Stability of Two Reserve Antibiotics in Elastomeric Pumps: Ceftazidime-Avibactam and Ceftolozane-Tazobactam
Abstract
1. Introduction
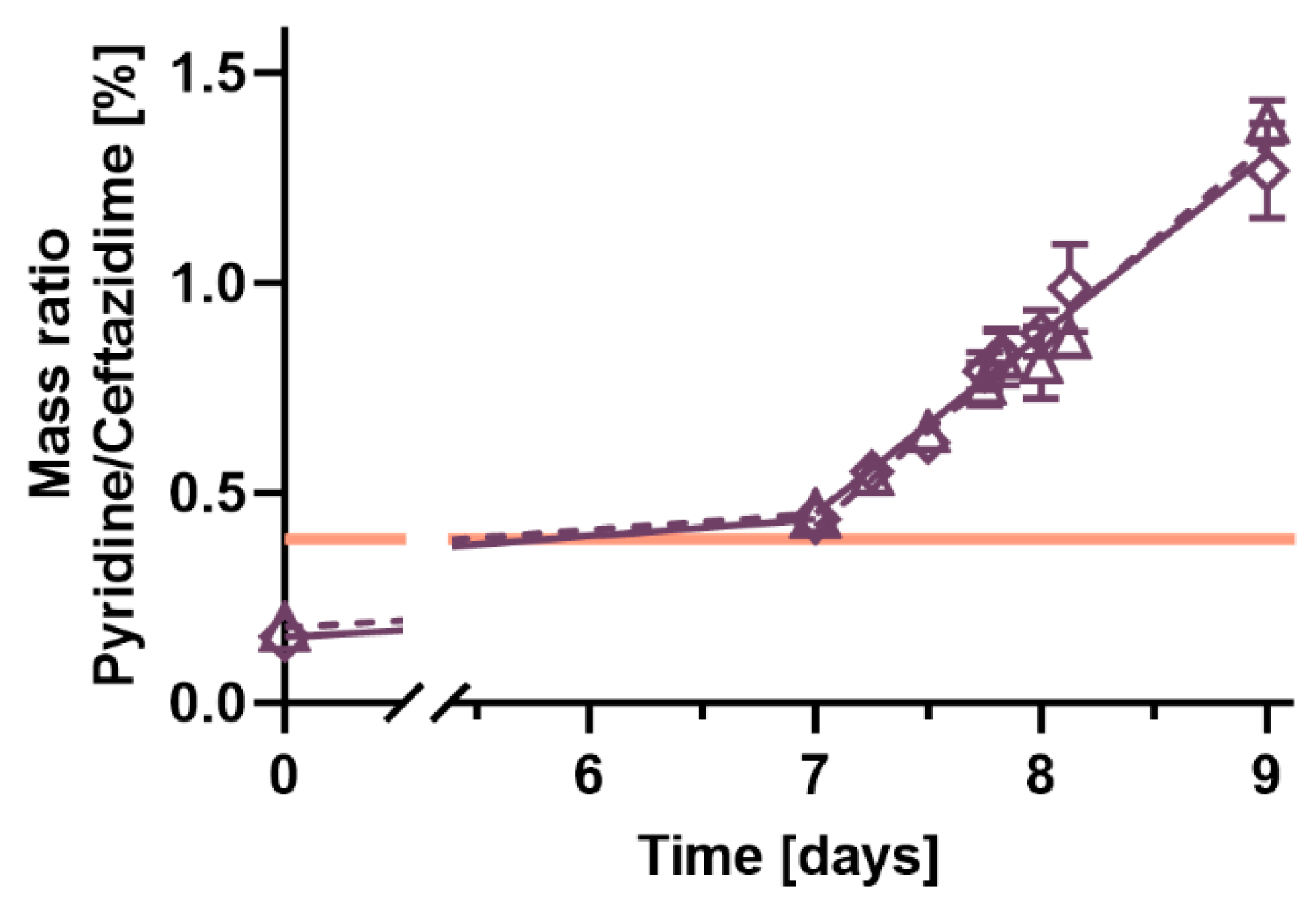
2. Results
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OPAT | Outpatient parenteral antimicrobial therapy |
| Ph. Eur. | European Pharmacopoeia |
| HPLC | High-performance liquid chromatography |
| MS | Mass spectrometer |
| QC | Quality control |
References
- van Duin, D.; Bonomo, R.A. Ceftazidime/Avibactam and Ceftolozane/Tazobactam: Second-generation beta-Lactam/beta-Lactamase Inhibitor Combinations. Clin. Infect. Dis. 2016, 63, 234–241. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Alffenaar, J.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- Erba, A.; Beuret, M.; Daly, M.L.; Khanna, N.; Osthoff, M. OPAT in Switzerland: Single-center experience of a model to treat complicated infections. Infection 2020, 48, 231–240. [Google Scholar] [CrossRef]
- Burch, A.R.; Ledergerber, B.; Ringer, M.; Padrutt, M.; Reiber, C.; Mayer, F.; Zinkernagel, A.S.; Eberhard, N.; Kaelin, M.B.; Hasse, B. Improving antimicrobial treatment in terms of antimicrobial stewardship and health costs by an OPAT service. Infection 2024, 52, 1367–1376. [Google Scholar] [CrossRef]
- Briquet, C.; Cornu, O.; Servais, V.; Blasson, C.; Vandeleene, B.; Yildiz, H.; Stainier, A.; Yombie, J.C. Clinical characteristics and outcomes of patients receiving outpatient parenteral antibiotic therapy in a Belgian setting: A single-center pilot study. Acta Clin. Belg. 2020, 75, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.L.N.; Seaton, R.A.; Cooper, M.A.; Hedderwick, S.; Goodall, V.; Reed, C.; Sanderson, F.; Nathwani, D.; Good, B.B.O.P. Good practice recommendations for outpatient parenteral antimicrobial therapy (OPAT) in adults in the UK: A consensus statement. J. Antimicrob. Chemother. 2012, 67, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Voumard, R.; Van Neyghem, N.; Cochet, C.; Gardiol, C.; Decosterd, L.; Buclin, T.; de Valliere, S. Antibiotic stability related to temperature variations in elastomeric pumps used for outpatient parenteral antimicrobial therapy (OPAT). J. Antimicrob. Chemother. 2017, 72, 1462–1465. [Google Scholar] [CrossRef] [PubMed]
- Loeuille, G.; D’Huart, E.; Vigneron, J.; Nisse, Y.E.; Beiler, B.; Polo, C.; Ayari, G.; Sacrez, M.; Demore, B.; Charmillon, A. Stability Studies of 16 Antibiotics for Continuous Infusion in Intensive Care Units and for Performing Outpatient Parenteral Antimicrobial Therapy. Antibiotics 2022, 11, 458. [Google Scholar] [CrossRef]
- Naicker, S.; Roberts, J.A.; Won, H.; Wallis, S.C.; Unwin, S.; Jamieson, C.; Hills, T.; Gilchrist, M.; Santillo, M.; Seaton, R.A.; et al. Evaluation of the stability of ceftazidime/avibactam in elastomeric infusion devices used for outpatient parenteral antimicrobial therapy utilizing a national stability protocol framework. JAC Antimicrob. Resist. 2024, 6, dlae056. [Google Scholar] [CrossRef]
- Fernández-Rubio, B.; Herrera-Hidalgo, L.; de Alarcón, A.; Luque-Márquez, R.; López-Cortés, L.E.; Luque, S.; Gutiérrez-Urbón, J.M.; Fernández-Polo, A.; Gutiérrez-Valencia, A.; Gil-Navarro, M.V. Stability Studies of Antipseudomonal Beta Lactam Agents for Outpatient Therapy. Pharmaceutics 2023, 15, 2705. [Google Scholar] [CrossRef]
- Viaene, E.; Chanteux, H.; Servais, H.; Mingeot-Leclercq, M.P.; Tulkens, P.M. Comparative stability studies of antipseudomonal beta-lactams for potential administration through portable elastomeric pumps (home therapy for cystic fibrosis patients) and motor-operated syringes (intensive care units). Antimicrob. Agents Chemother. 2002, 46, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Menten, L.; Spriet, I.; Quintens, C.; Van Schepdael, A.; Adams, E. Liquid chromatographic method to follow-up ceftazidime and pyridine in portable elastomeric infusion pumps over 24 h. Electrophoresis 2022, 43, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Stendal, T.L.; Klem, W.; Tonnesen, H.H.; Kjonniksen, I. Drug stability and pyridine generation in ceftazidime injection stored in an elastomeric infusion device. Am. J. Health Syst. Pharm. 1998, 55, 683–685. [Google Scholar] [CrossRef]
- Walker, S.E.; Iazzetta, J.; Law, S.; Biniecki, K. Stability of commonly used antibiotic solutions in an elastomeric infusion device. Can. J. Hosp. Pharm. 2010, 63, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Zajac, M.; Siwek, J.; Muszalska, I. The mechanism of ceftazidime degradation in aqueous solutions. Acta Pol. Pharm. 1998, 55, 275–278. [Google Scholar]
- Bourget, P.; Amin, A.; Dupont, C.; Abely, M.; Desmazes-Dufeu, N.; Dubus, J.C.; Jouani, B.L.; Merlette, C.; Nove-Josserand, R.; Pages, J.; et al. How To Minimize Toxic Exposure to Pyridine during Continuous Infusion of Ceftazidime in Patients with Cystic Fibrosis? Antimicrob. Agents Chemother. 2014, 58, 2849–2855. [Google Scholar] [CrossRef]
- Ceftazidimum pentahydricum et natrii carbonas ad iniectabile, monograph 2344. In European Pharmacopoeia, 10.0; Council of Europe: Strasbourg, France, 2020; pp. 3200–3203.
- Raby, E.; Naicker, S.; Sime, F.B.; Manning, L.; Wallis, S.C.; Pandey, S.; Roberts, J.A. Ceftolozane-tazobactam in an elastomeric infusion device for ambulatory care: An in vitro stability study. Eur. J. Hosp. Pharm. 2020, 27, e84–e86. [Google Scholar] [CrossRef]
- Jamieson, C.; Drummond, F.; Hills, T.; Ozolina, L.; Gilchrist, M.; Seaton, R.A.; Santillo, M.; Wilkinson, A.S.; Allwood, M.C. Assessment of ceftolozane/tazobactam stability in elastomeric devices and suitability for continuous infusion via outpatient parenteral antimicrobial therapy. JAC Antimicrob. Resist. 2021, 3, dlab141. [Google Scholar] [CrossRef]
- Terracciano, J.; Rhee, E.G.; Walsh, J. Chemical Stability of Ceftolozane/Tazobactam in Polyvinylchloride Bags and Elastomeric Pumps. Curr. Ther. Res. Clin. Exp. 2017, 84, 22–25. [Google Scholar] [CrossRef]
- Jamieson, C.; Drummond, F.; Ozolina, L.; Wilkinson, A.S. Stability Testing of Ceftazidime Solutions for Injection in Elastomeric Devices at 12 mg/mL and 25 mg/mL in 0.9% w/v Saline for Safe Use in Outpatient Parenteral Antimicrobial Therapy (OPAT). BSAC OPAT Conference 2019, 11 December 2019. Available online: https://e-opat.com/wp-content/uploads/2020/01/OPAT2019-CAZPoster-28Nov.pdf (accessed on 16 January 2023).
- Jones, T.E.; Selby, P.R.; Mellor, C.S.; Cheam, D.B. Ceftazidime stability and pyridine toxicity during continuous i.v. infusion. Am. J. Health-Syst. Pharm. 2019, 76, 200–205. [Google Scholar] [CrossRef]
- Negrier, L.; Mena, A.M.; Dupont, C.; Gamache, P.; Zimbril, J.O.; Abdoune, Y.; Karrout, Y.; Odou, P.; Genay, S.; Decaudin, B. The Infusion of Piperacillin/Tazobactam with an Elastomeric Device: A Combined 24-H Stability Study and Drug Solution Flow Rate Analysis. Pharmaceuticals 2024, 17, 1085. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Cartelle, B.; Vicente-Oliveros, N.; Menéndez-Conde, C.P.; Serrano, D.R.; Martín-Dávila, P.; Fortún-Abete, J.; León-Gil, L.A.; Alvarez-Díaz, A. Antibiotic stability in portable elastomeric infusion devices: A systematic review. Am. J. Health-Syst. Pharm. 2022, 79, 1355–1368. [Google Scholar] [CrossRef] [PubMed]
- Committee for Medicinal Products for Human Use. ICH Guideline M10 on Bioanalytical Method Validation and Study Sample Analysis Step 5; EMA/CHMP/ICH/172948/2019; Committee for Medicinal Products for Human Use: Amsterdam, The Netherlands, 2022. [Google Scholar]

| Analyte | Spiked Concentration [mg/L] | Intraday | Interday | ||
|---|---|---|---|---|---|
| Accuracy [%] | Coefficient of Variation [%] | Accuracy [%] | Coefficient of Variation [%] | ||
| Ceftolozane | 2.0 | 112 | 5.0 | 105 | 12 |
| 5.0 | 96.6 | 4.3 | 96.2 | 4.4 | |
| 21.0 | 96.5 | 2.9 | 97.8 | 5.0 | |
| 52.5 | 101 | 1.4 | 101 | 2.7 | |
| Tazobactam | 1.0 | 97.0 | 6.9 | 99.0 | 2.7 |
| 2.5 | 95.4 | 5.2 | 100 | 2.4 | |
| 10.5 | 95.8 | 4.0 | 97.6 | 9.8 | |
| 26.3 | 94.0 | 3.5 | 105 | 5.6 | |
| Ceftazidime | 2.0 | 92.6 | 4.0 | 94.2 | 3.5 |
| 5.0 | 93.4 | 1.2 | 94.3 | 0.7 | |
| 21.0 | 96.8 | 1.2 | 97.7 | 0.7 | |
| 52.5 | 97.5 | 0.6 | 96.6 | 1.1 | |
| Avibactam | 1.0 | 95.4 | 4.4 | 100 | 6.5 |
| 2.5 | 99.2 | 2.5 | 98.7 | 2.4 | |
| 6.6 | 100 | 3.1 | 101 | 2.9 | |
| 16.5 | 98.7 | 3.6 | 101 | 4.6 | |
| Pyridine | 0.005 | 104 | 8.6 | 93.3 | 12 |
| 0.015 | 101 | 9.8 | 104 | 7.4 | |
| 0.095 | 97.1 | 2.7 | 97.5 | 0.6 | |
| 0.195 | 94.8 | 1.9 | 98.3 | 4.1 | |
| 0.400 | 98.4 | 2.1 | 98.8 | 1.0 | |
| Step | Duration [s] | Loading Pump | Eluting Pump | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Flow [mL/min] | A | B | C | Flow [mL/min] | A | B | C | ||
| 1 | 30 | 1.5 | 100 | 0 | 0 | 0.6 | 95 | 5 | 0 |
| 2 | 30 | 0.3 | 100 | 0 | 0 | 0.3 | 95 | 5 | 0 |
| 3 | 90 | 1.5 | 100 → 0 | 0 → 100 | 0 | 0.6 | 95 → 50 | 5 → 50 | 0 |
| 4 | 90 | 0.5 | 0 | 100 | 0 | 0.6 | 50 | 50 | 0 |
| 5 | 60 | 0.5 | 0 | 0 | 100 | 0.6 | 50 → 20 | 50 → 80 | 0 |
| 6 | 60 | 1.5 | 0 | 100 | 0 | 0.6 | 20 | 80 | 0 |
| 7 | 30 | 0.5 | 0 | 100 | 0 | 0.6 | 0 | 0 | 100 |
| 8 | 60 | 1.5 | 80 | 20 | 0 | 0.6 | 95 | 5 | 0 |
| 9 | 120 | 1.5 | 100 | 0 | 0 | 0.6 | 95 | 5 | 0 |
| Step | Duration [s] | Loading Pump | Eluting Pump | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Flow [mL/min] | A | B | C | Flow [mL/min] | A | B | C | ||
| 1 | 30 | 1.5 | 100 | 0 | 0 | 0.5 | 99 | 1 | 0 |
| 2 | 30 | 0.3 | 100 | 0 | 0 | 0.2 | 99 | 1 | 0 |
| 3 | 60 | 1.5 | 100 → 0 | 0 → 100 | 0 | 0.5 | 99 | 1 | 0 |
| 4 | 60 | 1.5 | 0 | 100 | 0 | 0.5 | 99 → 50 | 1 → 50 | 0 |
| 5 | 60 | 0.5 | 0 | 0 | 100 | 0.5 | 50 | 50 | 0 |
| 6 | 30 | 0.5 | 0 | 100 | 0 | 0.5 | 50 | 50 | 0 |
| 7 | 30 | 0.5 | 0 | 100 | 0 | 0.5 | 0 | 0 | 100 |
| 8 | 60 | 1.5 | 70 | 30 | 0 | 0.5 | 99 | 1 | 0 |
| 9 | 120 | 0.5 | 100 | 0 | 0 | 0.5 | 99 | 1 | 0 |
| Step | Duration [s] | Loading Pump | Eluting Pump | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Flow [mL/min] | A | B | C | D | Flow [mL/min] | A | B | C | ||
| 1 | 15 | 1.5 | 0 | 0 | 0 | 100 | 0.6 | 98 | 2 | 0 |
| 2 | 30 | 0.4 | 98 | 2 | 0 | 0 | 0.1 | 98 | 2 | 0 |
| 3 | 180 | 0.5 | 0 | 100 | 0 | 0 | 0.6 | 98 → 10 | 2 → 90 | 0 |
| 4 | 105 | 0.5 | 0 | 0 | 100 | 0 | 0.6 | 10 | 90 | 0 |
| 5 | 30 | 0.5 | 0 | 100 | 0 | 0 | 0.6 | 0 | 0 | 100 |
| 6 | 60 | 1.5 | 90 | 10 | 0 | 0 | 0.6 | 98 | 2 | 0 |
| 7 | 120 | 1.5 | 0 | 0 | 0 | 100 | 0.6 | 98 | 2 | 0 |
| HPLC-MS Method | Analyte | Polarity | Precursor m/z | Quantifier m/z Qualifier m/z | Collision Energy [V] |
|---|---|---|---|---|---|
| Ceftazidime-avibactam T1 | Ceftazidime | Positive | 547 | 396 277 468 | 19.7 16.3 11.9 |
| Ceftazidime-d5 | Positive | 552 | 396 277 468 | 19.2 15.5 10.3 | |
| Avibactam | Negative | 264 | 96.0 80.2 97.1 | 24.1 26.0 18.9 | |
| 13C5-Avibactam | Negative | 269 | 96.0 80.2 97.0 | 29.7 26.1 19.7 | |
| Ceftolozane-tazobactam T2 | Ceftolozane | Positive | 667 | 199 397 607 | 10.3 17.8 14.4 |
| Ceftolozane-d6 | Positive | 673 | 205 139 607 | 33.5 11.1 25.1 | |
| Tazobactam | Positive | 301 | 168 207 283 | 13.3 14.1 10.3 | |
| 15N3-Tazobactam | Positive | 304 | 208 168 286 | 13.1 14.5 10.3 | |
| Pyridine O | Pyridine | Positive | 80.0500 | Precursor m/z in full scan | |
| Pyridine-d5 | Positive | 85.0500 | Precursor m/z in full scan | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdmann, J.; Vu, L.A.T.; Halbeisen, D.; Rentsch, K.M. Stability of Two Reserve Antibiotics in Elastomeric Pumps: Ceftazidime-Avibactam and Ceftolozane-Tazobactam. Antibiotics 2025, 14, 966. https://doi.org/10.3390/antibiotics14100966
Erdmann J, Vu LAT, Halbeisen D, Rentsch KM. Stability of Two Reserve Antibiotics in Elastomeric Pumps: Ceftazidime-Avibactam and Ceftolozane-Tazobactam. Antibiotics. 2025; 14(10):966. https://doi.org/10.3390/antibiotics14100966
Chicago/Turabian StyleErdmann, Joana, Linh Anna Trúc Vu, Delia Halbeisen, and Katharina M. Rentsch. 2025. "Stability of Two Reserve Antibiotics in Elastomeric Pumps: Ceftazidime-Avibactam and Ceftolozane-Tazobactam" Antibiotics 14, no. 10: 966. https://doi.org/10.3390/antibiotics14100966
APA StyleErdmann, J., Vu, L. A. T., Halbeisen, D., & Rentsch, K. M. (2025). Stability of Two Reserve Antibiotics in Elastomeric Pumps: Ceftazidime-Avibactam and Ceftolozane-Tazobactam. Antibiotics, 14(10), 966. https://doi.org/10.3390/antibiotics14100966






