Silver Nanoparticle–Antibiotic Combinations: A Strategy to Overcome Bacterial Resistance in Escherichia coli, Salmonella Enteritidis and Staphylococcus aureus
Abstract
1. Introduction
2. Results
2.1. Minimal Inhibitory Concentration and Minimal Bactericidal Concentration
2.2. Checkerboard Assay
2.3. Time–Kill
2.4. Prolonged Exposure of Bacteria to Antimicrobials
2.5. Analysis of Cross-Resistance
2.6. FTIR Analysis of Bio-AgNP Combined with Conventional Antibiotics
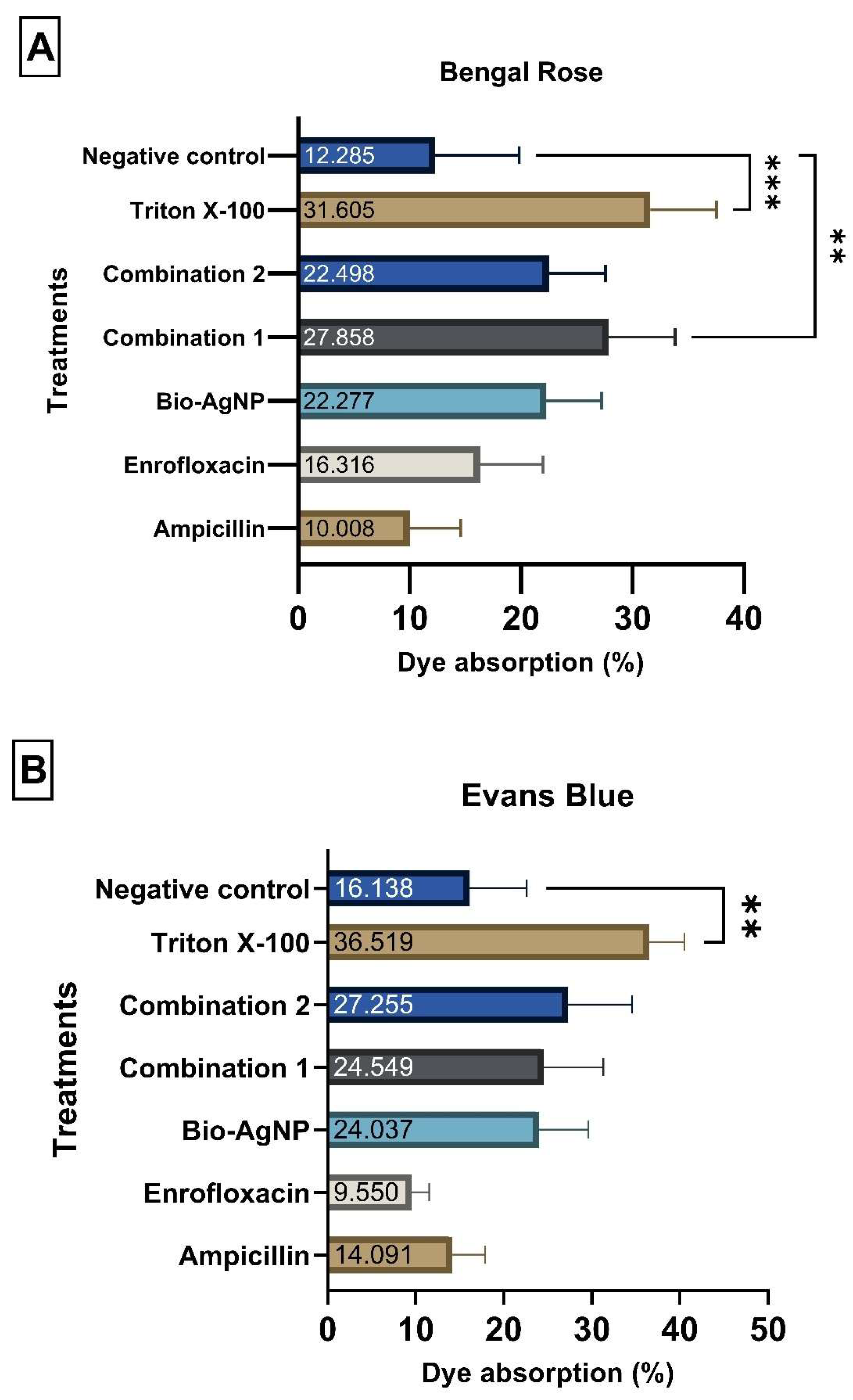
2.7. Alterations in Cytoplasmic Membrane Permeability
2.8. Detection of ROS
2.9. Inhibition of Efflux Pump
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Antimicrobials
4.3. Antibacterial Activity
4.3.1. MIC and MBC Determination
4.3.2. Checkerboard Assay
4.3.3. Time–Kill
4.3.4. Prolonged Exposure of Bacteria to Antimicrobials
4.3.5. Disk Diffusion Test
4.4. Characterization of Mechanism of Action of Antimicrobials
4.4.1. Dye Absorption Assay
4.4.2. Leakage of Cytoplasmic Contents
4.4.3. Measurement of Reactive Oxygen Species
4.4.4. Efflux Pump Inhibition
4.5. AgNP–Antibiotic Interaction Characterization by Optical Analysis
4.6. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OMS. Antimicrobial Resistance: Briefing to WHO Member States; OMS: Geneva, Switzerland, 2023. [Google Scholar]
- Zhou, Z.C.; Liu, Y.; Lin, Z.J.; Shuai, X.Y.; Zhu, L.; Xu, L.; Meng, L.X.; Sun, Y.J.; Chen, H. Spread of Antibiotic Resistance Genes and Microbiota in Airborne Particulate Matter, Dust, and Human Airways in the Urban Hospital. Environ. Int. 2021, 153, 106501. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Jampilek, J.; Kralova, K. Advances in Nanostructures for Antimicrobial Therapy. Materials 2022, 15, 2388. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver Nanoparticles: Various Methods of Synthesis, Size Affecting Factors and Their Potential Applications–a Review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Joshi, A.; Borkotoky, S.; Mehra, A.; Kaushik, V.; Sahu, R.; Farooq, A. Medical Applications of Functional Antimicrobial Nanoparticles. In Antiviral and Antimicrobial Coatings Based on Functionalized Nanomaterials: Design, Applications, and Devices; Elsevier: Amsterdam, The Netherlands, 2023; pp. 515–541. [Google Scholar] [CrossRef]
- Desai, P.P.; Radha, M.J.; Savitha, G.; Boregowda, R. Versatile Strategies for Multifaceted Nanoparticle Synthesis—An Overview. In Nanotechnology and In Silico Tools: Natural Remedies and Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2024; pp. 155–168. [Google Scholar] [CrossRef]
- Vishveshwaraiah, C.K.; Kirankumar, G.B.; Harshitha, M.; Madhu, B.K. A Review on Silver Nanoparticles: Synthesis Approaches, Properties, Characterization and Applications. Pharm. Nanotechnol. 2024, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Salas-Orozco, M.F.; Lorenzo-Leal, A.C.; de Alba Montero, I.; Marín, N.P.; Santana, M.A.C.; Bach, H. Mechanism of Escape from the Antibacterial Activity of Metal-Based Nanoparticles in Clinically Relevant Bacteria: A Systematic Review. Nanomedicine 2024, 55, 102715. [Google Scholar] [CrossRef]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver Nanoparticles: A New View on Mechanistic Aspects on Antimicrobial Activity. Nanomedicine 2016, 12, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Baran, A.; Kwiatkowska, A.; Potocki, L. Antibiotics and Bacterial Resistance—A Short Story of an Endless Arms Race. Int. J. Mol. Sci. 2023, 24, 5777. [Google Scholar] [CrossRef]
- Siqueira, P. Antibióticos Beta-Lactâmicos 2022. Available online: https://www.academia.edu/39300646/Antibi%C3%B3ticos_beta_lact%C3%A2micos (accessed on 22 September 2025).
- Iwu, C.D.; Korsten, L.; Okoh, A.I. The Incidence of Antibiotic Resistance within and beyond the Agricultural Ecosystem: A Concern for Public Health. Microbiologyopen 2020, 9, e1035. [Google Scholar] [CrossRef]
- Ruiz, J. Transferable Mechanisms of Quinolone Resistance from 1998 Onward. Clin. Microbiol. Rev. 2019, 32, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Rogério, R.; De Sousa, F. Pesquisa de Genes de Resistência a Quinolonas Em Bacilos Gram Negativos de Origem Clínica e Ambiental. Doctoral Dissertation, Universidade de São Paulo, São Paulo, Brazil, 2014. [Google Scholar]
- Scandorieiro, S.; Teixeira, F.M.M.B.; Nogueira, M.C.L.; Panagio, L.A.; de Oliveira, A.G.; Durán, N.; Nakazato, G.; Kobayashi, R.K.T. Antibiofilm Effect of Biogenic Silver Nanoparticles Combined with Oregano Derivatives against Carbapenem-Resistant Klebsiella Pneumoniae. Antibiotics 2023, 12, 756. [Google Scholar] [CrossRef]
- Şirin, M.C.; Cezaroğlu, Y.; Sesli Çetin, E.; Arıdoğan, B.; Trak, D.; Arslan, Y. Antibacterial and Antibiofilm Efficacy of Colistin & Meropenem Conjugated Silver Nanoparticles against Escherichia coli and Klebsiella pneumoniae. J. Basic Microbiol. 2023, 63, 1397–1411. [Google Scholar] [CrossRef]
- Tyers, M.; Wright, G.D. Drug Combinations: A Strategy to Extend the Life of Antibiotics in the 21st Century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef]
- Wypij, M.; Świecimska, M.; Czarnecka, J.; Dahm, H.; Rai, M.; Golinska, P. Antimicrobial and Cytotoxic Activity of Silver Nanoparticles Synthesized from Two Haloalkaliphilic Actinobacterial Strains Alone and in Combination with Antibiotics. J. Appl. Microbiol. 2018, 124, 1411–1424. [Google Scholar] [CrossRef]
- Lamrabet, O.; Martin, M.; Lenski, R.E.; Schneider, D. Changes in Intrinsic Antibiotic Susceptibility during a Long-Term Evolution Experiment with Escherichia coli. mBio 2019, 10, e00189-19. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D. Microbiological Effects of Sublethal Levels of Antibiotics. Nat. Rev. Microbiol. 2014, 12, 465–478. [Google Scholar] [CrossRef]
- Kaweeteerawat, C.; Na Ubol, P.; Sangmuang, S.; Aueviriyavit, S.; Maniratanachote, R. Mechanisms of Antibiotic Resistance in Bacteria Mediated by Silver Nanoparticles. J. Toxicol. Environ. Health A 2017, 80, 1276–1289. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Muñoz, R.; Meza-Villezcas, A.; Fournier, P.G.J.; Soria-Castro, E.; Juarez-Moreno, K.; Gallego-Hernández, A.L.; Bogdanchikova, N.; Vazquez-Duhalt, R.; Huerta-Saquero, A. Enhancement of Antibiotics Antimicrobial Activity Due to the Silver Nanoparticles Impact on the Cell Membrane. PLoS ONE 2019, 14, e0224904. [Google Scholar] [CrossRef] [PubMed]
- Graves, J.L.; Tajkarimi, M.; Cunningham, Q.; Campbell, A.; Nonga, H.; Harrison, S.H.; Barrick, J.E. Rapid Evolution of Silver Nanoparticle Resistance in Escherichia coli. Front. Genet. 2015, 6, 42. [Google Scholar] [CrossRef]
- Khorrami, S.; Zarrabi, A.; Khaleghi, M.; Danaei, M.; Mozafari, M.R. Selective Cytotoxicity of Green Synthesized Silver Nanoparticles against the MCF-7 Tumor Cell Line and Their Enhanced Antioxidant and Antimicrobial Properties. Int. J. Nanomed. 2018, 13, 8013–8024. [Google Scholar] [CrossRef]
- Lee, H.-S.; Ryu, D.-S.; Choi, S.-J.; Lee, D.-S. Antibacterial Activity of Silver-Nanoparticles Against Staphylococcus aureus and Escherichia coli. Korean J. Microbiol. Biotechnol. 2011, 39, 77–85. [Google Scholar]
- Li, P.; Li, J.; Wu, C.; Wu, Q.; Li, J. Synergistic Antibacterial Effects of β-Lactam Antibiotic Combined with Silver Nanoparticles. Nanotechnology 2005, 16, 1912–1917. [Google Scholar] [CrossRef]
- Mueller-Spitz, S.R.; Crawford, K.D. Silver Nanoparticle Inhibition of Polycyclic Aromatic Hydrocarbons Degradation by Mycobacterium Species RJGII-135. Lett. Appl. Microbiol. 2014, 58, 330–337. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia Coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Roy, P.; Jeevanandam, P. Antibacterial Activity Studies of ZnO Nanostructures with Different Morphologies against E. coli and S. aureus. Appl. Phys. A Mater. Sci. Process 2023, 129, 244. [Google Scholar] [CrossRef]
- Dwyer, D.J.; Belenky, P.A.; Yang, J.H.; Cody MacDonald, I.; Martell, J.D.; Takahashi, N.; Chan, C.T.Y.; Lobritz, M.A.; Braff, D.; Schwarz, E.G.; et al. Antibiotics Induce Redox-Related Physi. Proc. Natl. Acad. Sci. USA 2014, 111, E2100–E2109. [Google Scholar] [CrossRef]
- Longhini, R.; Lonni, A.A.S.G.; Sereia, A.L.; Krzyzaniak, L.M.; Lopes, G.C.; de Mello, J.C.P. Trichilia Catigua: Therapeutic and Cosmetic Values. Rev. Bras. Farmacogn. 2017, 27, 254–271. [Google Scholar] [CrossRef]
- Hassanisaadi, M.; Bonjar, G.H.S.; Rahdar, A.; Pandey, S.; Hosseinipour, A.; Abdolshahi, R. Environmentally Safe Biosynthesis of Gold Nanoparticles Using Plant Water Extracts. Nanomaterials 2021, 11, 2033. [Google Scholar] [CrossRef]
- Fafal, T.; Taştan, P.; Tüzün, B.S.; Ozyazici, M.; Kivcak, B. Synthesis, Characterization and Studies on Antioxidant Activity of Silver Nanoparticles Using Asphodelus Aestivus Brot. Aerial Part Extract. S. Afr. J. Bot. 2017, 112, 346–353. [Google Scholar] [CrossRef]
- Khan, A.N.; Ali Aldowairy, N.N.; Saad Alorfi, H.S.; Aslam, M.; Bawazir, W.A.B.; Hameed, A.; Soomro, M.T. Excellent Antimicrobial, Antioxidant, and Catalytic Activities of Medicinal Plant Aqueous Leaf Extract Derived Silver Nanoparticles. Processes 2022, 10, 1949. [Google Scholar] [CrossRef]
- Nakazato, G.; Celidonio, A.P.S.; Kobayashi, R.K.T.; Panagio, L.A.; Lonni, A.A.G.S.; De Campos, A.C.L.P.; Goncalves, M.C.; Okino, G.A.K. Patente—Processo de Produção de Nanopartículas Biogênicas de Prata, Nanopartículas Biogênicas de Prata e Usos Das Nanopartículas Biogênicas de Prata—GRAL Bioativos LTDA 2021. Available online: http://www.inpi.gov.br (accessed on 22 September 2022).
- CLSI. M07—Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; ISBN 1562388363. [Google Scholar]
- NCCLS. M26-A: Methods for Determining Bactericidal Activity of Antimicrobial Agents: Approved Guideline; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 1999; Volume 12, ISBN 1562383841. [Google Scholar]
- Domínguez, A.V.; Panadero, I.M.; Smani, Y. In Vitro and In Vivo Evaluation of Two Combined β-Lactamase Inhibitors against Carbapenem-Resistant Acinetobacter Baumannii. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 1317–1325. [Google Scholar] [CrossRef]
- CLSI. M02-A12: Performance Standards for Antimicrobial Disk Susceptibility Tests, 12th ed.; Approved Standard; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015; Volume 35, p. 73. [Google Scholar]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an Essential Oil of Clove) Acts as an Antibacterial Agent against Salmonella Typhi by Disrupting the Cellular Membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef]
- Halder, S.; Yadav, K.K.; Sarkar, R.; Mukherjee, S.; Saha, P.; Haldar, S.; Karmakar, S.; Sen, T. Alteration of Zeta Potential and Membrane Permeability in Bacteria: A Study with Cationic Agents. Springerplus 2015, 4, 672. [Google Scholar] [CrossRef] [PubMed]
- Cirino, I.C.S.; Menezes-Silva, S.M.P.; Silva, H.T.D.; De Souza, E.L.; Siqueira-Júnior, J.P. The Essential Oil from Origanum Vulgare L. and Its Individual Constituents Carvacrol and Thymol Enhance the Effect of Tetracycline against Staphylococcus Aureus. Chemotherapy 2015, 60, 290–293. [Google Scholar] [CrossRef] [PubMed]
- De Sousa Silveira, Z.; MacÊdo, N.S.; Dos Santos, J.F.S.; De Freitas, T.S.; Dos Santos Barbosa, C.R.; De Sousa, D.L.; Muniz, D.F.; De Oliveira, L.C.C.; Siqueira, J.P.; Da Cunha, F.A.B.; et al. Evaluation of the Antibacterial Activity and Efflux Pump Reversal of Thymol and Carvacrol against Staphylococcus Aureus and Their Toxicity in Drosophila Melanogaster. Molecules 2020, 25, 2103. [Google Scholar] [CrossRef] [PubMed]





| Bacterial Strain | AMP (μg/mL) | ENRO (μg/mL) | Bio-AgNP (μM) |
|---|---|---|---|
| E. coli ATCC 25922 | 8 | 0.01 | 62.5 |
| S. Enteritidis ATCC 13076 | 8 | 0.03 | 125 |
| S. aureus ATCC 25923 | 0.5 | 0.12 | 62.5 |
| E. coli 5616 | 128 | 0.015 | 62.5 |
| S. Typhimurium 685 | 8 | 0.03 | 125 |
| S. aureus N315 | 128 | 0.12 | 250 |
| Bacterial Strain | MIC before Induction of Resistance | MIC after Induction of Resistance | Increase in MIC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AMP μg/mL | ENRO μg/mL | Bio-AgNP μM | AMP μg/mL | ENRO μg/mL | Bio-AgNP μM | AMP | ENRO | Bio-AgNP | |
| E. coli ATCC 25922 | 8 | 0.015 | 31.2 | 16 | 0.32 | 2000 | 2× | 21.3× | 64.1× |
| S. Enteritidis ATCC 13076 | 8 | 0.03 | 125 | 64 | 1.92 | 125 | 8× | 64× | - |
| S. aureus ATCC 25923 | 0.5 | 0.12 | 31.2 | 20 | 10 | 125 | 40× | 83.3× | 4× |
| Before Induction of Resistance | ||||
|---|---|---|---|---|
| Bacterial Strain |
AMP
(μg/mL) |
Bio-AgNP
(μM) | ||
| MIC in Combination | MIC in Combination | FICI | Antibacterial Interaction | |
| E. coli ATCC 25922 | 0.25 | 15.6 | 0.53 | Additive |
| S. Enteritidis ATCC 13076 | 0.12 | 62.5 | 0.51 | Additive |
| S. aureus ATCC 25923 | 0.12 | 15.6 | 0.48 | Synergism |
| After Induction of Resistance | ||||
| E. coli ATCC 25922 | 1 | 15.6 | 0.55 | Additive |
| S. Enteritidis ATCC 13076 | 8 | 31.2 | 0.75 | Additive |
| S. aureus ATCC 25923 | 0.12 | 62.5 | 0.98 | Additive |
| Before Induction of Resistance | ||||
|---|---|---|---|---|
| Bacterial Strain |
ENRO
(μg/mL) |
Bio-AgNP
(μM) | ||
| MIC in Combination | MIC in Combination | FICI | Antibacterial Interaction | |
| E. coli ATCC 25922 | 0.0005 | 15.6 | 0.55 | Additive |
| S. Enteritidis ATCC 13076 | 0.003 | 62.5 | 0.55 | Additive |
| S. aureus ATCC 25923 | 0.06 | 31.2 | 1 | Additive |
| After Induction of Resistance | ||||
| E. coli ATCC 25922 | 0.001 | 62.5 | 0.51 | Additive |
| S. Enteritidis ATCC 13076 | 0.06 | 62.5 | 0.74 | Additive |
| S. aureus ATCC 25923 | 0.5 | 31.2 | 0.98 | Additive |
| Antibiotic | AMP (mm) | ENRO (mm) | Bio-AgNP (mm) | C1 (mm) | C2 (mm) | S/R (mm) |
|---|---|---|---|---|---|---|
| Amoxicillin-clavulanate | 18 S | 20 S | 26 S | 24 S | 22 S | ≥18/≤13 |
| Ampicillin | 10 R | 20 S | 20 S | 16 S | 10 R | ≥17/≤13 |
| Cefazolin | 21 I | 25 S | 16 R | 22 I | 11 R | ≥23/≤19 |
| Cefepime | 31 S | 32 S | 30 S | 30 S | 25 S | ≥25/≤18 |
| Cefoxitin | 25 S | 27 S | 20 S | 25 S | 25 S | ≥18/≤14 |
| Ceftriaxone | 30 S | 30 S | 30 S | 30 S | 30 S | ≥26/≤22 |
| Ciprofloxacin | 40 S | 33 S | 38 S | 40 S | 34 S | ≥26/≤21 |
| Chloramphenicol | 30 S | 25 S | 30 S | 22 S | 18 S | ≥18/≤12 |
| Enrofloxacin | 31 S | 20 I | 37 S | 35 S | 26 S | ≥23/≤16 |
| Fosfomycin | 25 S | 27 S | 24 S | 25 S | 25 S | ≥16/≤12 |
| Gentamicin | 27 S | 25 S | 30 S | 28 S | 27 S | ≥15/≤12 |
| Imipenem | 32 S | 30 S | 32 S | 30 S | 34 S | ≥23/≤19 |
| Nitrofurantoin | 25 S | 10 R | 25 S | 20 S | 24 S | ≥17/≤14 |
| Sulfamethoxazole/Trimethoprim | 27 S | 30 S | 31 S | 27 S | 21 S | ≥16/≤10 |
| Tetracycline | 25 S | 25 S | 25 S | 27 S | 23 S | ≥15/≤11 |
| Tobramycin | 23 S | 20 S | 20 S | 25 S | 25 S | ≥15/≤12 |
| Antibiotic | AMP (mm) | ENRO (mm) | Bio-AgNP (mm) | C1 (mm) | C2 (mm) | S/R (mm) |
|---|---|---|---|---|---|---|
| Amoxicillin-clavulanate | 22 S | 28 S | 25 S | 28 S | 30 S | ≥18/≤13 |
| Ampicillin | 10 R | 22 S | 24 S | 10 R | 20 S | ≥17/≤13 |
| Cefazolin | 10 R | 24 S | 20 I | 12 R | 25 S | ≥23/≤19 |
| Cefepime | 24 I | 30 S | 35 S | 25 S | 34 S | ≥25/≤18 |
| Cefoxitin | 10 R | 24 S | 20 S | 10 R | 25 S | ≥18/≤14 |
| Ceftriaxone | 30 S | 32 S | 30 S | 24 I | 30 S | ≥26/≤22 |
| Ciprofloxacin | 30 S | 22 I | 40 S | 32 S | 30 S | ≥26/≤21 |
| Chloramphenicol | 14 I | 24 S | 30 S | 12 R | 26 S | ≥18/≤12 |
| Enrofloxacin | 25 S | 16 R | 30 S | 22 I | 30 S | ≥23/≤16 |
| Fosfomycin | 24 S | 30 S | 26 S | 30 S | 30 S | ≥16/≤12 |
| Gentamicin | 17 S | 25 S | 25 S | 12 R | 30 S | ≥15/≤12 |
| Imipenem | 30 S | 35 S | 30 S | 35 S | 33 S | ≥23/≤19 |
| Nitrofurantoin | 20 S | 19 S | 19 S | 22 S | 22 S | ≥17/≤14 |
| Sulfamethoxazole/Trimethoprim | 20 S | 28 S | 29 S | 25 S | 30 S | ≥16/≤10 |
| Tetracycline | 20 S | 20 S | 21 S | 21 S | 23 S | ≥15/≤11 |
| Tobramycin | 16 I | 22 S | 20 S | 11 R | 20 S | ≥15/≤12 |
| Antibiotic | AMP (mm) | ENRO (mm) | Bio-AgNP (mm) | C1 (mm) | C2 (mm) | S/R (mm) |
|---|---|---|---|---|---|---|
| Ampicillin | 0 R | 40 S | 40 S | 20 S | 40 S | ≥18/≤18 |
| Azithromycin | 0 R | 25 S | 25 S | 25 S | 25 S | ≥18/≤13 |
| Cefoxitin | 0 R | 30 S | 25 S | 32 S | 30 S | ≥22/≤21 |
| Ciprofloxacin | 30 S | 0 R | 27 S | 30 S | 25 S | ≥21/≤15 |
| Clindamycin | 0 R | 30 S | 10 R | 20 R | 30 S | ≥21/≤14 |
| Chloramphenicol | 15 I | 30 S | 30 S | 30 S | 30 S | ≥18/≤12 |
| Enrofloxacin | 25 S | 10 R | 28 S | 30 S | 25 S | ≥23/≤16 |
| Gentamicin | 25 S | 30 S | 30 S | 30 S | 30 S | ≥15/≤12 |
| Nitrofurantoin | 0 R | 25 S | 25 S | 30 S | 25 S | ≥17/≤14 |
| Sulfamethoxazole-Trimethoprim | 0 R | 30 S | 26 S | 35 S | 35 S | ≥16/≤10 |
| Tetracycline | 20 R | 30 S | 30 S | 35 S | 30 S | ≥19/≤14 |
| Vancomycin | 0 R | 25 S | 20 S | 20 S | 20 S | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, M.H.d.M.; Endo, T.H.; Scandorieiro, S.; Pavanelli, W.R.; Kobayashi, R.K.T.; Nakazato, G. Silver Nanoparticle–Antibiotic Combinations: A Strategy to Overcome Bacterial Resistance in Escherichia coli, Salmonella Enteritidis and Staphylococcus aureus. Antibiotics 2025, 14, 960. https://doi.org/10.3390/antibiotics14100960
Santos MHdM, Endo TH, Scandorieiro S, Pavanelli WR, Kobayashi RKT, Nakazato G. Silver Nanoparticle–Antibiotic Combinations: A Strategy to Overcome Bacterial Resistance in Escherichia coli, Salmonella Enteritidis and Staphylococcus aureus. Antibiotics. 2025; 14(10):960. https://doi.org/10.3390/antibiotics14100960
Chicago/Turabian StyleSantos, Mariana Homem de Mello, Thiago Hideo Endo, Sara Scandorieiro, Wander Rogério Pavanelli, Renata Katsuko Takayama Kobayashi, and Gerson Nakazato. 2025. "Silver Nanoparticle–Antibiotic Combinations: A Strategy to Overcome Bacterial Resistance in Escherichia coli, Salmonella Enteritidis and Staphylococcus aureus" Antibiotics 14, no. 10: 960. https://doi.org/10.3390/antibiotics14100960
APA StyleSantos, M. H. d. M., Endo, T. H., Scandorieiro, S., Pavanelli, W. R., Kobayashi, R. K. T., & Nakazato, G. (2025). Silver Nanoparticle–Antibiotic Combinations: A Strategy to Overcome Bacterial Resistance in Escherichia coli, Salmonella Enteritidis and Staphylococcus aureus. Antibiotics, 14(10), 960. https://doi.org/10.3390/antibiotics14100960







