nat. Commun. 2024, 15, 7551. [Google Scholar] [CrossRef] [PubMed]
- Davoudabadi, S.; Goudarzi, M.; Hashemi, A. Detection of virulence factors and antibiotic resistance among Klebsiella pneumoniae isolates from Iran. Biomed Res. Int. 2023, 2023, 3624497. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, L.; Lin, Y.; Zhao, X.; Chen, H.; Zhong, Y.; Jiang, W.; Wu, X.; Lin, X. Unveiling the Antibacterial Mechanism of Resveratrol against Aeromonas hydrophila through Proteomics Analysis. Front. Cell. Infect. Microbiol. 2024, 14, 1378094. [Google Scholar] [CrossRef]
- Vázquez, X.; García-Fierro, R.; Fernández, J.; Bances, M.; Herrero-Fresno, A.; Olsen, J.E.; Rodicio, R.; Ladero, V.; García, V.; Rodicio, M.R. Incidence and genomic background of antibiotic resistance in Food-Borne and Clinical Isolates of Salmonella enterica serovar Derby from Spain. Antibiotics 2023, 12, 1204. [Google Scholar] [CrossRef]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile genetic elements associated with antimicrobial resistance. Clin. Microbiol. Rev. 2018, 31, e00088-17. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.-F. Antibiotic Resistance in the Environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- WHO. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Lai, W.; Xu, K.; Wang, L.; Zhang, Y.; Wang, Y.; Sun, S. Simultaneous and visual detection of KPC and NDM carbapenemase genes using asymmetric PCR barcoded primers and a CRISPR-Cas12a lateral-flow strip. BioMed Res. Int. 2023, 9975620. [Google Scholar] [CrossRef]
- Wong, F.; Zheng, E.J.; Valeri, J.A.; Donghia, N.M.; Anahtar, M.N.; Omori, S.; Li, A.; Cubillos-Ruiz, A.; Krishnan, A.; Jin, W.; et al. Discovery of a structural class of antibiotics with explainable deep learning. Nature 2024, 626, 177–185. [Google Scholar] [CrossRef]
- Li, M.; Wang, P.; Zhang, K.; Zhang, H.; Bao, Y.; Li, Y.; Zhan, S.; Crittenden, J.C. Single Cobalt Atoms Anchored on Ti_3C_2T_x with Dual Reaction Sites for Efficient Adsorption–Degradation of Antibiotic Resistance Genes. Proc. Natl. Acad. Sci. USA 2023, 120, e2305705120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Q.; Wang, T.; Xu, N.; Lu, T.; Hong, W.; Penuelas, J.; Gillings, M.; Wang, M.; Gao, W.; et al. Assessment of global health risk of antibiotic resistance genes. Nat. Commun. 2022, 13, 1553. [Google Scholar] [CrossRef] [PubMed]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6, ARBA-0009-2017. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2025; WHO: Geneva, Switzerland, 2025; Available online: https://www.who.int/publications/i/item/9789240108127 (accessed on 15 May 2025).
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Bürgmann, H.; Sørum, H.; Norström, M.; Pons, M.-N.; et al. Tackling antibiotic resistance: The environmental framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef]
- Haffiez, N.; Zakaria, B.S.; Mirsoleimani Azizi, S.M.; Dhar, B.R. Antibiotic Resistance Genes Proliferation under Anaerobic Degradation of polylactic acid and polyhydroxy butyrate Bioplastics. Environ. Int. 2023, 175, 107938. [Google Scholar] [CrossRef]
- Chang, P.-H.; Juhrend, B.; Olson, T.M.; Marrs, C.F.; Wigginton, K.R. Degradation of Extracellular Antibiotic Resistance Genes with UV254 Treatment. Environ. Sci. Technol. 2017, 51, 6185–6192. [Google Scholar] [CrossRef]

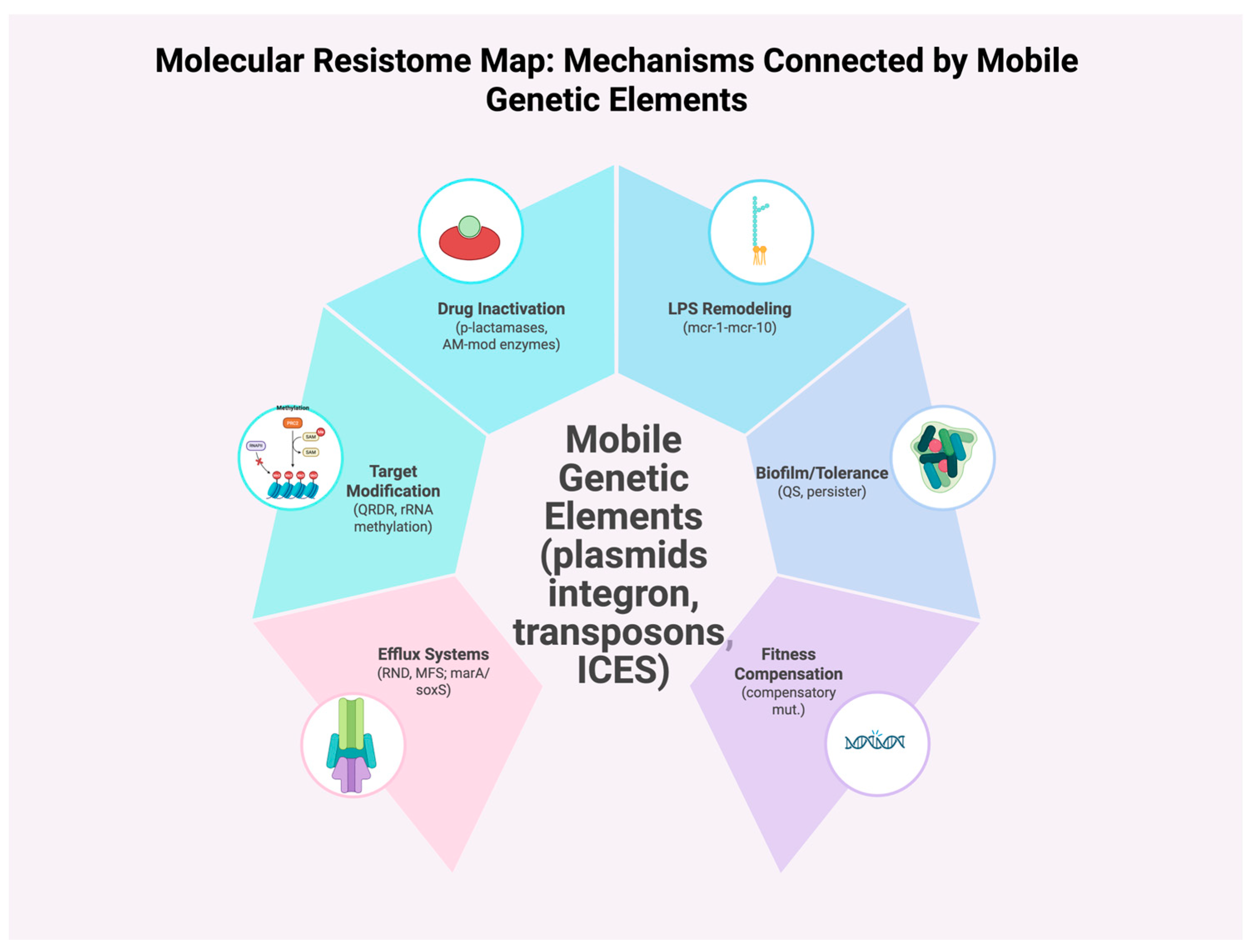
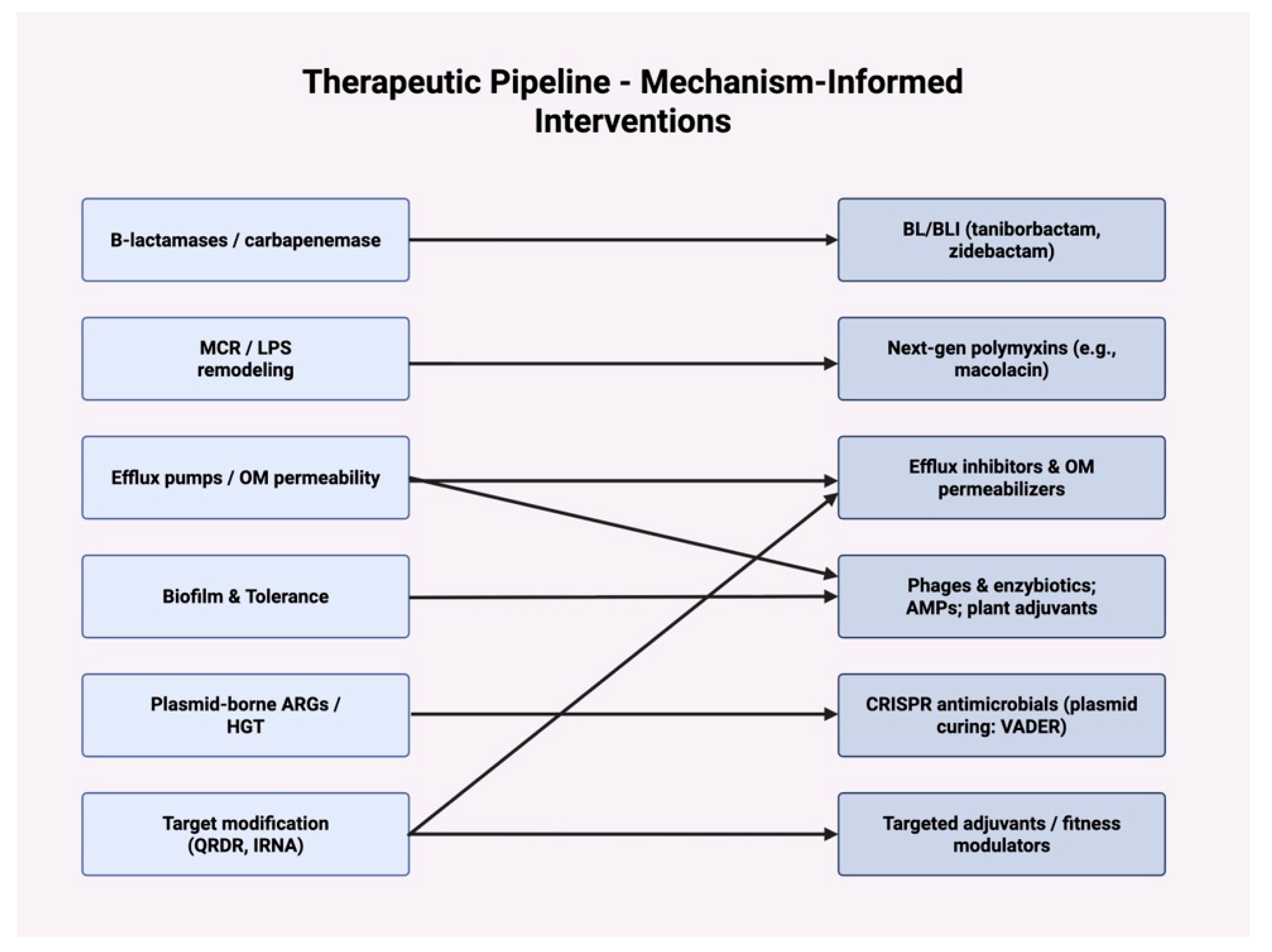
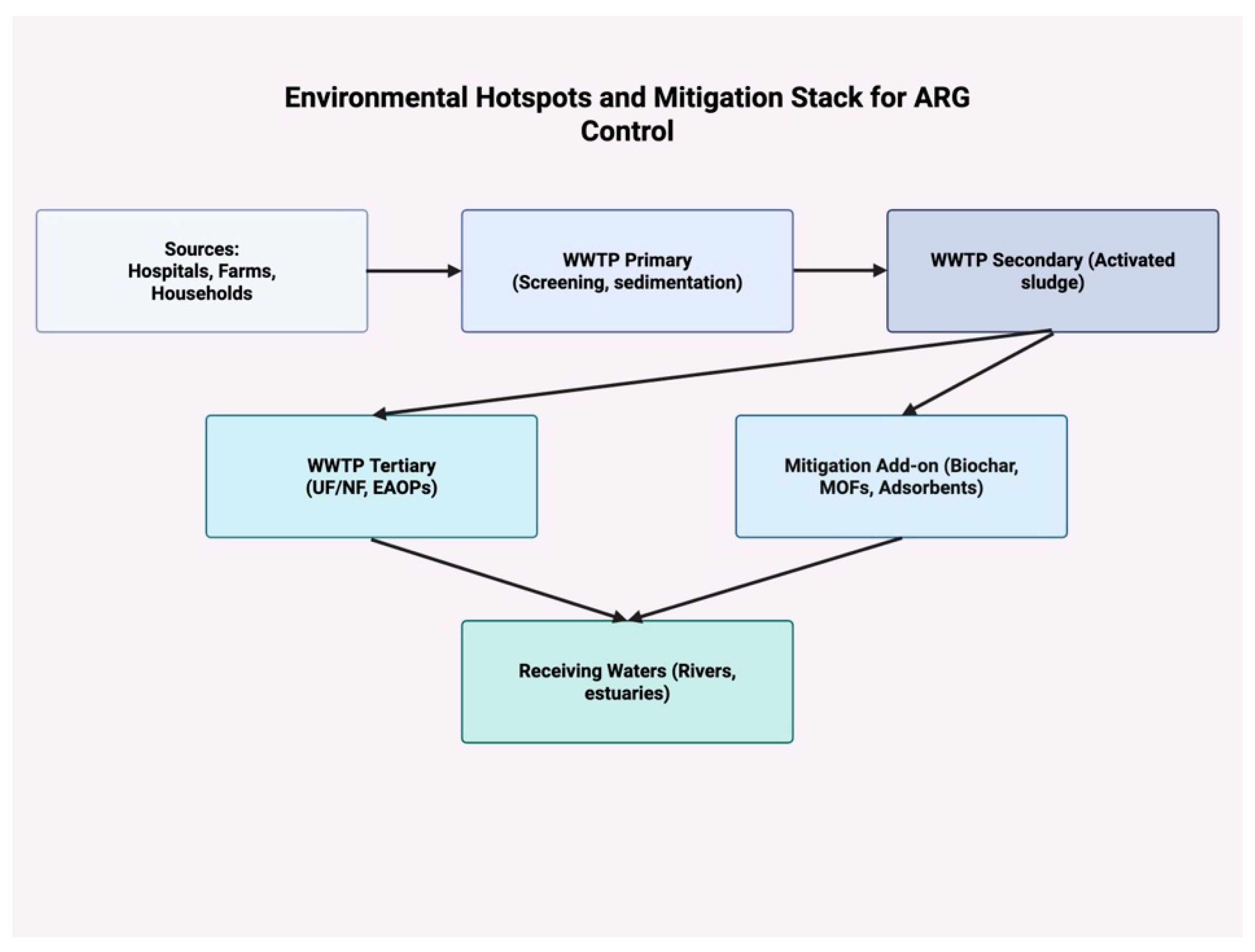
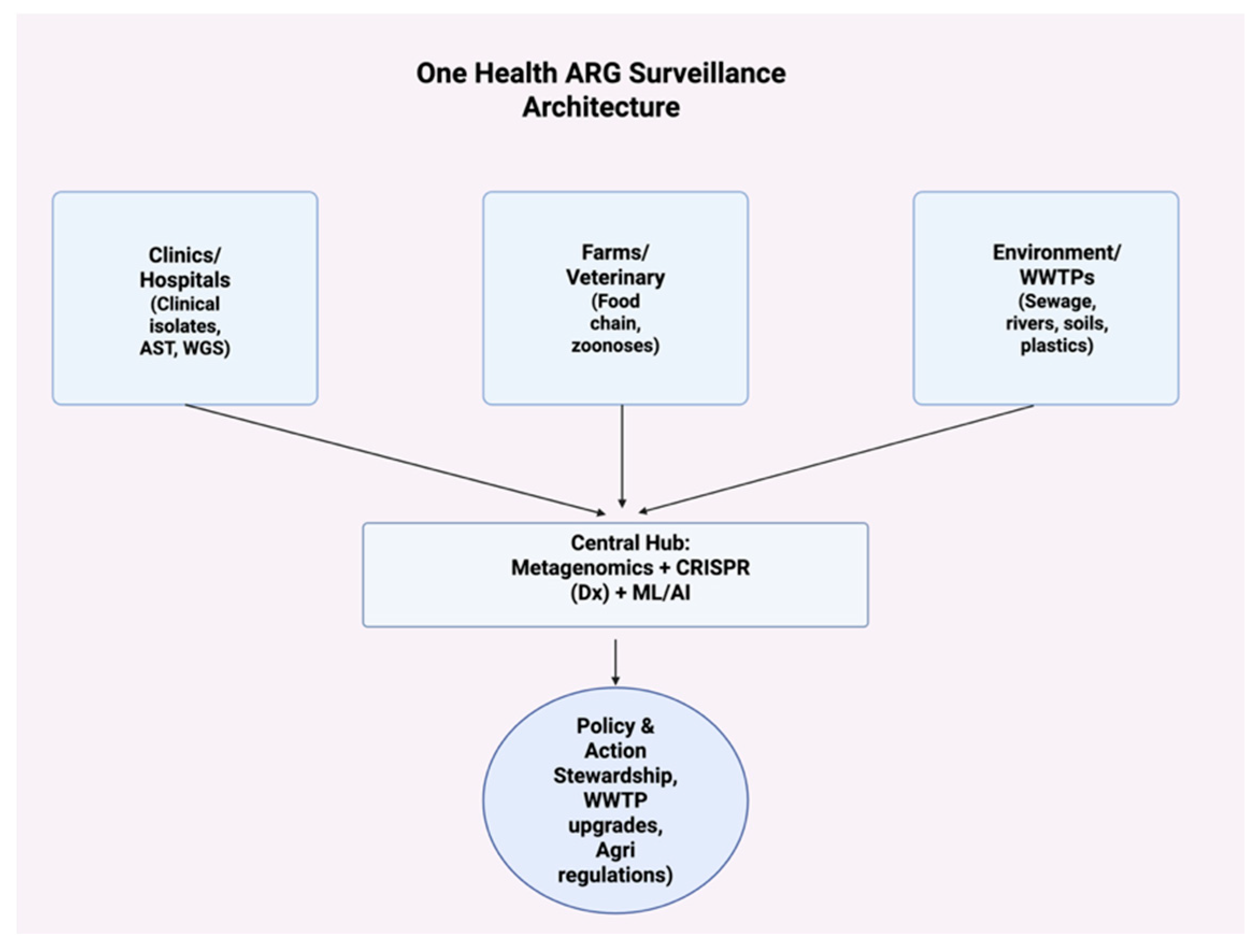
| Mechanism | Molecular Example(s) | Mobile Elements/ Regulation | Clinical Relevance | Key Refs |
|---|---|---|---|---|
| Drug inactivation (β-lactams) | Class A/C/D ESBLs; NDM/VIM/IMP metallo-β-lactamases | Plasmids, integrons; SOS-induced cassette exchange | Carbapenem and cephalosporin failure in Enterobacterales | [8,9,24,39,43,44,45] |
| Target protection/modification | QRDR mutations in gyrA/parC (FQs); rRNA methylation (erm) | IS activation, transposons | Fluoroquinolone and macrolide resistance across E. coli, S. aureus | [27,28,37] |
| Efflux overexpression | RND pumps (MexAB-OprM, AcrAB-TolC); regulators marA/soxS | sRNAs; global stress circuits | Broad MDR in P. aeruginosa, E. coli | [39,40,41,42] |
| Outer membrane/LPS remodeling | mcr-1–mcr-10 phosphoethanolamine transferases | Conjugative plasmids | Colistin failure; spread from agriculture→clinic | [10,35] |
| Bypass/synthesis | Altered PBPs; folate pathway detours | ICEs, integrons | β-lactam and TMP-SMX compromised | [43,44] |
| Biofilm-linked tolerance | QS, matrix genes; oxidative stress reprogramming | — | Persisters; device infections | [41,42] |
| Fitness compensation | Compensatory chromosomal/plasmid mutations | — | Persistence of ARGs without antibiotic pressure | [17,34,46,47,48,49,50] |
| Hotspot | Typical ARGs/Drivers | Key Findings (Performance) | Technology/Approach | Key Refs |
|---|---|---|---|---|
| WWTP secondary effluent | blaKPC, blaNDM, mcr, intI1; sub-MICs | Conventional steps often leave extracellular ARGs | Add tertiary barrier + EAOP/nanofiltration | [61,62,63] |
| WWTP tertiary (UF/NF) | Free-floating ARGs | UF → ARG passage; NF removed >99.9% total bacteria & ARGs in effluent | Nanofiltration polishing | [62,63] |
| Anaerobic digestion | tet, sul; co-selection | ARG persistence unless optimized; biochar/consortia recommended | Process optimization + amendments | [63] |
| Groundwater (nitrate/pesticides/antibiotics) | Mixed ARGs; co-pollutants | “Green” filtration reduced contaminants and ARG loads | Biofiltration + sorbents | [64] |
| Urban/agricultural soils | Metals, pesticides; manure | Biochar & soil management lowered ARG incidence | Bioremediation | [65] |
| Plastisphere (micro/nanoplastics) | blaTEM, qnrS, sulI, ermB; aged surfaces | Biofilms on MNPs enrich ARGs despite treatment | Plastic pollution control + targeted removal | [63] |
| Rivers/estuaries | Nutrients, metals | Nonlinear links between nutrients/metals and ARG abundance | Source control + adsorbents/MOFs | [66,67,68,69,70] |
| Bioplastics (PLA/PHB) | Proliferation under anaerobic degradation | Enhanced ARG proliferation during biodegradation | Life-cycle scrutiny & controls | [68] |
| Modality | Molecular Target/Concept | Status/Notes | Key Refs |
|---|---|---|---|
| Macolacin (polymyxin congener) | Active vs. mcr-positive GNB | In vivo efficacy in murine models | [62,71,72] |
| New BL/BLI pairs (e.g., taniborbactam, zidebactam) | broaden carbapenemase coverage | Late stage/updates 2023 | [18,73] |
| Antimicrobial peptides & plant bioactives (quercetin, rutin, andrographolide) | Anti-biofilm, QS, oxidative pathways; ARG expression down | Synergistic adjuvants | [20,66,74] |
| Phage therapy/engineered lysins | Pathogen-specific lysis; biofilm disruption | Clinical case expansion; standardization needed | [19,75,76,77] |
| CRISPR antimicrobials (e.g., VADER) | Plasmid curing/ARG excision | Demonstrated in bioreactors/WWTP contexts | [78,79,80] |
| Nanomaterial hybrid disinfection | ROS-based kill; extracellular DNA fragmentation | Outperforms UV/chlorination; pair with polishing | [57,81,82,83] |
| Tool | What It Detects | Deployment | LOD/Speed | Notes | Key Refs |
|---|---|---|---|---|---|
| Sewage metagenomics (shotgun) | Full ARGome + pathogens | WWTP influent/effluent | High depth; batch (hours–days) | Community-level early warning | [69,84,95] |
| CRISPR-Cas diagnostics (Cas12a) | Specific ARGs (e.g., blaCTX-M-15, floR, intI1) | Fieldable, lateral-flow | Attomolar; minutes–<1 h | Low-cost, portable | [66,80,92] |
| Viral metagenomics | Phage-borne ARGs | Rivers, WWTPs | Batch | Complements bacterial WGS | [68] |
| ML/AI prevalence prediction | ARG mobilization, regional risk | Dashboard | Real-time once trained | Decision support for policy | [69,78,79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nass, N.M.; Zaher, K.A. Beyond the Resistome: Molecular Insights, Emerging Therapies, and Environmental Drivers of Antibiotic Resistance. Antibiotics 2025, 14, 995. https://doi.org/10.3390/antibiotics14100995
Nass NM, Zaher KA. Beyond the Resistome: Molecular Insights, Emerging Therapies, and Environmental Drivers of Antibiotic Resistance. Antibiotics. 2025; 14(10):995. https://doi.org/10.3390/antibiotics14100995
Chicago/Turabian StyleNass, Nada M., and Kawther A. Zaher. 2025. "Beyond the Resistome: Molecular Insights, Emerging Therapies, and Environmental Drivers of Antibiotic Resistance" Antibiotics 14, no. 10: 995. https://doi.org/10.3390/antibiotics14100995
APA StyleNass, N. M., & Zaher, K. A. (2025). Beyond the Resistome: Molecular Insights, Emerging Therapies, and Environmental Drivers of Antibiotic Resistance. Antibiotics, 14(10), 995. https://doi.org/10.3390/antibiotics14100995






