Insight into the Roles of Albumin—Alone and in Combination with Either Voriconazole or Antimicrobial Peptides Derived from Chromogranin A—In the Growth of Different Microbial Species
Abstract
1. Introduction
2. Results
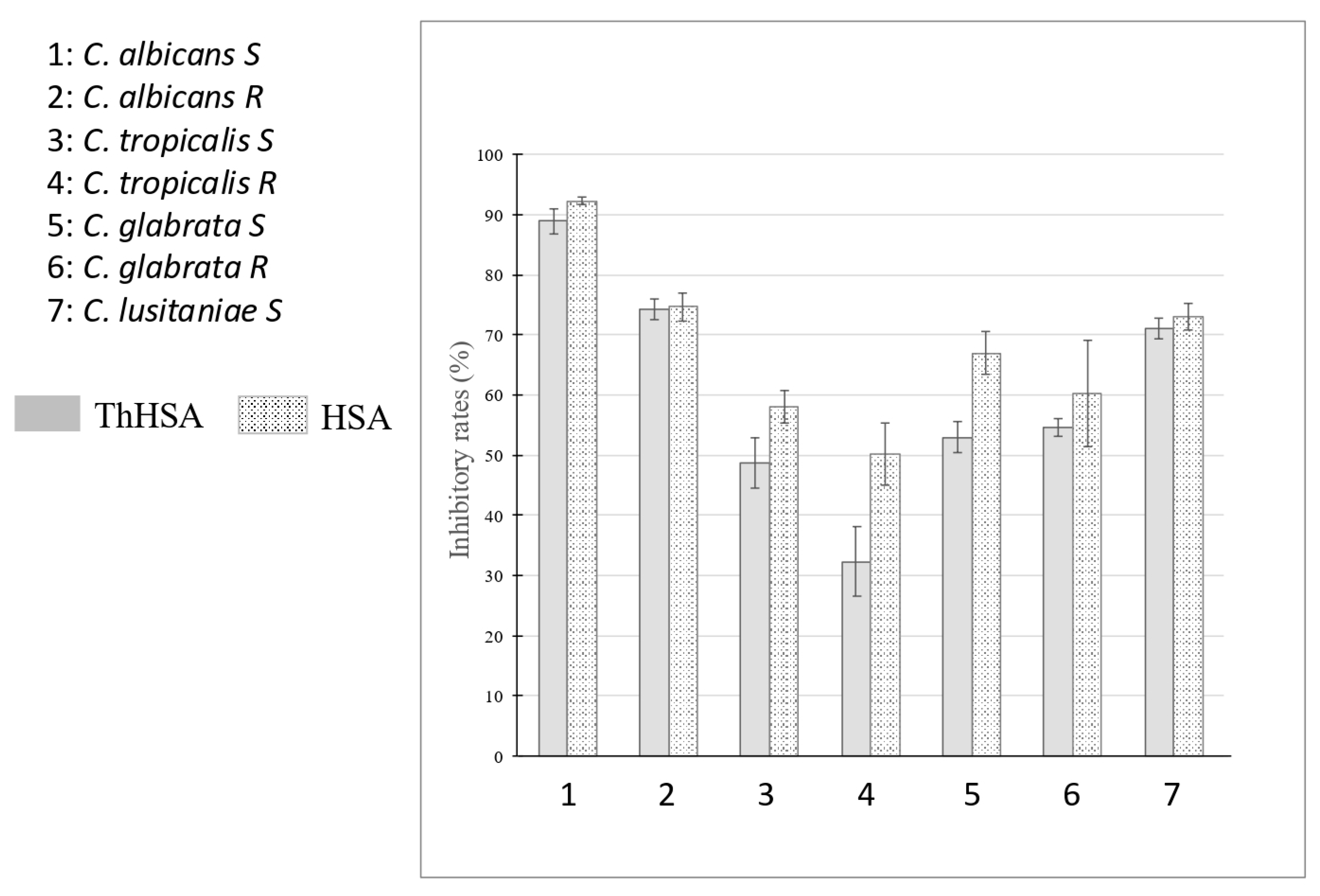
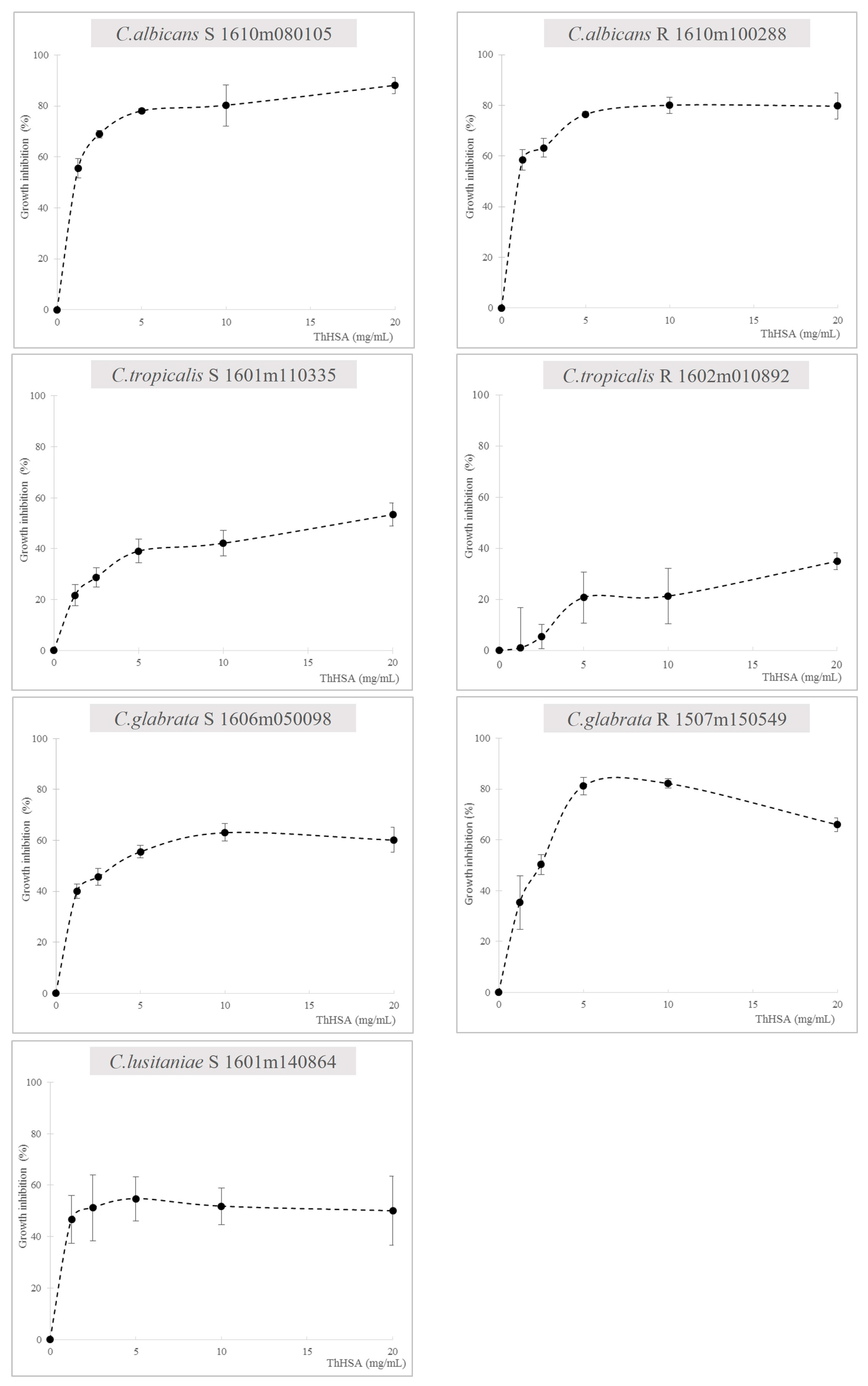
2.1. Antimicrobial Assays with ThHSA Alone or in Combination with VCZ
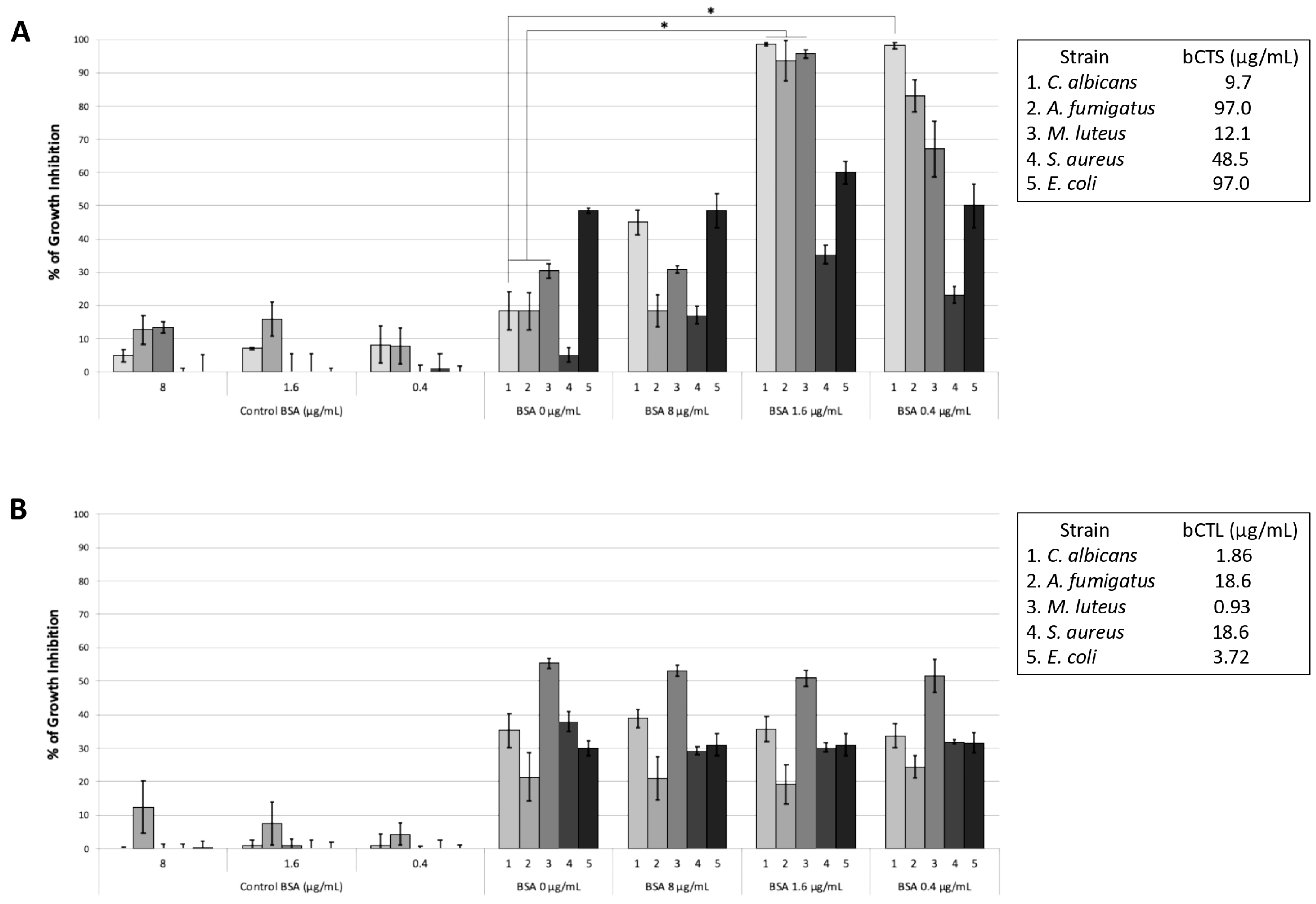
2.2. Influence of BSA on the Antimicrobial Activity of Chromogranin A-Derived Peptides
3. Discussion
4. Materials and Methods
4.1. Candida Strains
4.2. Characterization of Albumins (HSA and ThHSA)
4.3. Preparation of Voriconazole and MICs for Antibiotics
4.4. Growth Inhibition Assays of HSA, ThHSA, and Voriconazole Against Candida Strains
4.5. Analysis of the Antimicrobial Activity of ThHSA in Combination with Voriconazole
4.6. Preparation and Characterization of Synthetic CgA-Derived Peptides
4.7. Antimicrobial and Antifungal Assays for Albumin and CgA-Derived Peptides
4.8. Hemolysis Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A620 nm | American Type Culture Collector |
| Alb | Albumin |
| BSA | Bovine Serum Albumin |
| bCTS | Bovine Catestatin |
| bCTL | Bovine Cateslytin |
| HSA | Human Serum Albumin |
| HPLC | High-Performance Liquid Chromatography |
| ICU | Intensive Care Unit |
| MS | Mass Spectrometry |
| PDB | Potato Dextrose Broth |
| QCM | Quartz Crystal Microbalance |
| Th Alb | Therapeutic Albumin |
| Th HSA | Therapeutic Human Serum Albumin |
| VCZ | Voriconazole |
References
- Lass-Flörl, C.; Kanj, S.S.; Govender, N.P.; Thompson, G.R., 3rd; Ostrosky-Zeichner, L.; Govrins, M.A. Invasive candidiasis. Nat. Rev. Dis. Primers 2024, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Giacobbe, D.R.; Vena, A.; Trucchi, C.; Ansaldi, F.; Antonelli, M.; Adamkova, V.; Alicino, C.; Almyroudi, M.P.; Atchade, E.; et al. Incidence and outcome of invasive candidiasis in intensive care units (ICUs) in Europe: Results of the EUCANDICU project. Crit. Care 2019, 23, 219–226. [Google Scholar] [CrossRef]
- Marangos, M.; Ioannou, P.; Senn, L.; Spiliopoulou, A.; Tzalis, S.; Kolonitsiou, F.; Valta, M.; Kokkini, S.; Pagani, J.L.; Stafylaki, D.; et al. Role of source control in critically ill candidemic patients: A multicenter retrospective study. Infection 2024, 52, 1733–1743. [Google Scholar] [CrossRef]
- Ponde, N.O.; Lortal, L.; Ramage, G.; Naglik, J.R.; Richardson, J.P. Candida albicans biofilms and polymicrobial interactions. Crit. Rev. Microbiol. 2021, 47, 91–111. [Google Scholar] [CrossRef]
- Reginatto, P.; Bergamo, V.Z.; Berlitz, S.J.; Guerreiro, I.C.K.; de Andrade, S.F.; Fuentefria, A.M. Rational selection of antifungal drugs to propose a new formulation strategy to control Candida biofilm formation on venous catheters. Braz. J. Microbiol. 2020, 51, 1037–1049. [Google Scholar] [CrossRef]
- Thompson, G.R.; Jenks, J.D.; Baddley, J.W.; Lewis, J.S.; Egger, M.; Schwartz, I.S.; Boyer, J.; Patterson, T.F.; Chen, S.C.; Pappas, P.G.; et al. Fungal Endocarditis: Pathophysiology, Epidemiology, Clinical Presentation, Diagnosis, and Management. Clin. Microbiol. Rev. 2023, 36, e0001923. [Google Scholar] [CrossRef] [PubMed]
- Azim, A.; Ahmed, A. Diagnosis and management of invasive fungal diseases in non-neutropenic ICU patients with focus on candidiasis and aspergillosis: A comprehensive review. Front. Cell. Infect. Microbiol. 2024, 14, 125–158. [Google Scholar] [CrossRef]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef]
- Pea, F.; Lewis, R.E. Overview of antifungal dosing in invasive candidiasis. J. Antimicrob. Chemother. 2018, 73 (Suppl. S1), i33–i43. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2015, 62, e1–e50. [Google Scholar] [CrossRef]
- Tissot, F.; Agrawal, S.; Pagano, L.; Petrikkos, G.; Groll, A.H.; Skiada, A.; Lass-Flörl, C.; Calandra, T.; Viscoli, C.; Herbrecht, R. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica 2017, 102, 433–444. [Google Scholar] [CrossRef]
- Beredaki, M.I.; Georgiou, P.C.; Siopi, M.; Kanioura, L.; Arendrup, M.C.; Mouton, J.W.; Meletiadis, J. Voriconazole efficacy against Candida glabrata and Candida krusei: Preclinical data using a validated in vitro pharmacokinetic/pharmacodynamic model. J. Antimicrob. Chemother. 2020, 75, 140–148. [Google Scholar] [CrossRef]
- Schneider, F.; Dureau, A.F.; Hellé, S.; Betscha, C.; Senger, B.; Cremel, G.; Boulmedais, F.; Strub, J.M.; Corti, A.; Meyer, N.; et al. A Pilot Study on Continuous Infusion of 4% Albumin in Critically Ill Patients: Impact on Nosocomial Infection via a Reduction Mechanism for Oxidized Substrates. Crit. Care Explor. 2019, 1, e0044. [Google Scholar] [CrossRef]
- Zazeri, G.; Povinelli, A.P.R.; Bertozo, L.C.; Jones, A.M.; Ximenes, V.F. The role of medium polarity in the efficiency of albumin binding with hydrophobic ligands: Experimental studies and a molecular dynamics investigation. Int. J. Mol. Sci. 2024, 25, 12664–12680. [Google Scholar] [CrossRef]
- McCluskey, A.; Thomas, A.N.; Bowles, B.J. The prognostic value of serial measurements of serum albumin concentration in patients admitted to an intensive care unit. Anaesthesia 1996, 1, 724–727. [Google Scholar] [CrossRef]
- Abedi, F.; Zarei, B.; Elyasi, S. Albumin: A comprehensive review and practical guideline for clinical use. Eur. J. Clin. Pharmacol. 2024, 80, 1151–1169. [Google Scholar] [CrossRef]
- Jing-Yuan, X.; Qi-Hong, C.; Jian-Feng, X.; Chun, P.; Song-Qiao, L.; Li-Wei, H.; Cong-Shan, Y.; Ling, L.; Ying-Zi, H.; Feng-Mei, G.; et al. Comparison of the effects of albumin and crystalloid on mortality in adult patients with severe sepsis and septic shock: A meta-analysis of randomized clinical trials. Crit. Care 2014, 18, 702–709. [Google Scholar] [CrossRef]
- Svenson, J.; Brandsdal, B.O.; Stensen, W.; Svendsen, J.S. Albumin binding of short cationic antimicrobial micropeptides and its influence on the in vitro bactericidal effect. J. Med. Chem. 2007, 50, 3334–3339. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Wang, C.F.; Liao, Y.D. Fetal bovine serum albumin inhibits antimicrobial peptide activity and binds drug only in complex with α1-antitrypsin. Sci. Rep. 2021, 11, 1267–1279. [Google Scholar] [CrossRef] [PubMed]
- Mancino, D.; Kharouf, N.; Scavello, F.; Hellé, S.; Salloum-Yared, F.; Mutschler, A.; Mathieu, E.; Lavalle, P.; Metz-Boutigue, M.H.; Haïkel, Y. The Catestatin-Derived Peptides Are New Actors to Fight the Development of Oral Candidosis. Int. J. Mol. Sci. 2022, 23, 2066–2077. [Google Scholar] [CrossRef] [PubMed]
- Pricopie, A.I.; Ionuț, I.; Marc, G.; Arseniu, A.M.; Vlase, L.; Grozav, A.; Găină, L.I.; Vodnar, D.C.; Pîrnău, A.; Tiperciuc, B.; et al. Design and synthesis of novel 1,3-Thiazole and 2-Hydrazinyl-1,3-Thiazole derivatives as anti-Candida agents: In vitro antifungal screening, molecular docking study, and spectroscopic investigation of their binding interaction with bovine serum albumin. Molecules 2019, 24, 3435–3455. [Google Scholar] [CrossRef] [PubMed]
- Grela, E.; Stączek, S.; Nowak, M.; Pawlikowska-Pawlega, B.; Zdybicka-Barabas, A.; Janik, S.; Cytryńska, M.; Grudzinski, W.; Gruszecki, W.I.; Luchowski, R. Enhanced Antifungal Activity of Amphotericin B Bound to Albumin: A “Trojan Horse” Effect of the Protein. J. Phys. Chem. B 2023, 127, 3632–3640. [Google Scholar] [CrossRef]
- Arzumanyan, V.G.; Ozhovan, I.M.; Svitich, O.A. Antimicrobial effect of albumin on bacteria and yeast cells. Bull. Exp. Biol. Med. 2019, 167, 763–766. [Google Scholar] [CrossRef]
- Austermeier, S.; Pekmezović, M.; Porschitz, P.; Lee, S.; Kichik, N.; Moyes, D.L.; Ho, J.; Ko-towicz, N.K.; Naglik, J.R.; Hube, B.; et al. Albumin neutralizes hydrophobic toxins and modulates Candida albicans Pathogenicity. mBio 2021, 12, e0053121. [Google Scholar] [CrossRef]
- Kremer, H.; Baron-Menguy, C.; Tesse, A.; Gallois, Y.; Mercat, A.; Henrion, D.; Andriantsitohaina, R.; Asfar, P.; Meziani, F. Human serum albumin improves endothelial dysfunction and survival during experimental endotoxemia: Concentration-dependent properties. Crit. Care Med. 2011, 39, 1414–1422. [Google Scholar] [CrossRef]
- Schneider, F.; Castelain, V.; Morel, G.; Dureau, A.-F.; Poidevin, A.; Ludes, P.-O.; Fabacher, T.; Senger, B.; Meyer, N.; Metz-Boutigue, M.-H. Continuous 4 Percent Albumin Versus Intermittent 20 Percent Albumin in Adults with Septic Shock: A Prospective, Phase IV, Open-label Randomized Trial. Am. J. Intern. Med. 2020, 8, 89–100. [Google Scholar] [CrossRef]
- Scavello, F.; Kharouf, N.; Lavalle, P.; Haikel, Y.; Schneider, F.; Metz-Boutigue, M.H. The antimicrobial peptides secreted by the chromaffin cells of the adrenal medulla link the neuroendocrine and immune systems: From basic to clinical studies. Front. Immunol. 2022, 13, 977175–977187. [Google Scholar] [CrossRef]
- Gelamo, E.L.; Silva, C.H.; Imasato, H.; Tabak, M. Interaction of bovine (BSA) and human (HSA) serum albumins with ionic surfactants: Spectroscopy and modelling. Biochim. Biophys. Acta 2002, 1594, 84–99. [Google Scholar] [CrossRef]
- Ho, C.S.; Wong, C.T.H.; Aung, T.T.; Lakshminarayanan, R.; Mehta, J.S.; Rauz, S.; McNally, A.; Kintses, B.; Peacock, S.J.; de la Fuente-Nunez, C.; et al. Antimicrobial resistance: A concise update. Lancet Microbe 2025, 6, 100947–100960. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Simeonova, M.; Leung, V.; Lo, J.; Kan, T.; Raybardhan, S.; Sapin, M.E.; Mponponsuo, K.; Farrell, A.; et al. Antimicrobial resistance in patients with COVID-19: A systematic review and meta-analysis. Lancet Microbe 2023, 4, e179–e191. [Google Scholar] [CrossRef]
- Roux, D.; Gaudry, S.; Dreyfuss, D.; El-Benna, J.; de Prost, N.; Denamur, E.; Saumon, G.; Ricard, J.D. Candida albicans impairs macrophage function and facilitates Pseudomonas aeruginosa pneumonia in rat. Crit. Care Med. 2009, 37, 1062–1067. [Google Scholar] [CrossRef]
- Teevan-Hanman, C.; O’Shea, P. Candida albicans exhibit two classes of cell surface binding sites for serum albumin defined by their affinity, abundance and prospective role in interkingdom signaling. PLoS ONE 2021, 16, e0254593. [Google Scholar] [CrossRef]
- Schneider, F.; Clère-Jehl, R.; Scavello, F.; Lavigne, T.; Corti, A.; Angelone, T.; HaïKel, Y.; Lavalle, P. Chromogranin A and Its Fragments in the Critically Ill: An Expanding Domain of Interest for Better Care. Pharmaceutics 2022, 14, 2178. [Google Scholar] [CrossRef]
- Spada, A.; Emami, J.; Tuszynski, J.A.; Lavasanifar, A. The uniqueness of albumin as a carrier in nanodrug delivery. Mol. Pharm. 2021, 18, 1862–1894. [Google Scholar] [CrossRef]
- Vanstraelen, K.; Wauters, J.; De Loor, H.; Vercammen, I.; Annaert, P.; Lagrou, K.; Spriet, I. Protein-binding characteristics of voriconazole determined by high-throughput equilibrium dialysis. J. Pharm. Sci. 2014, 103, 2565–2570. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Daneshnia, F.; Hafez, A.; Khodavaisy, S.; Najafzadeh, M.J.; Charsizadeh, A.; Zarrinfar, H.; Salehi, M.; Shahrabadi, Z.Z.; Sasani, E.; et al. Antifungal susceptibility, genotyping, resistance mechanism, and clinical profile of Candida tropicalis blood isolates. Med. Mycol. 2020, 58, 766–773. [Google Scholar] [CrossRef]
- Pekmezovic, M.; Kaune, A.K.; Austermeier, S.; Hitzler, S.U.J.; Mogavero, S.; Hovhannisyan, H.; Gabaldón, T.; Gresnigt, M.S.; Hube, B. Human albumin enhances the pathogenic potential of Candida glabrata on vaginal epithelial cells. PLoS Pathog. 2021, 17, e1010037. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa-García, E.; Miramontes-Zapata, M.; Sánchez-Vargas, L.O.; Mondragón-Padilla, A. Oral colonisation and infection by Candida sp. in diabetic and non-diabetic patients with chronic kidney disease on dialysis. Nefrologia 2013, 33, 764–770. [Google Scholar]
- Marek, M.; Silvestro, D.; Fredslund, M.D.; Andersen, T.G.; Pomorski, T.G. Serum albumin promotes ATP-binding cassette transporter-dependent sterol uptake in yeast. FEMS Yeast Res. 2014, 14, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Riera Romo, M.; Pérez-Martínez, D.; Castillo Ferrer, C. Innate immunity in vertebrates: An overview. Immunology 2016, 148, 125–139. [Google Scholar] [CrossRef]
- Li, X.; Hu, Q.; Lin, Q.; Luo, J.; Xu, J.; Chen, L.; Xu, L.; Lin, X. Inhibition of Candida albicans in vivo and in vitro by antimicrobial peptides chromogranin A-N12 through microRNA-155/suppressor of cytokine signaling 1 axis. Bioengineered 2022, 13, 2513–2524. [Google Scholar] [CrossRef]
- Mahata, S.K.; O’Connor, D.T.; Mahata, M.; Yoo, S.H.; Taupenot, L.; Wu, H.; Gill, B.M.; Parmer, R.J. Novel autocrine feedback control of catecholamine release. A discrete chromogranin a fragment is a noncompetitive nicotinic cholinergic antagonist. J. Clin. Invest. 1997, 100, 1623–1633. [Google Scholar] [CrossRef]
- Jati, S.; Mahata, S.; Das, S.; Chatterjee, S.; Mahata, S.K. Catestatin: Antimicrobial Functions and Potential Therapeutics. Pharmaceutics 2023, 15, 1550–1568. [Google Scholar] [CrossRef]
- Briolat, J.; Wu, S.D.; Mahata, S.K.; Gonthier, B.; Bagnard, D.; Chasserot-Golaz, S.; Helle, K.B.; Aunis, D.; Metz-Boutigue, M.H. New antimicrobial activity for the catecholamine release-inhibitory peptide from chromogranin A. Cell. Mol. Life Sci. 2005, 62, 377–385. [Google Scholar] [CrossRef]
- Tsigelny, I.; Mahata, S.K.; Taupenot, L.; Preece, N.E.; Mahata, M.; Khan, I.; Parmer, R.J.; O’Connor, D.T. Mechanism of action of chromogranin A on catecholamine release: Molecular modeling of the catestatin region reveals a beta-strand/loop/beta-strand structure secured by hydrophobic interactions and predictive of activity. Regul. Pept. 1998, 77, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, M.; Resende, J.M.; Moraes, C.M.; Marquette, A.; Chich, J.F.; Metz-Boutigue, M.H.; Bechinger, B. Membrane structure and interactions of human catestatin by multidimensional solution and solid-state NMR spectroscopy. FASEB J. 2010, 24, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shooshtarizadeh, P.; Laventie, B.J.; Colin, D.A.; Chich, J.F.; Vidic, J.; de Barry, J.; Chasserot-Golaz, S.; Delalande, F.; Van Dorsselaer, A.; et al. Two chromogranin a-derived peptides induce calcium entry in human neutrophils by calmodulin-regulated calcium independent phospholipase A2. PLoS ONE 2009, 4, e4501. [Google Scholar] [CrossRef]
- Jean-François, F.; Castano, S.; Desbat, B.; Odaert, B.; Roux, M.; Metz-Boutigue, M.H.; Dufourc, E.J. Aggregation of cateslytin beta-sheets on negatively charged lipids promotes rigid membrane domains. A new mode of action for antimicrobial peptides? Biochemistry 2008, 47, 6394–6402. [Google Scholar] [CrossRef]
- Sivertsen, A.; Isaksson, J.; Leiros, H.K.; Svenson, J.; Svendsen, J.S.; Brandsdal, B.O. Synthetic cationic antimicro bial peptides bind with their hydrophobic parts to drug site II of human serum albumin. BMC Struct. Biol. 2014, 14, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Rabbi, M.F.; Eissa, N.; Munyaka, P.M.; Kermarrec, L.; Elgazzar, O.; Khafipour, E.; Bernstein, C.N.; Ghia, J.E. Reactivation of Intestinal Inflammation Is Suppressed by Catestatin in a Murine Model of Colitis via M1 Macrophages and Not the Gut Microbiota. Front. Immunol. 2017, 8, 985–1001. [Google Scholar] [CrossRef]
- Aslam, R.; Marban, C.; Corazzol, C.; Jehl, F.; Delalande, F.; Van Dorsselaer, A.; Prévost, G.; Haïkel, Y.; Taddei, C.; Schneider, F.; et al. Cateslytin, a chromogranin A derived peptide is active against Staphylococcus aureus and resistant to degradation by its proteases. PLoS ONE 2013, 8, e68993. [Google Scholar] [CrossRef] [PubMed]
| (A) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C. albicans (S) | C. tropicalis (S) | C. glabrata (S) | C. lusitaniae (S) | ||||||||||||||||
| ThHSA (mg/mL) | 0.0 | 1.25 | 2.50 | 5.00 | 0.0 | 1.25 | 2.50 | 5.00 | 0.0 | 1.25 | 2.50 | 5.00 | 10.00 | 0.0 | 1.25 | 2.50 | 5.00 | 10.00 | |
| VCZ (μg/mL) | |||||||||||||||||||
| 0.0000 | 0.0 | 55.5 | 68.9 | 77.9 | 0.0 | 21.7 | 28.7 | 39.1 | 0.0 | 38.9 | 45.6 | 55.5 | 63.1 | 0.0 | 46.8 | 51.2 | 54.7 | 51.8 | |
| 0.0156 | 6.6 | 31.9 | 56.5 | 72.6 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| 0.0312 | 19.8 | 34.8 | 64.3 | 73.5 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| 0.0625 | 18.7 | 21.9 | 73.9 | 75.3 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| 0.1250 | 44.8 | 50.1 | 67.1 | 70.6 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| 0.1560 | -- | -- | -- | -- | 10.1 | 7.4 | 29.3 | 38.7 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| 0.2500 | 83.6 | 97.9 | 99.8 | 95.6 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| 0.3120 | -- | -- | -- | -- | 7.6 | 7.4 | 31.3 | 42.1 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| 0.6250 | -- | -- | -- | -- | 8.2 | 10.9 | 34.5 | 40.4 | 8.2 | 63.7 | 44.5 | 52.1 | 58.6 | −25.1 | 29.3 | 57.1 | 41.7 | 64.9 | |
| 1.2500 | -- | -- | -- | -- | 10.8 | 8.4 | 20.4 | 42.3 | 30.2 | 55.1 | 42.3 | 53.1 | 61.9 | 34.1 | 35.5 | 50.7 | 50.9 | 72.7 | |
| 2.5000 | -- | -- | -- | -- | 45.3 | 52.5 | 51.3 | 56.9 | 60.5 | 49.9 | 50.2 | 57.8 | 64.7 | 68.6 | 68.5 | 57.3 | 70.7 | 65.5 | |
| 5.0000 | -- | -- | -- | -- | -- | -- | -- | -- | 84.1 | 75.2 | 77.1 | 86.6 | 84.9 | 85.9 | 97.7 | 98.4 | 97.4 | 95.8 | |
| 10.0000 | -- | -- | -- | -- | -- | -- | -- | -- | 93.3 | 89.0 | 90.0 | 92.9 | 93.4 | 89.2 | 98.9 | 100 | 100 | 100 | |
| (B) | |||||||||||||||||||
| C. albicans (R) | C. tropicalis (R) | C. glabrata (R) | |||||||||||||||||
| ThHSA (mg/mL) | 0.0 | 1.25 | 2.50 | 5.00 | 0.0 | 1.25 | 2.50 | 5.00 | 10.00 | 0.0 | 1.25 | 2.50 | 5.00 | 10.00 | |||||
| VCZ (μg/mL) | |||||||||||||||||||
| 0.0000 | 0.0 | 58.5 | 63.2 | 76.5 | 0.0 | 1.0 | 5.4 | 20.7 | 21.3 | 0.0 | 35.3 | 50.2 | 81.1 | 82.2 | |||||
| 0.0156 | 12.4 | 58.7 | 71.4 | 79.6 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |||||
| 0.0312 | 11.1 | 59.5 | 72.5 | 79.3 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |||||
| 0.0625 | 9.5 | 57.2 | 77.5 | 77.8 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |||||
| 0.1250 | 33.8 | 59.5 | 72.5 | 79.8 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |||||
| 0.2500 | 91.2 | 93.9 | 97.6 | 90.9 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |||||
| 0.6250 | -- | -- | -- | -- | 18.6 | −55.6 | −66.5 | 0.0 | 0.8 | −4.6 | 58.7 | 40.8 | 43.2 | 43.6 | |||||
| 1.2500 | -- | -- | -- | -- | 16.6 | −54.4 | −63.8 | −10.0 | 7.6 | −4.5 | 59.5 | 40.2 | 43.1 | 43.9 | |||||
| 2.5000 | -- | -- | -- | -- | 18.4 | −60.3 | −59.1 | 1.0 | 14.6 | −0.1 | 57.2 | 34.9 | 45.6 | 49.2 | |||||
| 5.0000 | -- | -- | -- | -- | 17.4 | −64.9 | −52.6 | 1.6 | 14.0 | −3.0 | 59.5 | 44.6 | 36.9 | 50.1 | |||||
| 10.0000 | -- | -- | -- | -- | 15.7 | −55.5 | −55.4 | 1.4 | 21.8 | −3.5 | 43.9 | 60.3 | 55.2 | 65.2 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider, F.; Hellé, S.; Strub, J.-M.; von Hunolstein, F.-X.; Schaaf, P.; Lavalle, P.; Scavello, F.; Metz-Boutigue, M.-H. Insight into the Roles of Albumin—Alone and in Combination with Either Voriconazole or Antimicrobial Peptides Derived from Chromogranin A—In the Growth of Different Microbial Species. Antibiotics 2025, 14, 974. https://doi.org/10.3390/antibiotics14100974
Schneider F, Hellé S, Strub J-M, von Hunolstein F-X, Schaaf P, Lavalle P, Scavello F, Metz-Boutigue M-H. Insight into the Roles of Albumin—Alone and in Combination with Either Voriconazole or Antimicrobial Peptides Derived from Chromogranin A—In the Growth of Different Microbial Species. Antibiotics. 2025; 14(10):974. https://doi.org/10.3390/antibiotics14100974
Chicago/Turabian StyleSchneider, Francis, Sophie Hellé, Jean-Marc Strub, François-Xavier von Hunolstein, Pierre Schaaf, Philippe Lavalle, Francesco Scavello, and Marie-Hélène Metz-Boutigue. 2025. "Insight into the Roles of Albumin—Alone and in Combination with Either Voriconazole or Antimicrobial Peptides Derived from Chromogranin A—In the Growth of Different Microbial Species" Antibiotics 14, no. 10: 974. https://doi.org/10.3390/antibiotics14100974
APA StyleSchneider, F., Hellé, S., Strub, J.-M., von Hunolstein, F.-X., Schaaf, P., Lavalle, P., Scavello, F., & Metz-Boutigue, M.-H. (2025). Insight into the Roles of Albumin—Alone and in Combination with Either Voriconazole or Antimicrobial Peptides Derived from Chromogranin A—In the Growth of Different Microbial Species. Antibiotics, 14(10), 974. https://doi.org/10.3390/antibiotics14100974







