Microalgae, Cell Factories for Antimicrobial Peptides: A Promising Response to Antibiotic Resistance
Abstract
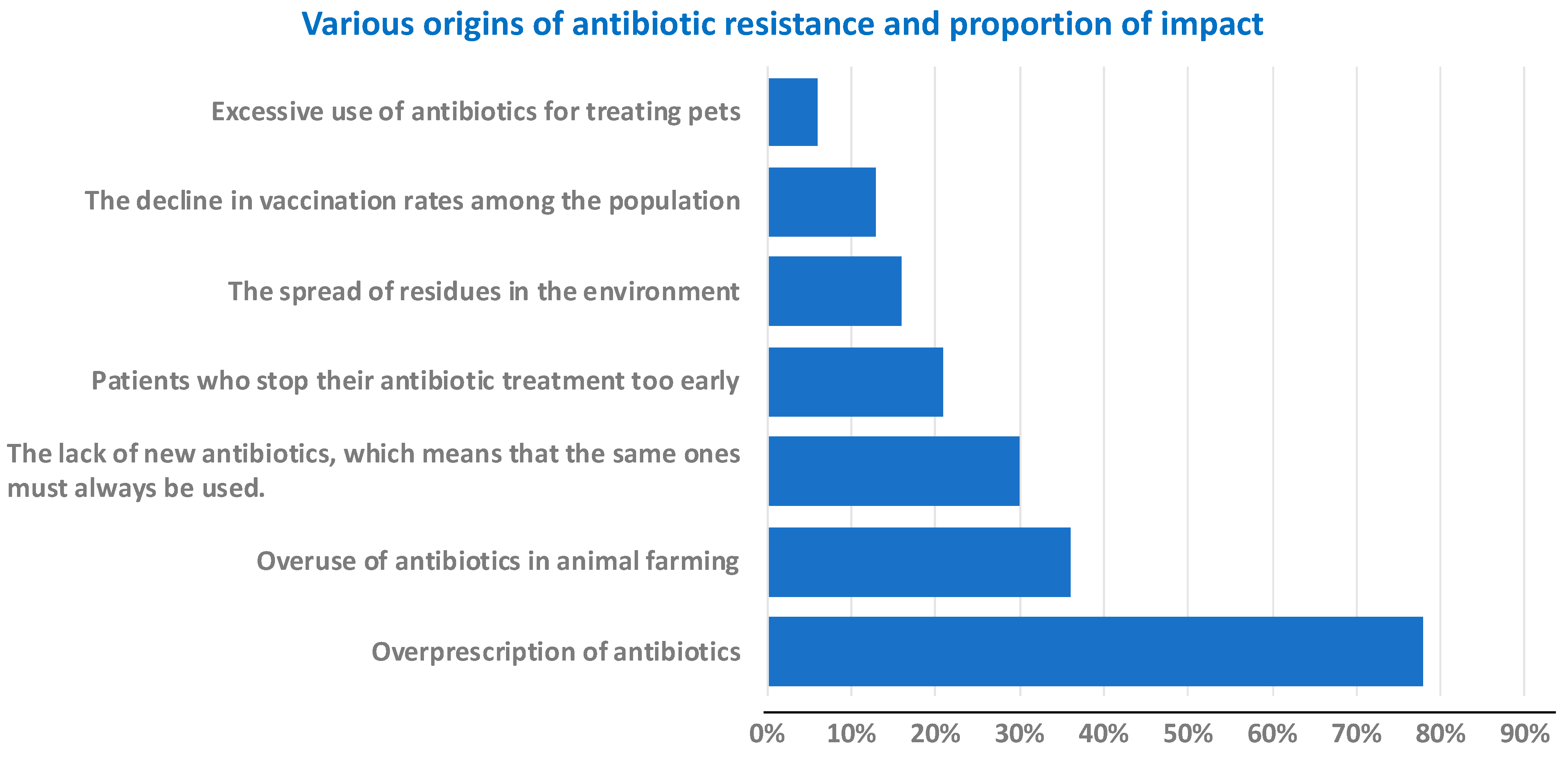
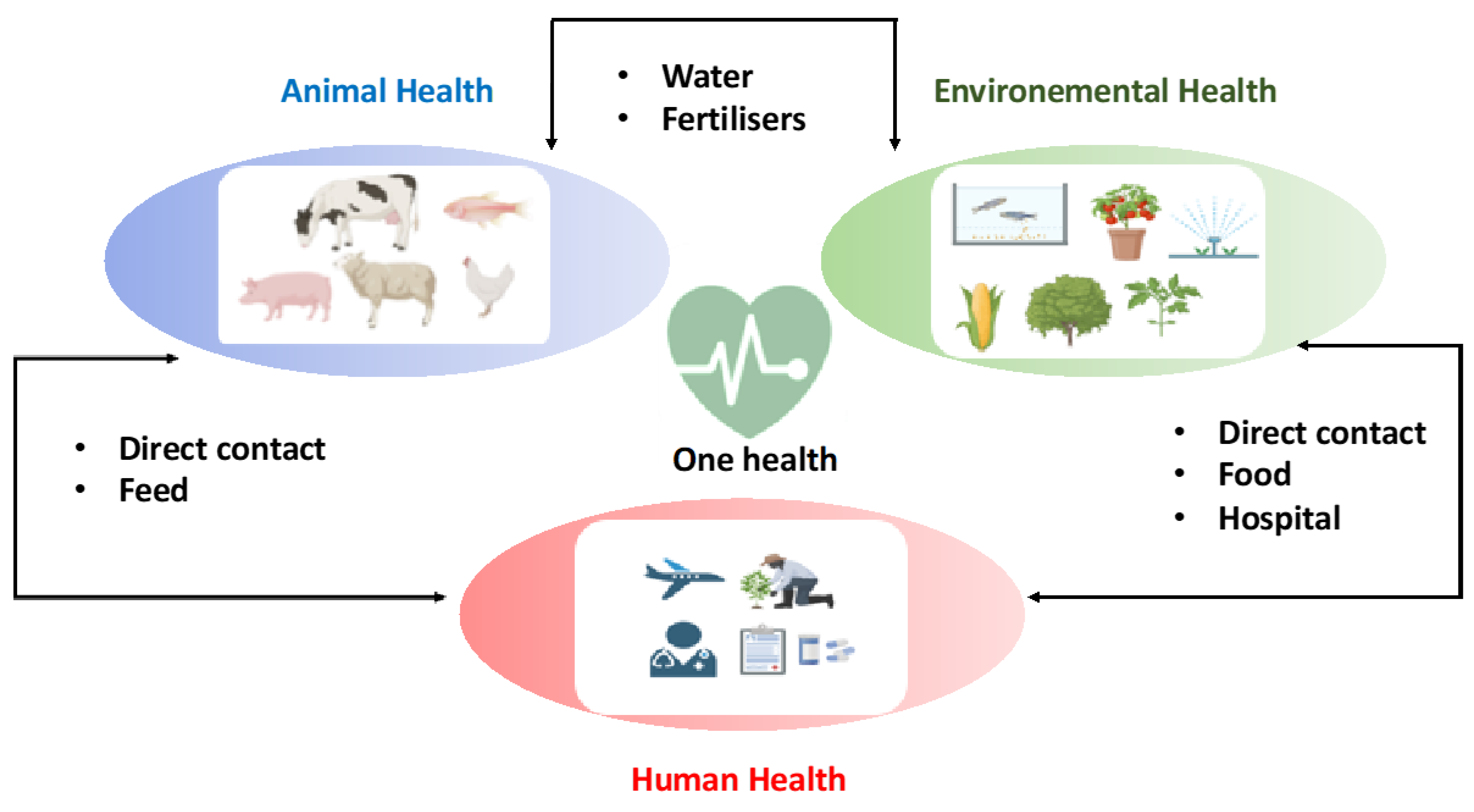
1. Introduction
2. Results and Discussion
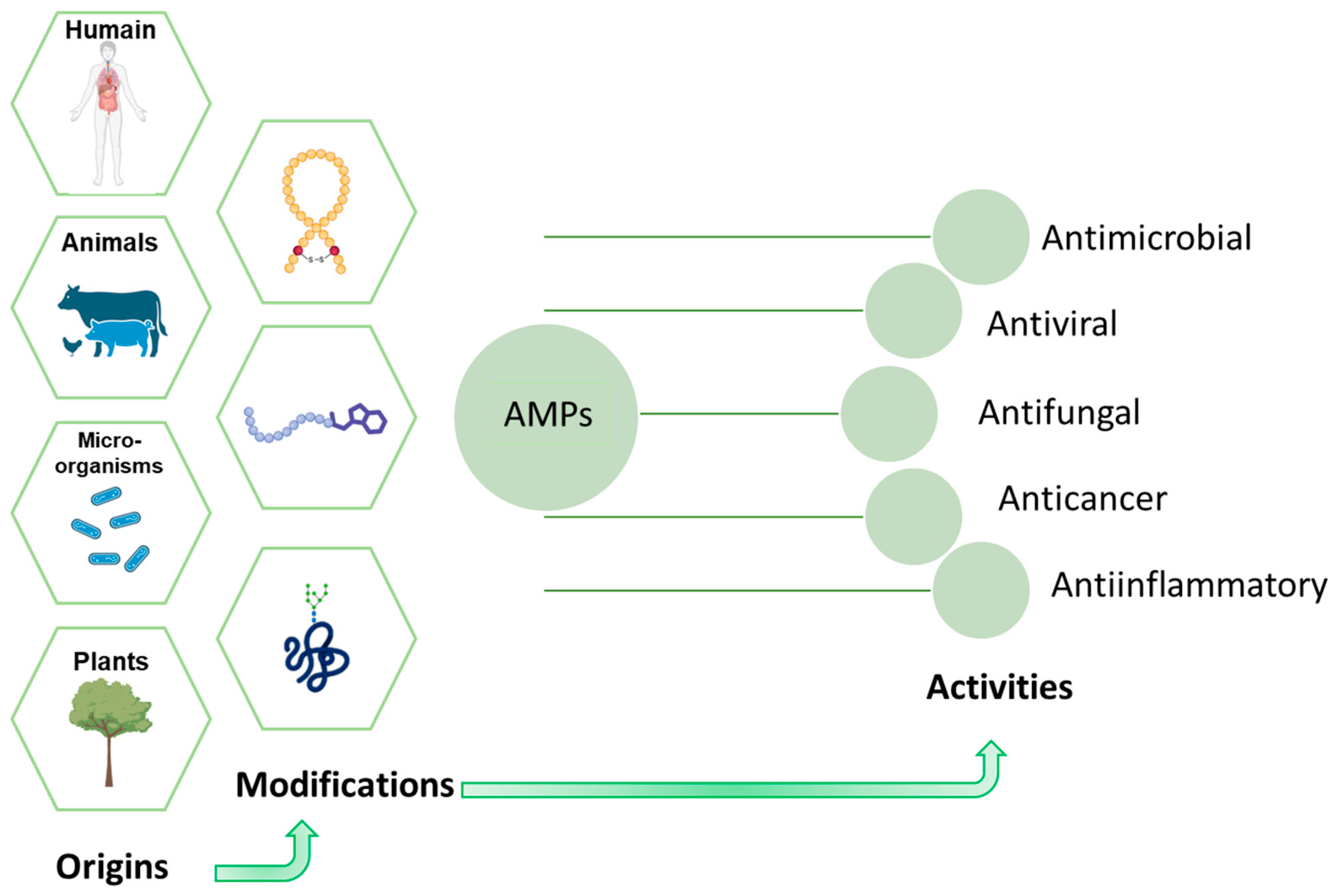
2.1. Antimicrobial Peptides (AMPs)
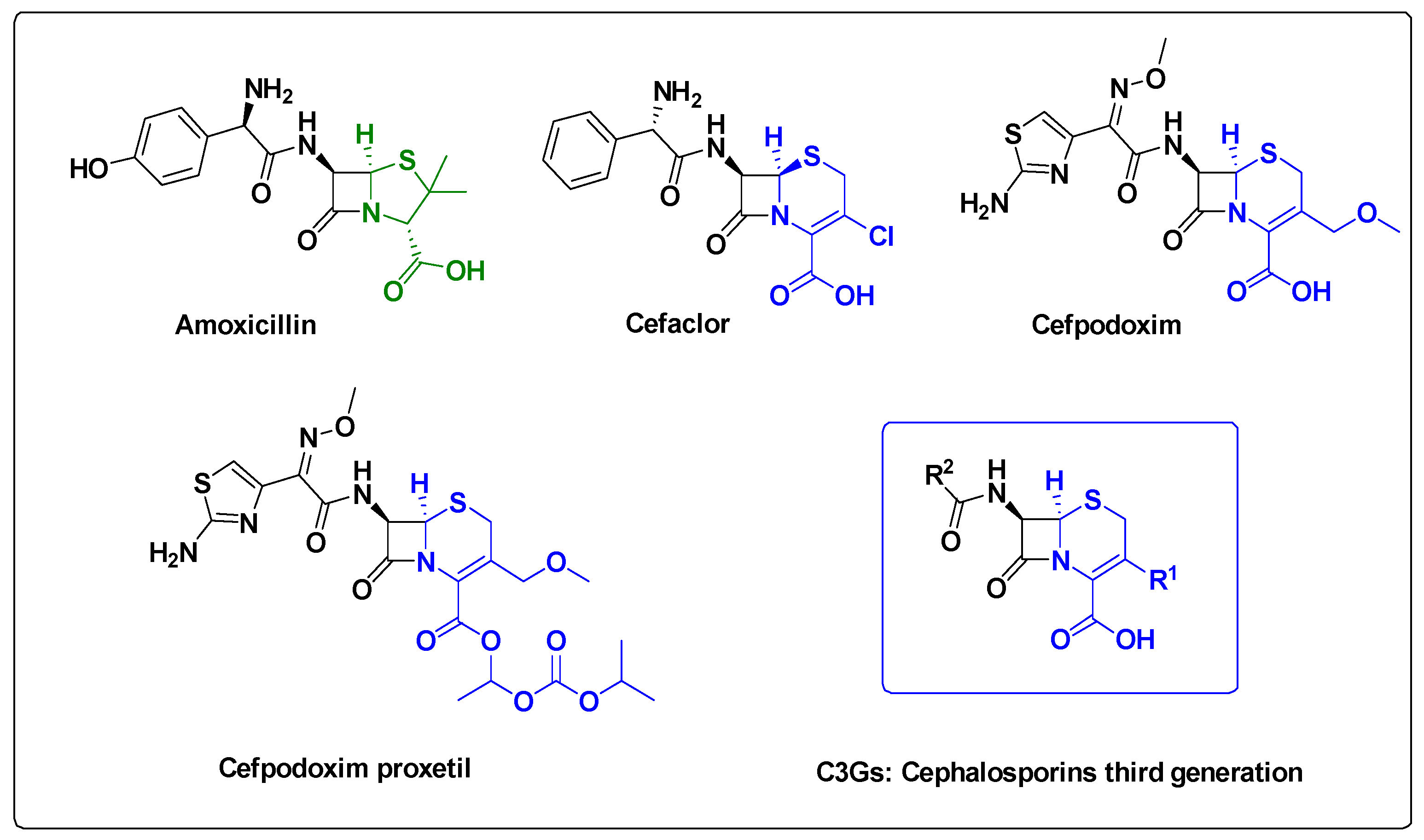
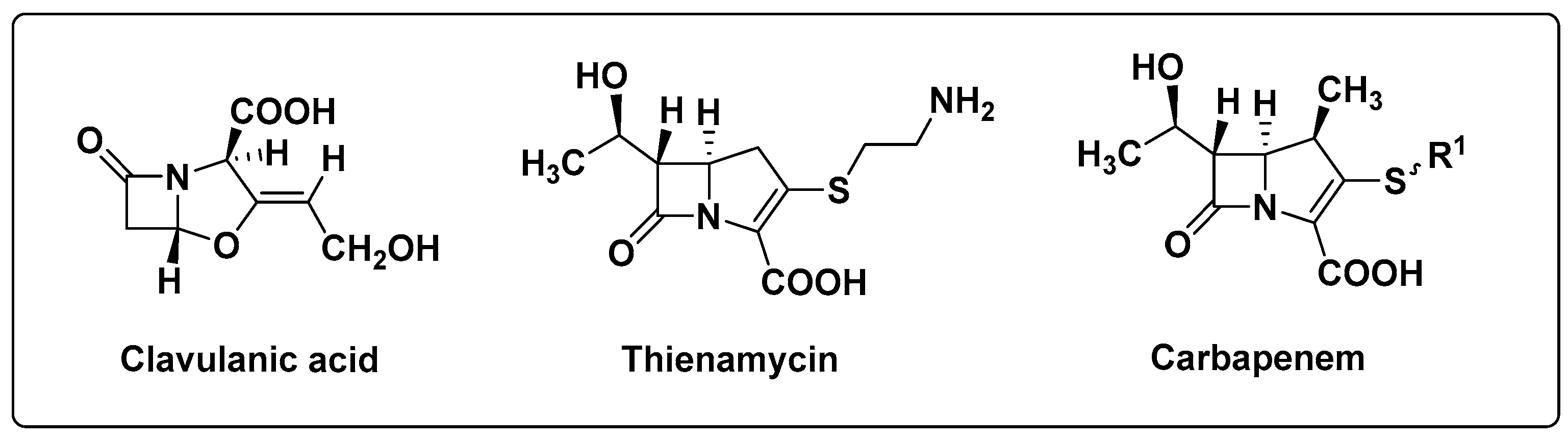
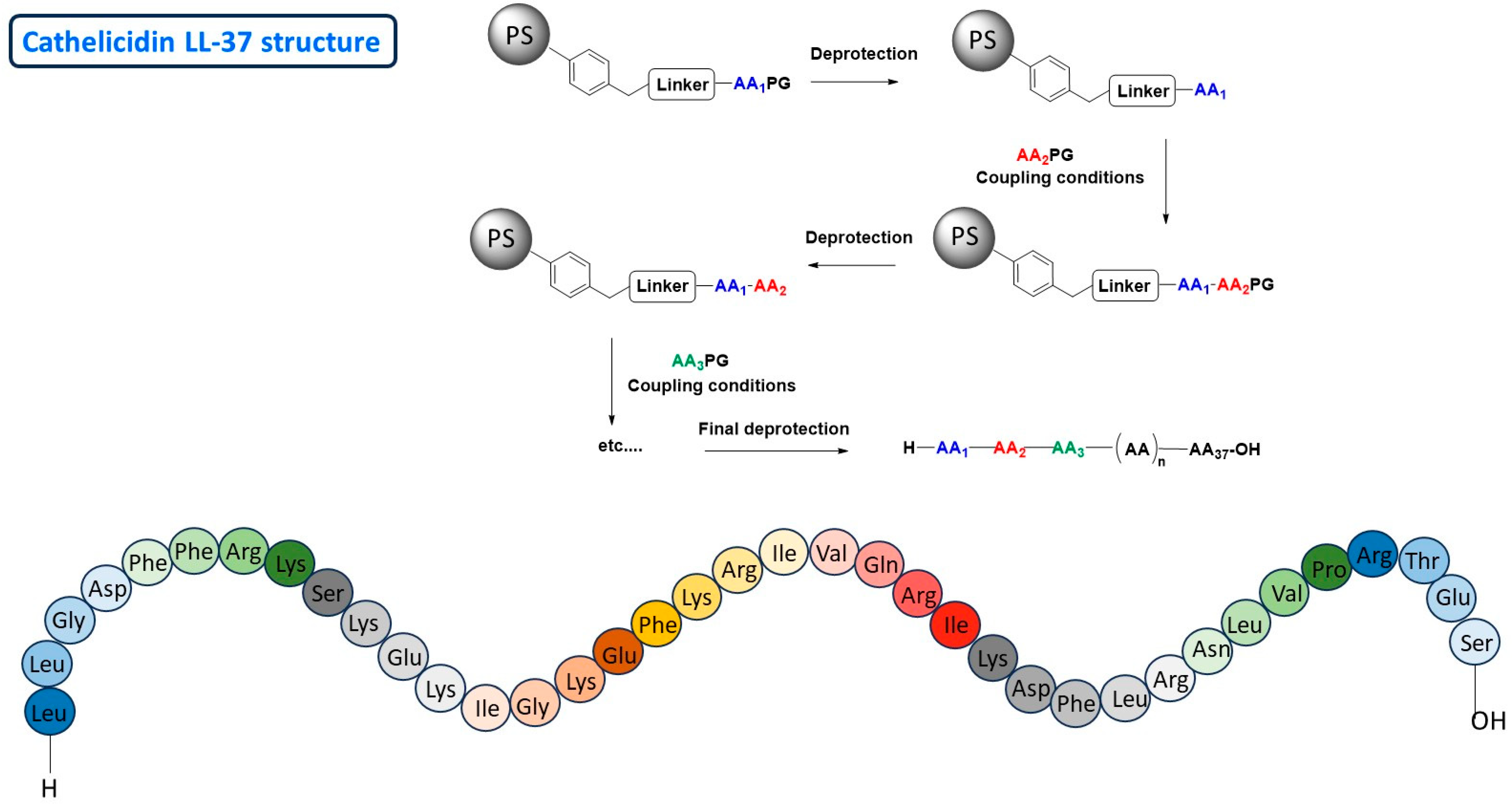
2.1.1. Chemical Synthesis Approach
2.1.2. Bioproduction Approach
2.2. Microalgae
2.2.1. Microalgae as Sustainable Biotechnology Platforms: Cultivation, Safety and Biotechnological Potential
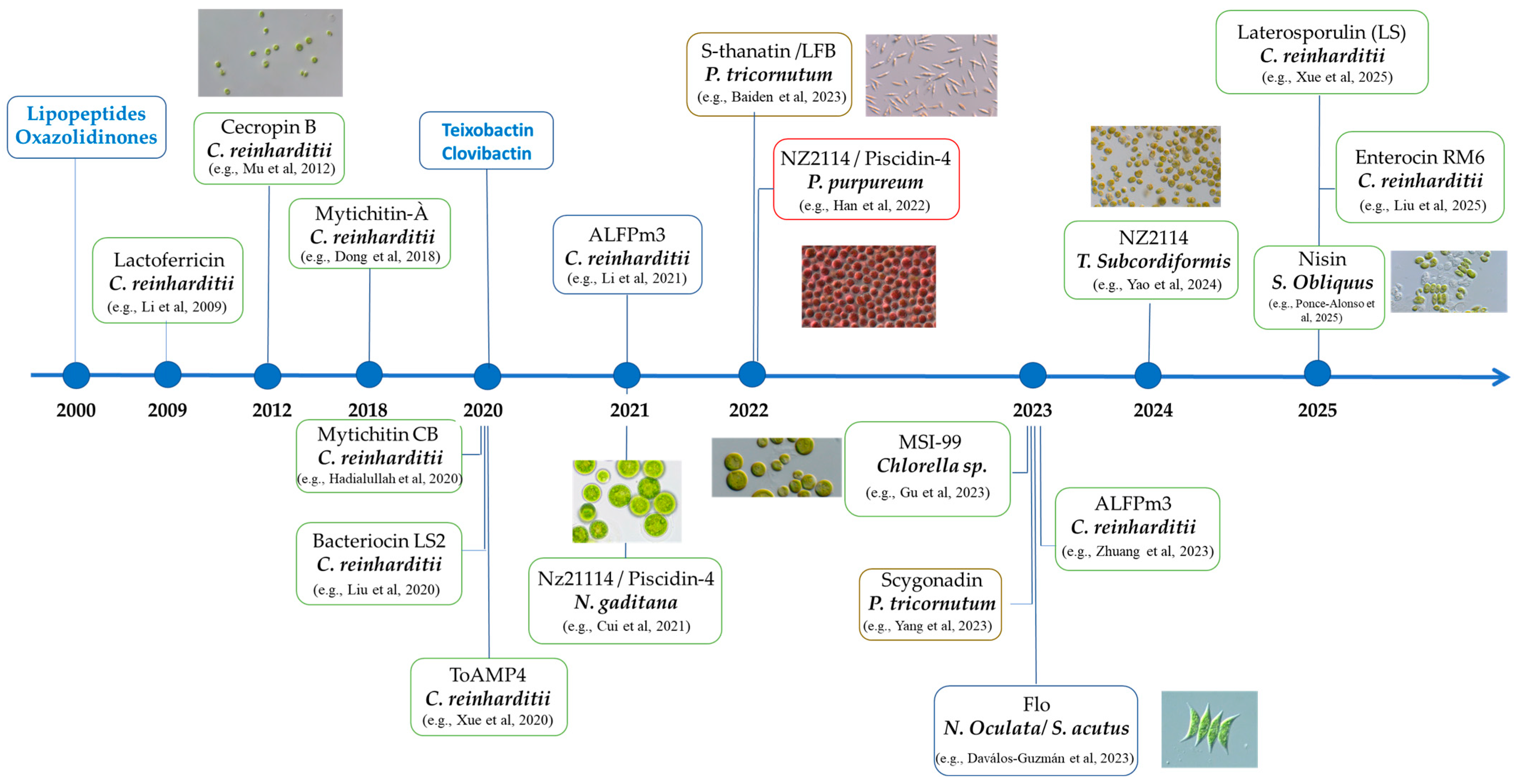
2.2.2. Microalgae Platforms for AMP Expression: Comparative Overview
2.2.3. Application of Microalgae Derived AMPs Towards Multi-Sector Exploitation
3. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lieberman, J.M. Appropriate antibiotic use and why it is important: The challenges of bacterial resistance. Pediat. Infect. Dis. J. 2023, 22, 1143–1151. [Google Scholar] [CrossRef]
- Bottalico, L.; Charitos, I.A.; Potenza, M.A.; Montagnani, M.; Santacroce, L. The war against bacteria, from the past to present and beyond. Expert Rev. Anti-Infect. Ther. 2022, 20, 681–706. [Google Scholar] [CrossRef]
- Colomb-Cotinat, M.; Lacoste, J.; Brun-Buisson, C.; Jarlier, V.; Coignard, B.; Vaux, S. Estimating the morbidity and mortality associated with infections due to multidrug-resistant bacteria (MDRB), France, 2012. Antimicrob. Resist. Infect. Control 2016, 5, 56. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Alain, L.F.A. Antibiotics and Antibiotic Resistance. Biomed. J. Sci. Tech. Res. 2017, 1, 117. [Google Scholar] [CrossRef]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef] [PubMed]
- Berteina-Raboin, S. Comprehensive Overview of Antibacterial Drugs and Natural Antibacterial Compounds Found in Food Plants. Antibiotics 2025, 14, 185. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 21 November 2023).
- Desoubeaux, G.; Pelegrin, M. Anticorps monoclonaux en infectiologie: Des nouveaux partenaires dans l’arsenal thérapeutique. Med. Sci. M/S 2019, 35, 1008–1013. [Google Scholar] [CrossRef]
- Lowy, I.; Molrine, D.C.; Leav, B.A.; Blair, B.M.; Baxter, R.; Gerding, D.N.; Nichol, G.; Thomas, W.D., Jr.; Leney, M.; Sloan, S.; et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N. Engl. J. Med. 2010, 362, 197–205. [Google Scholar] [CrossRef]
- Delmas, Y.; Vendrely, B.; Clouzeau, B.; Bachir, H.; Bui, H.N.; Lacraz, A.; Hélou, S.; Bordes, C.; Reffet, A.; Llanas, B.; et al. Outbreak of Escherichia coli O104:H4 haemolytic uraemic syndrome in France: Outcome with eculizumab. Nephrol. Dial. Transplant. 2014, 29, 565–572. [Google Scholar] [CrossRef]
- Auta, A.; Hadi, M.A.; Oga, E.; Adewuyi, E.O.; Abdu-Aguye, S.N.; Adeloye, D.; Strickland-Hodge, B.; Morgan, D.J. Global access to antibiotics without prescription in community pharmacies: A systematic review and meta-analysis. J. Infect. 2019, 78, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Milani, R.V.; Wilt, J.K.; Entwisle, J.; Hand, J.; Cazabon, P.; Bohan, J.G. Reducing inappropriate outpatient antibiotic prescribing: Normative comparison using unblinded provider reports. BMJ Open Qual. 2019, 8, e000351. [Google Scholar] [CrossRef] [PubMed]
- Ierano, C.; Thursky, K.; Marshall, C.; Koning, S.; James, R.; Johnson, S.; Imam, N.; Worth, L.J.; Peel, T. Appropriateness of Surgical Antimicrobial Prophylaxis Practices in Australia. JAMA Netw. Open 2019, 2, e1915003. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 481–511. [Google Scholar] [CrossRef]
- Thomas, C.M.; Nielsen, K.M. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat. Rev. Microbiol. 2005, 3, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.G.; Ochman, H. Amelioration of bacterial genomes: Rates of change and exchange. J. Mol. Evol. 1997, 44, 383–397. [Google Scholar] [CrossRef]
- Shukla, R.; Peoples, A.J.; Ludwig, K.C.; Maity, S.; Derks, M.G.N.; De Benedetti, S.; Krueger, A.M.; Vermeulen, B.J.A.; Harbig, T.; Lavore, F.; et al. An antibiotic from an uncultured bacterium binds to an immutable target. Cell 2023, 186, 4059–4073.e27. [Google Scholar] [CrossRef] [PubMed]
- DGS/IFOP—Les Français et L’antibiorésistance—Novembre 2017. Available online: https://sante.gouv.fr/IMG/pdf/3_laymand_les_francais_et_l_antibioresistance_-_colloque_16_nov_2017.pdf (accessed on 16 November 2017).
- Egorov, A.M.; Ulyashova, M.M.; Rubtsova, M.Y. Bacterial Enzymes and Antibiotic Resistance. Acta Nat. 2018, 10, 33–48. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, A.G.; Butterworth, D.; Cole, M.; Hanscomb, G.; Hood, J.D.; Reading, C.; Rolinson, G.N. Naturally-occurring beta-lactamase inhibitors with antibacterial activity. J. Antibiot. 1976, 29, 668–669. [Google Scholar] [CrossRef]
- Etebu, E.; Arikekpar, I. Antibiotics: Classification and mechanisms of action with emphasis on molecular perspectives. Int. J. Appl. Microbiol. Biotechnol. Res. 2016, 4, 90–101. [Google Scholar]
- Torres, J.A.; Villegas, M.V.; Quinn, J.P. Current concepts in antibiotic-resistant gram-negative bacteria. Expert Rev. Anti-Infect. Ther. 2007, 5, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Parodi, A.L. Le concept «One Health», une seule santé: Réalité et perspectives. Bull. Acad. Natl. Med. 2021, 205, 659–661. (In French) [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santos, L.H.M.L.M.; Araújo, A.N.; Fachini, A.; Pena, A.; Delerue-Matos, C.; Montenegro, M.C.B.S.M. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J. Hazard. Mater. 2010, 175, 45–95. [Google Scholar] [CrossRef] [PubMed]
- Serreau, R.; Amirouche, A.; Benyamina, A.; Berteina-Raboin, S. Propranolol Hydrochloride Psychiatric Effectiveness and Oxidative Stress: An Update. Oxygen 2024, 4, 139–149. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Björn, C.; Ekblom, J. Antimicrobial Peptides as Therapeutic Agents: Opportunities and Challenges. Crit. Rev. Biotechnol. 2020, 40, 978–992. [Google Scholar] [CrossRef]
- Jenssen, H.; Hamill, P.; Hancock, R.E. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef]
- Pushpanathan, M.; Gunasekaran, P.; Rajendhran, J. Antimicrobial peptides: Versatile biological properties. Int. J. Pept. 2013, 2013, 675391. [Google Scholar] [CrossRef]
- Diamond, G.; Beckloff, N.; Weinberg, A.; Kisich, K.O. The roles of antimicrobial peptides in innate host defense. Curr. Pharm. Des. 2009, 15, 2377–2392. [Google Scholar] [CrossRef]
- Zhang, L.J.; Gallo, R.L. Antimicrobial peptides. Curr. Biol. 2016, 26, R14–R19. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar] [PubMed] [PubMed Central]
- Browne, K.; Chakraborty, S.; Chen, R.; Willcox, M.D.P.; StClair Black, D.; Walsh, W.R.; Kumar, N. A New Era of Antibiotics: The Clinical Potential of Antimicrobial Peptides. Int. J. Mol. Sci. 2020, 21, 7047. [Google Scholar] [CrossRef]
- Gaspar, D.; Veiga, A.S.; Castanho, M.A. From antimicrobial to anticancer peptides. A review. Front. Microbiol. 2013, 4, 294. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Rossetti, P.; Trollmann, M.F.W.; Wichmann, C.; Gutsmann, T.; Eggeling, C.; Böckmann, R.A. From Membrane Composition to Antimicrobial Strategies: Experimental and Computational Approaches to AMP Design and Selectivity. Small 2025, e2411476. [Google Scholar] [CrossRef]
- Cardoso, P.; Glossop, H.; Meikle, T.G.; Aburto-Medina, A.; Conn, C.E.; Sarojini, V.; Valery, C. Molecular Engineering of Antimicrobial Peptides: Microbial Targets, Peptide Motifs and Translation Opportunities. Biophys. Rev. 2021, 13, 35–69. [Google Scholar] [CrossRef]
- Joo, H.-S.; Fu, C.-I.; Otto, M. Bacterial Strategies of Resistance to Antimicrobial Peptides. Phil. Trans. R. Soc. B 2016, 371, 20150292. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Misawa, T.; Goto, C.; Demizu, Y.; Hara-Kudo, Y.; Kikuchi, Y. The Effects of Magainin 2-Derived and Rationally Designed Antimicrobial Peptides on Mycoplasma Pneumoniae. PLoS ONE 2022, 17, e0261893. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; MacDonald, D.L.; Holroyd, K.J.; Thornsberry, C.; Wexler, H.; Zasloff, M. In Vitro Antibacterial Properties of Pexiganan, an Analog of Magainin. Antimicrob. Agents Chemother. 1999, 43, 782–788. [Google Scholar] [CrossRef]
- Zasloff, M. Magainins, a Class of Antimicrobial Peptides from Xenopus Skin: Isolation, Characterization of Two Active Forms, and Partial cDNA Sequence of a Precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 5449–5453. [Google Scholar] [CrossRef]
- Li, B.; Lyu, P.; Xie, S.; Qin, H.; Pu, W.; Xu, H.; Chen, T.; Shaw, C.; Ge, L.; Kwok, H.F. LFB: A Novel Antimicrobial Brevinin-Like Peptide from the Skin Secretion of the Fujian Large Headed Frog, Limnonectes fujianensi. Biomolecules 2019, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Jekhmane, S.; Derks, M.G.N.; Maity, S.; Slingerland, C.J.; Tehrani, K.H.M.E.; Medeiros-Silva, J.; Charitou, V.; Ammerlaan, D.; Fetz, C.; Consoli, N.A.; et al. Host Defence Peptide Plectasin Targets Bacterial Cell Wall Precursor Lipid II by a Calcium-Sensitive Supramolecular Mechanism. Nat. Microbiol. 2024, 9, 1778–1791. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.; Oedairadjsingh, T.; Ludwig, I.S.; Wood, T.M.; Martin, N.I.; Broere, F.; Weingarth, M.H.; Veldhuizen, E.J.A. Antimicrobial and Immunomodulatory Activities of Porcine Cathelicidin Protegrin-1. Mol. Immunol. 2024, 173, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Merrifield, R.B. Solid phase peptide synthesis I. The synthesis of a tetraeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Bucki, R.; Leszczynska, K.; Namiot, A.; Sokolowski, W. Cathelicidin LL-37: A multitask antimicrobial peptide. Arch. Immunol. Ther. Exp. 2010, 58, 15–25. [Google Scholar] [CrossRef]
- Agier, J.; Efenberger, M.; Brzezinska-Blaszczyk, E. Cathelicidin impact on inflammatory cells. Cent. Eur. J. Immunol. 2015, 40, 225–235. [Google Scholar] [CrossRef]
- Hancock, R.E. Cationic peptides: Effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 2001, 1, 156–164. [Google Scholar] [CrossRef]
- Radek, K.; Gallo, R. Antimicrobial peptides: Natural effectors of the innate immune system. Semin. Immunopathol. 2007, 29, 27–43. [Google Scholar] [CrossRef]
- Marxer, M.; Vollenweider, V.; Schmid-Hempel, P. Insect antimicrobial peptides act synergistically to inhibit a trypanosome parasite. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150302. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Adhikari, P.; Anaikutti, P. Design, synthesis, and characterization of non- hemolytic antimicrobial peptides related to human cathelicidin LL-37. RSC Adv. 2023, 13, 15594–15605. [Google Scholar] [CrossRef]
- Dutta, J.; Ramesh, S.; Radebe, S.M.; Somboro, A.M.; de la Torre, B.G.; Kruger, H.G.; Essack, S.Y.; Albericio, F.; Govender, T. Optimized Microwave Assisted Synthesis of LL37, a Cathelicidin Human Antimicrobial Peptide. Int. J. Pept. Res. Ther. 2014, 1, 13–20. [Google Scholar] [CrossRef]
- Madhavan, A.; Arun, K.B.; Sindhu, R.; Krishnamoorthy, J.; Reshmy, R.; Sirohi, R.; Pugazhendi, A.; Awasthi, M.K.; Szakacs, G.; Binod, P. Customized Yeast Cell Factories for Biopharmaceuticals: From Cell Engineering to Process Scale Up. Microb. Cell. Fact. 2021, 20, 124. [Google Scholar] [CrossRef] [PubMed]
- Bachhav, B.; De Rossi, J.; Llanos, C.D.; Segatori, L. Cell Factory Engineering: Challenges and Opportunities for Synthetic Biology Applications. Biotechnol. Bioeng. 2023, 120, 2441–2459. [Google Scholar] [CrossRef] [PubMed]
- Pavelka, A.; Vacek, L.; Norek, A.; Kobzova, S.; Janda, L. Recombinant production of human antimicrobial peptide LL-37 and its secondary structure. Biologia 2024, 79, 263–273. [Google Scholar] [CrossRef]
- Liang, X.; Jiang, H.; Si, X.; Xin, Q.; Meng, D.; Chen, P.; Mao, X. Boosting Expression Level of Plectasin in Recombinant Pichia Pastoris via 2A Self-Processing Peptide Assembly. Appl. Microbiol. Biotechnol. 2022, 106, 3669–3678. [Google Scholar] [CrossRef]
- Kharrat, O.; Yamaryo-Botté, Y.; Nasreddine, R.; Voisin, S.; Aumer, T.; Cammue, B.P.A.; Madinier, J.-B.; Knobloch, T.; Thevissen, K.; Nehmé, R.; et al. The Antimicrobial Activity of ETD151 Defensin Is Dictated by the Presence of Glycosphingolipids in the Targeted Organisms. Proc. Natl. Acad. Sci. USA 2025, 122, e2415524122. [Google Scholar] [CrossRef]
- Schneble, E.; Jinga, D.C.; Peoples, G. Breast cancer immunotherapy. Maedica 2015, 10, 185–191. [Google Scholar] [PubMed]
- Wang, H.; Nie, C.; Xu, W.; Li, J.; Gou, H.; Lv, H.; Chen, B.; Wang, J.; Liu, Y.; He, Y.; et al. In era of immunotherapy: The value of trastuzumab beyond progression in patients with trastuzumab-resistant HER2-positive advanced or metastatic gastric cancer. Therap. Adv. Gastroenterol. 2024, 17, 17562848241245455. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, K.; Deng, L.; Wu, H.; Fan, J. Towards Green Biomanufacturing of High-Value Recombinant Proteins Using Promising Cell Factory: Chlamydomonas Reinhardtii Chloroplast. Bioresour. Bioprocess. 2022, 9, 83. [Google Scholar] [CrossRef]
- Gong, Y.; Hu, H.; Gao, Y.; Xu, X.; Gao, H. Microalgae as Platforms for Production of Recombinant Proteins and Valuable Compounds: Progress and Prospects. J. Ind. Microbiol. Biotechnol. 2011, 38, 1879–1890. [Google Scholar] [CrossRef]
- Onyeaka, H.; Miri, T.; Obileke, K.; Hart, A.; Anumudu, C.; Al-Sharify, Z.T. Minimizing Carbon Footprint via Microalgae as a Biological Capture. Carbon Capture Sci. Technol. 2021, 1, 100007. [Google Scholar] [CrossRef]
- Maeda, Y.; Yoshino, T.; Matsunaga, T.; Matsumoto, M.; Tanaka, T. Marine Microalgae for Production of Biofuels and Chemicals. Curr. Opin. Biotechnol. 2018, 50, 111–120. [Google Scholar] [CrossRef]
- Rojas, V.; Rivas, L.; Cárdenas, C.; Guzmán, F. Cyanobacteria and Eukaryotic Microalgae as Emerging Sources of Antibacterial Peptides. Molecules 2020, 25, 5804. [Google Scholar] [CrossRef]
- Keeling, P.J. The number, speed, and impact of plastid endosymbioses in eukaryotic evolution. Annu. Rev. Plant Biol. 2013, 64, 583–607. [Google Scholar] [CrossRef]
- Gentil, J.; Hempel, F.; Moog, D.; Zauner, S.; Maier, U.G. Review: Origin of Complex Algae by Secondary Endosymbiosis: A Journey through Time. Protoplasma 2017, 254, 1835–1843. [Google Scholar] [CrossRef]
- Dougan, K.E.; González-Pech, R.A.; Stephens, T.G.; Shah, S.; Chen, Y.; Ragan, M.A.; Bhattacharya, D.; Chan, C.X. Genome-Powered Classification of Microbial Eukaryotes: Focus on Coral Algal Symbionts. Trends Microbiol. 2022, 30, 831–840. [Google Scholar] [CrossRef]
- Barboza-Rodríguez, R.; Rodríguez-Jasso, R.M.; Rosero-Chasoy, G.; Rosales Aguado, M.L.; Ruiz, H.A. Photobioreactor Configurations in Cultivating Microalgae Biomass for Biorefinery. Bioresour. Technol. 2024, 394, 130208. [Google Scholar] [CrossRef]
- Wiatrowski, M.; Klein, B.C.; Davis, R.W.; Quiroz-Arita, C.; Tan, E.C.D.; Hunt, R.W.; Davis, R.E. Techno-Economic Assessment for the Production of Algal Fuels and Value-Added Products: Opportunities for High-Protein Microalgae Conversion. Biotechnol. Biofuels Bioprod. 2022, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L.; Vaishnav, P. Production of recombinant proteins by microbes and higher organisms. Technol. Adv. 2009, 27, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.R.; Purton, S. Genetic engineering of eukaryotic algae: Progress and prospects. J. Phycol. 1997, 33, 713–722. [Google Scholar] [CrossRef]
- Leon-Banares, R.; Gonzalez-Ballester, D.; Galvan, A.; Fernandez, E. Transgenic microalgae as green cell-factories. Trends Biotechnol. 2004, 22, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.E.; Mayfield, S.P. Recent developments in the production of human therapeutic proteins in eukaryotic algae. Expert Opin. Biol. Ther. 2005, 5, 225–235. [Google Scholar] [CrossRef]
- Walker, T.L.; Collet, C.; Purton, S. Algal transgenics in the genomic ERA. J. Phycol. 2005, 41, 1077–1093. [Google Scholar] [CrossRef]
- Walker, T.L.; Purton, S.; Becker, D.K.; Collet, C. Microalgae as bioreactors. Plant Cell Rep. 2005, 24, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Goshtasbi, H.; Okolodkov, Y.B.; Movafeghi, A.; Awale, S.; Safary, A.; Barar, J.; Omidi, Y. Harnessing Microalgae as Sustainable Cellular Factories for Biopharmaceutical Production. Algal Res. 2023, 74, 103237. [Google Scholar] [CrossRef]
- Vasquez-Moscoso, C.A.; Merlano, J.A.R.; Olivera Gálvez, A.; Volcan Almeida, D. Antimicrobial Peptides (AMPs) from Microalgae as an Alternative to Conventional Antibiotics in Aquaculture. Prep. Biochem. Biotechnol. 2024, 55, 26–35. [Google Scholar] [CrossRef]
- Banerjee, A.; Ward, V. Production of Recombinant and Therapeutic Proteins in Microalgae. Curr. Opin. Biotechnol. 2022, 78, 102784. [Google Scholar] [CrossRef]
- Toustou, C.; Boulogne, I.; Gonzalez, A.-A.; Bardor, M. Comparative RNA-Seq of Ten Phaeodactylum Tricornutum Accessions: Unravelling Criteria for Robust Strain Selection from a Bioproduction Point of View. Mar. Drugs 2024, 22, 353. [Google Scholar] [CrossRef]
- Deo, S.; Turton, K.L.; Kainth, T.; Kumar, A.; Wieden, H.-J. Strategies for Improving Antimicrobial Peptide Production. Biotechnol. Adv. 2022, 59, 107968. [Google Scholar] [CrossRef]
- Harris, E.H. Chlamydomonas as a model organism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 363–406. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Cheng, R.-Q.; Liu, Q.-Y.; Wang, J.; Fan, Z.-C. Multimer of the Antimicrobial Peptide Mytichitin-A Expressed in Chlamydomonas Reinhardtii Exerts a Broader Antibacterial Spectrum and Increased Potency. J. Biosci. Bioeng. 2018, 125, 175–179. [Google Scholar] [CrossRef]
- Mu, F.-Y.; Li, H.; Hu, Z.-L. Expression of Tandem Repeat Cecropin B in Chlamydomonas reinhardtii and Its Antibacterial Effect. Prog. Biochem. Biophys. 2012, 39, 344–351. [Google Scholar] [CrossRef]
- Li, A.; Huang, R.; Wang, C.; Hu, Q.; Li, H.; Li, X. Expression of Anti-Lipopolysaccharide Factor Isoform 3 in Chlamydomonas Reinhardtii Showing High Antimicrobial Activity. Mar. Drugs 2021, 19, 239. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-X.; Zhang, Y.-Y.; Fan, Z.-C. Antibacterial Efficacy of Chlamydomonas Reinhardtii–Expressed Enterocin RM6 Against Gram-Positive and Gram-Negative Bacteria. Probiot. Antimicro. Prot. 2025. [Google Scholar] [CrossRef]
- Li, S.S.; Tsai, H.J. Transgenic microalgae as a non-antibiotic bactericide producer to defend against bacterial pathogen infection in the fish digestive tract. Fish Shellfish. Immunol. 2009, 26, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Dong, C.M.; Hu, H.H.; Dong, B.; Fan, Z.C. Chlamydomonas reinhardtii-expressed multimer of ToAMP4 inhibits the growth of bacteria of both Gram-positive and Gram-negative. Process Biochem. 2020, 91, 311–318. [Google Scholar] [CrossRef]
- Hadiatullah, H.; Wang, H.; Liu, Y.X.; Fan, Z.C. Chlamydomonas reinhardtii-derived multimer Mytichitin-CB possesses potent antibacterial properties. Process Biochem. 2020, 91, 21–29. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, K.; Xu, W.; Wang, Y.; Gao, Z.; Cui, H.; Meng, C.; Qin, S. Plastid Engineering of a Marine Alga, Nannochloropsis gaditana, for Co-Expression of Two Recombinant Peptides. J. Phycol. 2021, 57, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Ou, Y.; Chen, R.; Huang, D.; Wang, C. Comparing the Ability of Secretory Signal Peptides for Heterologous Expression of Anti-Lipopolysaccharide Factor 3 in Chlamydomonas reinhardtii. Mar. Drugs 2023, 21, 346. [Google Scholar] [CrossRef]
- Xue, B.; Li, Y.-Y.; Zheng, B.-F.-C.; Zhang, C.; Hadiatullah, H.; Dai, W.-T.; Wang, Y.-J.; Fan, Z.-C. Expression and Characterization of Recombinant Triple Laterosporulin in Chlamydomonas Reinhardtii. Probiot. Antimicro. Prot. 2025. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Li, Z.-F.; Lv, Y.-J.; Dong, B.; Fan, Z.-C. Chlamydomonas Reinhardtii-Expressed Multimer of Bacteriocin LS2 Potently Inhibits the Growth of Bacteria. Process Biochem. 2020, 95, 139–147. [Google Scholar] [CrossRef]
- Han, S.; Zhao, J.; Liu, P.; Wang, K.; Qin, S.; Zhao, Z.; Cui, Y. Two Foreign Antimicrobial Peptides Expressed in the Chloroplast of Porphyridium Purpureum Possessed Antibacterial Properties. Mar. Drugs 2022, 20, 484. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.A.; De Vries, R.; Biswaro, L.S.; Vasconcelos, I.M.; Franco, O.L.; Dias, S.C. Fusion of Plectasin Derivative NZ2114 with Hydrophilic Random Coil Polypeptide: Recombinant Production in Pichia Pastoris and Antimicrobial Activity against Clinical Strain MRSA. Pept. Sci. 2018, 110, e23034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Teng, D.; Mao, R.; Wang, X.; Xi, D.; Hu, X.; Wang, J. High expression of a plectasin-derived peptide NZ2114 in Pichia pastoris and its pharmacodynamics, postantibiotic and synergy against Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2014, 98, 681–694. [Google Scholar] [CrossRef]
- Baiden, N.; Gandini, C.; Goddard, P.; Sayanova, O. Heterologous expression of antimicrobial peptides S-thanatin and bovine Lactoferricin in the Marine Diatom Phaeodactylum Tricornutum Enhances Native Antimicrobial Activity against Gram-Negative Bacteria. Algal Res. 2023, 69, 102927. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, L.; Zhuo, J.; Li, X.; Wu, S.; Yu, G.; Gao, S.; Wang, G. Expression of Secreted Antimicrobial Peptide in a Marine Diatom—Phaeodactylum Tricornutum. Algal Res. 2023, 75, 103270. [Google Scholar] [CrossRef]
- Dávalos-Guzmán, S.D.; Martinez-Gutierrez, F.; Martínez-González, L.; Quezada-Rivera, J.J.; Lorenzo-Leal, A.C.; Bach, H.; Morales-Domínguez, J.F.; Soria-Guerra, R.E. Antimicrobial Activity of the Flo Peptide Produced in Scenedesmus Acutus and Nannochloropsis Oculata. World J. Microbiol. Biotechnol. 2023, 39, 211. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Deng, Y.; Wang, A.; Gan, Q.; Xin, Y.; Paithoonrangsarid, K.; Lu, Y. Engineering a Marine Microalga chlorella Sp. as the Cell Factory. Biotechnol. Biofuels 2023, 16, 133. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Alonso, J.; Martínez-González, L.; Alpuche-Solís, Á.G.; Martinez-Gutierrez, F.; Lorenzo-Leal, A.C.; Bach, H.; Soria-Guerra, R.E. Expression of nisin in Scenedesmus acutus and evaluation of its antimicrobial activity. Biotechnol. Lett. 2025, 47, 60. [Google Scholar] [CrossRef] [PubMed]
- Mathieu-Rivet, E.; Mati-Baouche, N.; Walet-Balieu, M.-L.; Lerouge, P.; Bardor, M. N-and O-Glycosylation Pathways in the Microalgae Polyphylic Group. Front. Plant Sci. 2020, 11, 609993. [Google Scholar] [CrossRef]
- Mócsai, R.; Figl, R.; Troschl, C.; Strasser, R.; Svehla, E.; Windwarder, M.; Thader, A.; Altmann, F. N-Glycans of the Microalga Chlorella Vulgaris Are of the Oligomannosidic Type but Highly Methylated. Sci. Rep. 2019, 9, 331. [Google Scholar] [CrossRef]
- Dehghani, J.; Balieu, J.; Perruchon, O.; Mathieu-Rivet, E.; Mati-Baouche, N.; Lerouge, P.; Bardor, M. Exploring Protein N-Glycosylation in the Green Microalga Dunaliella Salina. Algal. Res. 2024, 83, 103711. [Google Scholar] [CrossRef]
- Schulze, S.; Urzica, E.; Reijnders, M.J.M.F.; Van De Geest, H.; Warris, S.; Bakker, L.V.; Fufezan, C.; Martins Dos Santos, V.A.P.; Schaap, P.J.; Peters, S.A.; et al. Identification of Methylated GnTI-dependent N-glycans in Botryococcus Brauni. New Phytol. 2017, 215, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Levy-Ontman, O.; Arad, S.; Harvey, D.J.; Parsons, T.B.; Fairbanks, A.; Tekoah, Y. Unique N-Glycan Moieties of the 66-kDa Cell Wall Glycoprotein from the Red Microalga porphyridium sp. J. Biol. Chem. 2011, 286, 21340–21352. [Google Scholar] [CrossRef]
- Xie, X.; Du, H.; Chen, J.; Aslam, M.; Wang, W.; Chen, W.; Li, P.; Du, H.; Liu, X. Global Profiling of N-Glycoproteins and N-Glycans in the Diatom Phaeodactylum Tricornutum. Front. Plant Sci. 2021, 12, 779307. [Google Scholar] [CrossRef]
- Shi, Q.; Chen, C.; Zhang, W.; Wu, P.; Sun, M.; Wu, H.; Wu, H.; Fu, P.; Fan, J. Transgenic Eukaryotic Microalgae as Green Factories: Providing New Ideas for the Production of Biologically Active Substances. J. Appl. Phycol. 2021, 33, 705–728. [Google Scholar] [CrossRef]
- Yang, R.; Wei, D.; Xie, J. Diatoms as Cell Factories for High-Value Products: Chrysolaminarin, Eicosapentaenoic Acid, and Fucoxanthin. Crit. Rev. Biotechnol. 2020, 40, 993–1009. [Google Scholar] [CrossRef]
- Toustou, C.; Plasson, C.; Kiefer-Meyer, M.-C.; Bardor, M. Characterization of the VOC Promoter That Is Active Under Low-Salinity Conditions in the Diatom Phaeodactylum Tricornutum. Mar. Drugs 2025, 23, 185. [Google Scholar] [CrossRef] [PubMed]
- Behzadnia, A.; Moosavi-Nasab, M.; Oliyaei, N. Anti-Biofilm Activity of Marine Algae-Derived Bioactive Compounds. Front. Microbiol. 2024, 15, 1270174. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.A.; Kumari, P.; Kumar, P.; Yadav, A.; Bhardwaj, R.; Swapnil, P.; Meena, M. Microalgae-derived antioxidants and antimicrobials: A sustainable approach for natural food preservatives. Front. Sustain. Food Syst. 2025, 9, 1669731. [Google Scholar] [CrossRef]
- Yao, T.; Sun, F.; Zhu, B.; Han, S.; Zhang, H.; Meng, C.; Gao, Z.; Cui, Y. Oral Administration of Antimicrobial Peptide NZ2114 Through the Microalgal Bait Tetraselmis Subcordiformis (Wille) Butcher for Improving the Immunity and Gut Health in Turbot (Scophthalmus maximus L.). Mar. Biotechnol. 2024, 26, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Toustou, C.; Mekhalfi, M.; Kiefer-Meyer, M.C.; Bardor, M. Production of Therapeutic Antibodies by the Microalgae Phaeodactylum Triconutum. WO2023:208883, 2 November 2023. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mekhalfi, M.; Berteina-Raboin, S. Microalgae, Cell Factories for Antimicrobial Peptides: A Promising Response to Antibiotic Resistance. Antibiotics 2025, 14, 959. https://doi.org/10.3390/antibiotics14100959
Mekhalfi M, Berteina-Raboin S. Microalgae, Cell Factories for Antimicrobial Peptides: A Promising Response to Antibiotic Resistance. Antibiotics. 2025; 14(10):959. https://doi.org/10.3390/antibiotics14100959
Chicago/Turabian StyleMekhalfi, Malika, and Sabine Berteina-Raboin. 2025. "Microalgae, Cell Factories for Antimicrobial Peptides: A Promising Response to Antibiotic Resistance" Antibiotics 14, no. 10: 959. https://doi.org/10.3390/antibiotics14100959
APA StyleMekhalfi, M., & Berteina-Raboin, S. (2025). Microalgae, Cell Factories for Antimicrobial Peptides: A Promising Response to Antibiotic Resistance. Antibiotics, 14(10), 959. https://doi.org/10.3390/antibiotics14100959